Over the previous few decades, nuclear energy has been regarded as a clean and efficient way to provide a stable electricity supply to the major developed countries and it is an indispensable part of the world's energy supply[1]. As shown in Fig. 1 and Fig. 2, current nuclear power plants are mainly distributed in the coastal developed areas and statistics indicate that as of 2022, there were approximately 400 nuclear power plants under operation on a global scale, mainly in countries such as the United States (94), China (80), France (57), Russia (40) and Japan (13), with a total installed capacity exceeding 400 GW[2-3]. Since nuclear energy possesses advantages such as low greenhouse gas emissions, and stable energy supply, there are over 30 nuclear reactors under construction worldwide[4-5]. As displayed in Fig. 3 and Fig. 4, USA, France, and Japan represented the main global energy consumers in the world between 2000 and 2011. The number of nuclear reactors reached a peak with nuclear consumption settled at around 2 130.8 TWh between 2013 and 2022[6-7]. Japan's energy consumption was 108-166 TWh between 2018 and 2022. The future of world's nuclear energy consumption is witnessing a transition period to achieve low-carbon and a cleaner development[4]. The core aim of the nuclear power use is to enhance sustainable development requirements[4]. However, there are still important challenges for the nuclear energy application to achieve sustainable development such as management of the radioactive waste, and prevention of nuclear proliferation[1]. Tab. 1 illustrates how past nuclear accidents at the Kyshtym, Chernobyl, and Fukushima Daiichi nuclear power plants have caused a negative impact on the surrounding ecological system both immediately and over time[8-22]. They also endanger the environment, food safety, and the mental and physical health of the general populace[8, 23-25]. The Kyshtym nuclear accident was a severe nuclear leaking accident occurred in 1957 near Kishtam, Russia, caused by an explosion of a storage container[26-27]. A significant amount of radioactive material was released, including 137cesium (137Cs), 90strontium, and (90Sr) into the surrounding soil and water system[28]. Previous studies revealed that the total radionuclide content of the soil close to the Chernobyl catastrophy site is still in high level, with 90Sr of 810 TBq and 137Cs of 4 000 TBq, respectively[29]. In Novozybkov district, a highly influenced area by the Chernobyl accident, activity concentration of 90Sr ranged from 70 to 600 Bq/kg in topsoils[30]in 2013, and Petrovi Ac'G2 et al[31] unveiled that the activity concentration of 137Cs in surface soils in Serbia was maximumly 180 Bq/kg, with a mean value of about 30 Bq/kg[31]. An 8-year survey from 2011 to 2019 on crop grain samples collected from Ivankiv district revealed that 90Sr and 137Cs activity concentrations outweighed the Ukrainian standard limitation in 50% of the samples and 90Sr in 75% of the pine tree surpassed the limits on firewood (60 Bq/kg)[32]. A survey from 2011 to 2016 revealed that 137Cs concentration in the milk collected from the Rivne region exceeded the national permissible level of 100 Bq/L and residents suffered from annual intake doses of over 1 mSv/a through the food chain[33]. Fukushima Daiichi Nuclear Power Plant (FDNPP) accident happened in March 2011, mainly caused by the Tōhoku earthquake and tsunami (Tab. 1 and Fig. 5). As a result of the Fukushima catastrophe, the liquid that was rich in 11 different types of radionuclides, including 3H, 14C, 137Cs, and 90Sr, was released[34].The accident was a top grade (grade 7) by the International Nuclear and Radiological Event Scale. Approximately 15-20 PBq of 137Cs or 134Cs and 0.04-1.00 PB q90Sr were FDNPP accident into the North Pacific Ocean[35-36]. The groundwater system also suffered from the radionuclide impact, in which the tritium concentration reached 52.7 Bq/L and 16.5 Bq/L in areas 25 and 50 km away from FDNPP[35-38]. In fact, following the FDNPP accident, Hamada et al[39] found radioactive contamination in various food (such as milk, vegetables, and seafood) and revealed that radionuclides in drinking water in Japan was higher than the levels permitted by the provisional regulations (1-2 000 Bq/kg). Studies[40] revealed that radionuclides originated from anthropogenic sources entered the environment and led a serious risk to humans being by way of the food chain. Other studies[41-43] have pointed out that 90Sr and radioactive Cs concentration also maintained in paddy soil around 300 Bq/kg. A 0.68-0.96 134Cs/137Cs activity ratio was found in all soils, indicating that the radio Cs discharged from the FDNPP was deposited in these regions[43-44].
表 1(Tab. 1)
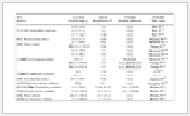
Tab. 1 Concentrations of Cs and Sr in environment near historical nuclear accidents
| Nuclear accident |
Location |
137Cs concentration |
90Sr concentration |
Reference |
| Kyshtym (1957) |
Techa River |
Fish: 18±8 Bq/kg |
Sylvaemus uralensis:
0.5 Bq/kg
Fish: >1.5×103 Bq/kg |
[8-10] |
|
| Sverdlovsk region |
Betula verrucosa leaves: 4.7+1.2 Bq/kg
Centaurea scabiosa: 37.0±12 Bq/kg |
Betula verrucosa leaves:
269.0 +24 Bq/kg Centaurea scabiosa:
4.5×104 Bq/kg |
[11-12] |
| Chernobyl (1986) |
Glubokoye Lake and Borschi watershed |
Carassius gibelio:
5.8 kBq/kg
Glubokoye water:
3.6 ±1.0 Bq/L |
Borschi water: 1.6-41.0 Bq/L
Glubokoye water:
100.0 ±11 Bq/L |
[13-14] |
|
|
| Myodes glareolus: 1.9×103 Bq/kg |
Myodes glareolus:
4.0 ×105 Bq/kg |
[15-17] |
| Fukushima (2011) |
Seawater near coast |
Seawater (within 6 km): 124.0±3 Bq/m3 |
10.0 Bq/m3-9.0 kBq/m3 |
[18-19] |
|
| Pacific Ocean |
WPO (1-2 year): 100.0-1 000.0 Bq/m3 |
NWP: < 2.7×105 Bq/m3 |
[20-21] |
|
| Land |
Mushroom: 2.5±93 Bq/kg Rice: 0.8±17 Bq/kg |
Mushroom < 0.4 Bq/kg
Rice: < 0.8 Bq/kg |
[22] |
| Notes: NWP and WPO stands for Northwest Pacific Ocean and Western Pacific Ocean respectively. |
|
Tab. 1 Concentrations of Cs and Sr in environment near historical nuclear accidents
|
Due to its geochemical similarities to calcium, 90Sr it is frequently found in the bones of marine fauna, and studies have showed that 90Sr accumulated in demersal fish (0.17-0.22 Bq/kg) from central and southern sites of the Fukushima coastal regions[45]. Prior to the FDNPP, the 90Sr activity in aquatic creatures (marine fish) in the surrounding areas ranged from 0.025 to 0.021 Bq/kg (fresh weight) and was about 2.0 Bq/kg within 20 km after the FDNPP[46]. The radionuclide bio-accomulation effect threatens the local ecological system and human health.
Radionuclides in wastewater can be migrated and diffused in water systems. Therefore, the treatment of nuclear wastewater is particularly necessary for the environment and ecological system. Current treatment methods of nuclear wastewater include chemical precipitation, physical adsorption, ion exchange, membrane technology, and bioremediation. Chemical precipitation is a fine method of high efficiency by converting radioactive substances into solid waste but with the disadvantage of complexity and subsequent treatment cost of these by-products[47]. Physical adsorption usually has high treatment efficiency for radioactive pollutants, but adsorbents have certain selectivity and saturation capacity[48]. Ion exchange is adsorbed from nuclear wastewater using ion exchange resin and they are desorbed through regeneration, possessing advantage of high removal rate for multiple radioactive nuclides but also the disadvantage of large amount of resin requirement and higher costs[49]. There are still issues in nuclear wastewater treatment and practical treatment strategies to reduce discharge levels. It is, however, difficult to find cost-effective way to treat various radioactive substances. The plan and decision of nuclear wastewater discharging is controversial and complex and requires long-term and sustained multi-party communication to effectively solve the issue. The current disposal strategy applied by the Japanese government may result in high expenses and future potential environmental risks. Sr, Cs, and other radionuclides having nearly 30-year half-lives, are examples of radionuclides that must be stored throughout an extended duration in storage tanks. The nuclear effluent that has been treated still contains reflection nuclides. There are few studies on the financial aspects of these technologies, even though many studies have proposed Sr treatment technologies and the evaluation of their removal efficiency. Some methods are not considered cost-effective solutions. For instance, nanotechnology and membrane technology can treat other radioactive elements like Sr, but their high cost restricts their use.
The region's principal emphasis in soil and water conservation is on the management of 137Cs, a significant pollutant, with less attention given to 90Sr. In marine environments, Cs and Sr may accumulate in coastal sediments, affecting both surface and deep soils. Japan's ocean discharge program also encounters soil contamination issues. Radionuclides, including Sr and Cs, may return to coastal soils due to weather conditions. This can lead to increased soil contamination and erosion, potentially spreading Cs and Sr to neighboring areas. The top few centimeters of soil in Fukushima's forests still contain significant amounts of 137Cs, which could continue to enter waterways[50]. Previous studies have demonstrated the potential for radioactive sediment to re-mobilize in coastal rivers following the 2013 typhoons[51]. These powerful storms caused soil erosion and the remobilization of contaminated material in dam reservoirs or alluvial plains. Soil exposure is influenced by rainfall intensity, potentially polluting downstream watersheds[51]. Rainfall and snowmelt runoff events transport particle-bound radiocesium downstream[52]. Typhoons in the fall exposed the entire watershed's soil to fresh material, with radiation exposures in newly formed sediment layers generally lower than those of adjacent soils[51]. The preferential delivery of particulate matter, which concentrates radioactive contamination in watercourses, reflects the fresh sediment supply from soil to rivers. Thus, it is expected that the degree of contamination in newly formed sediment deposits from recent erosion events is higher than the dose rate in adjacent soils[51]. The application of soil and water conservation techniques can mitigate soil transport, thereby reducing the spread of 137Cs and 90Sr in soils[53]. Within an 80-km radius, 137Cs rapidly moved from the litter layer to the mineral soil surface in forest settings[50]. Soil and water conservation is critical in mitigating the long-term impacts of radioactive contamination. The technologies can prevent pollutants (Cs and Sr) migration in different landuse areas and media.
This paper aims to (ⅰ) analyze the main pollutants and environmental hazards of Fukushima nuclear wastewater discharge, (ⅱ) summarize the current nuclear wastewater treatment technologies for Cs and Sr, and (ⅲ) provide a feasible nuclear wastewater treatment/discharge strategy based on technical, and environmental assessment, (ⅳ) identify potential feasible solutions to address the pollutants (Cs and Sr) in wastewater and the surrounding affected areas of Fukushima accident. The study will provide reliable scientific basis for the treatment of Fukushima nuclear wastewater and emergency response to related nuclear accidents at other sites worldwide, thus, to contribute to the world's nuclear energy industry sustainable development.
1 Methods
A search of the primary literature and the five main recent approaches of treating water pollution and preventing wind and water erosion (migration of pollutants into different media) at the scale of the contaminated area was carried out. This article reviews the main chemical, physical ion exchange and phytoremediation studies and applications in the previous fifteen years, and to compares the advantages and disadvantages of the different research methods, and to find potential ways of treating and preventing the migration of Sr and Cs.
2 Effects of Japan nuclear wastewater discharge
2.1 Fukushima nuclear accident
In some tanks'storage areas, four main elements (including 14C, tritium, Cs, and Sr) exceed the safe range. 3H, 14C, 99Tc, 125Sb, 60Co, 106Ru, 137Cs, 134Cs, 90Sr, and 129I were highly concerned by researchers, governments, and the public because of their high levels of concentration (especially for 90Sr and 137Cs, reaching up to 1575 Bq/L and 2.6Bq/L, respectively)[54-60]. The wastewater is considered a weak β-emitter and the ten main radionuclides were monitored by TEPCO[51-53]. The ocean absorbed about 80% of the radiation emitted into the atmosphere during the March 2011 FDNPP accident[57, 58, 60]. The FDNPP released radioactive fallout into the ocean and over a wide swath of Japan and global[52-55]. During the first several months, a high concentration of radionuclides in seawater and bottom sediments was detected[56]. To cool the nuclear reactor cores to a high temperature and avoid a meltdown, seawater was continuously applied by the TEPCO. The contaminated water was stored in about 1 000 tanks to prevent radionuclide releases and decrease the radionuclide concentration with multiple nuclear wastewater treatment systems containing the Advanced Liquid Processing System (ALPS) during the first months of the accident[55]. ALPS plans to decrease about 62 different radionuclides concentration with an effective efficiency. The water in the tank is a mixture of intentionally injected cooling waters and retrieved groundwater, both of which were contaminated by their interactions with the extremely radioactive nuclear reactor cores. As shown in Fig. 6, in 2011, Japan's nuclear power plants were reviewed and refurbished, leading to a drastic reduction in the number of nuclear reactors. Consequently, Japan's nuclear energy consumption percentage declined, and reactors that had been reviewed and approved for use began to be commissioned after 2015. The sharp decrease of Japan's nuclear energy consumption after the nuclear accident may cause significant impact on Japan's energy structure.
2.2 Treatment of radioactive wastewater
ALPS systems and the Kurion system were applied in radioactive wastewater treatment, which aim to remove the 62 radionuclides in the tank water. The Kurion zeolite system and the Simplified Active Water Retrieve and Recovery System (SARRY system), both of them use silicotitanate, are the two fundamental treatment systems that reduce the content of radiocesium from water supplied back to reactors for cooling. The ALPS pumping and filtration system try to eliminate 62 radionuclides from contaminated water through a sequence of chemical processes. However, tritium cannot be removed from the wastewater only by ALPS system[60]. The Kurion system includes adsorption beds that are specifically designed to separate radiocesium, an ultafiltration unit (UF) to remove colloidal materials, and stainless steel filters (SS filters) to remove coarse particles[60]. The transition metal hexacyanoferrate products CsTreat and sodium titanate SrTreat, respectively, are key players in the elimination of radiocesium and radiostrontium in ALPS[60]. However, monitoring of the nuclear wastewater in the storage tanks did not meet expectations. A few news reports also mentioned the unstable treatment of the ALPS system. According to TEPCO data, ALPS's efficacy in treating 90Sr was incredibly unpredictable. 90Sr activity concentrations in parts of the water exceeded the discharge concentration limit by a significant margin, reaching 104 Bq/L. (Japanese domestic standard: 30 Bq/L)[55, 61-62]. The Japanese domestic maximum release criteria for 137Cs and 90Sr are 90 Bq/L and 30 Bq/L respectively. Both 137Cs and 90Sr in WHO standards are 10 Bq/L[55, 62]. The average of 90Sr concentration in area B, G1 South, G3, J2, J5, G3, J1, and K2 is at a high level, which is 6 414, 1 324, 598, 33 543.3, 398, 478, 1 454, and 147 Bq/L respectively. Some tanks' Sr and Cs concentrations exceed Japanese domestic and WHO standards, like G1 in J1 area (61 and 4 550 Bq/L) and A1 in G3 area (82 and 30 500 Bq/L). Multiple excess radionuclides were detected in the tank water, thus TEPCO undertook secondary treatment of a portion of the nuclear wastewater with the ALPS systems. More than 60 elements exist in storage tanks, including Cs, and Sr, exceeded the intended safe range. As shown in Fig. 7, it compares the areas where both Cs and Sr exceeded the standard of WHO international drinking water (10 Bq/m3). The Cs and Sr concentration in tanks (nearly 500 tanks and 36 areas) were detected by TEPCO on September 30, 2023. The number of measured tanks in H6(I), J2, J1, G3, and K1 was 4, 6, 20, and 4 respectively. Both Cs and Sr concentration in 5 of 36 detected areas (H6(I), J2, J1, G3, and K1) exceeds the international drinking water standard. The H6(I) and G3 areas have the most serious percentage of exceedances. In addition, the number of barrels with Cs concentration exceeding the international drinking water standard reached 10 tanks and areas in J2, J1, H6(I), G3, and K1. The number of barrels with Sr concentrations exceeding the international drinking water standard reached 68 tanks, which were mainly distributed in areas B, G1, G3, J1, H6(I), J2, J4, J5, K1, and K2.
2.3 Discharge of radioactive wastewater
Fig. 8 shows structure and scheme of radionuclide liquid storage and discharge plan in Japan. The ALPS treated water is transferred into a treated water pump tank and diluted with seawater. Based on the discharging program, the diluted wastewater is emitted through a 1 km length pipe into the ocean. The government gave TEPCO permission to discharge the treated wastewater into the ocean over a 30-year period on April 13th. About 31, 200 tons of wastewater tanks were planned to be discharged into the ocean until March 2024, when just 10 of the 1 000 tanks will be emptied and the empty tanks will be continuously filled with wastewater from the FDNPP[63]. According to the latest data, the mean value of 90Sr and 137Cs in tanks achieves 1 575 Bq/L and 2.6 Bq/L, respectively[64]. For the concentration of 90Sr, it does not meet the Japanese government criteria or WHO drinking water standard. The concentration of 137Cs meets the criteria of the Japanese government and WHO criteria. However, analysis of accurate data still needs further confirmation and evaluation.
2.4 Impact on the fishing industry and marine ecosystem
The fishing industry will face the problem of reduced exports and a long-term reduction in seafood consumption. The study by Guo et al[65] confirms this view that the continued discharge into the sea led to decrease the exportation of Japanese seafood and caused around 259 million USD in losses.
A range of economic and environmental impacts have been studied in relation to the ocean discharge[66-67]. However, the study[68] pointed out that while concentrations and annual human intake of the major radioactive elements of tritium (1 μSv/year) did not have a direct negative impact on fish and humans in a short time, there was still concern for the potential effects of radioactive heavy metals, especially Sr and Cs, which are the two of the ten main radionuclides in the tanks. Cs is mainly deposited in the ocean because of its high distribution coefficient KD value. Although the concentration of Cs in nuclear wastewater in the storage tanks was very low, according to the data, low concentrations of radioactive Cs remaining in sediments may still be accumulated in benthic organisms. The Cs may accumulate in the bodies of predators through the food web. Some studies have shown that the radiation activity of nuclear wastewater in the storage tanks can be completely reduced to a biologically acceptable level after 40 years.
Another species in the region that has shown accumulation of Cs+ is crab. Bendriss et al[69] found that crab's intestines may reach 13 600 Bq90Sr and 1 300 Bq137Cs. Activity concentrations of 90Sr Macroalgae in surrounding marines may reach 170 Bq/kg[70]. Therefore, if radioactive wastewater is dumped into the ocean, there is a risk of environmental harm for hundreds or thousands of years to come. Through predation, seafood networks establish strong connections amongst marine organisms. Besides the Fukushima industry, further environmental recovery and sustainable development may be prevented by the nuclear wastewater discharging program[71]. Because of the 30-year discharge procedure, local businesses may suffer, and the post-disaster socioeconomic recovery may be hampered by the spread of false information among customers[71]. This phenomenon could be caused by the distrust of the government discharging program and the concern about water quality.
Thus, the decision by the government and TEPCO will have an impact on the export and import of marine products both domestically and internationally. Wu et al[66]showed that that nuclear wastewater discharging had an impact on a total loss of 2 348×106 US$ in 23 countries under scenario 1 (the Japanese fishery products final demand may decline by 34.4%). The effects of the radioactive wastewater were amplified and diluted by the ocean currents. Fisheries in neighboring nations and areas across the Pacific Rim may be directly impacted by Japan's release of radioactive effluent[64].
3 Development of nuclear wastewater treatment technology
The potential capacities of different technologies for the Cs+ and Sr2+ removal can be evaluated by[67, 72-73]:
where Qe is the equilibrium sorption capacity[72-73], mg/g. The Re indicates the removal rates[67, 74] of Cs+ and Sr2+. V is the solution volume for the test and m(g) is the mass of (ion exchanger or chemical and physical absorbent) that participate in Cs+ and Sr2+ removal processes.
3.1 Chemical precipitation
Chemical precipitation involves the interaction between dissolved metals and a precipitation agent in aqueous environment, leading to the formation of metal precipitation, which is widely applied in industry scale[75]. Further application of flocculation using coagulants can increase the size of precipitation particles to separate the particles from wastewater and transform them into sludge[76]. As shown in Tab. 2[77-83], in a study of Bengiat et al[84], it was discovered that aqueous organic ligand can promote the Supramolecular complexation between 137Cs and the rigid ligand, resulting in the selective precipitation of 137Cs in solution. Concerning Sr precipitation, the phosphate and carbonate pathways are predominantly targeted, leading to the formation of Sr3(PO4)2 and SrCO3. The study highlighted that approximately 1 kg of solid residue with a total beta activity of 100 MBq can be obtained by wastewater treatment of one cubic metre. The resulting effluent exhibited extremely low radioactivity levels, with 0.6 kBq/L for Sr and 0.2 kBq/L for 14C[85]. Tokunaga et al[85] utilized barite (BaSO4) to remove more than 90% Sr in a marine water environment. This material (BaSO4) shows potential for application in nuclear wastewater treatment. The study of Hodkin et al[80] offers the possibility of coprecipitation of 14C and Sr, but the resulting high intensity solid residue treatment has not been investigated which may limited methods application in industry. In addition, the method is applicable to nuclear wastewater treatment in groundwater, but further studies are still needed for the crystallization and occurring recrystallization of carbonates in different aqueous environments (e.g., treatment of Sr in Fukushima wastewater).
表 2(Tab. 2)

Tab. 2 Comparison of different nuclear wastewater treatment technologies
| Technology |
Mechanism or formula |
Contaminants |
Removal
rate/% |
Qm/
(mg·g-1) |
KD/
(ml·g-1) |
Reference |
| Membrane filtration |
GO and DTS membrane
One-step hydrothermal route to synthesize pharmacosiderite type titanosilicate combining Na+ and K+(DTS) |
Cs and Sr |
Cs+ (10 ppm):
99.3; (100 ppm): 94.0
Sr2+ (9 ppm): 99.7
Sr2+ (1 ppm): 92.0 |
Cs+: 499
Sr2+: 223 |
Cs+: > 105
Sr2+: > 105 |
[77] |
| Membrane filtration |
SbS-1K+Cs+→SbS-1Cs+K+(PH 2) SbS-
1K+Cs++Sr2+→SbS-1CsSr+K+ (PH 6)SbS-
1K/PTFE membrane (potassium
thioantimonate K2Sb4S7·2H2O) eliminating
and separating |
Cs and Sr |
Cs+: >99.9
Sr2+: >98.2 |
Cs+: 71.0
Sr2+: 49.5 |
Cs+:
1.1 ×105 Sr2+:
1.9 ×105 |
[78] |
| Chemical precipitation |
137Cs: (Fe4[Fe(CN)6]3) 4Fe3++3[Fe(CN)6]4-XH2O→Fe4[Fe(CN)6]3
(Chemical precipitation)
KFe[Fe(CN)6]+Cs+→CsFe[Fe(CN)6]++K+
Sr (BaSO4 co-precipitation)
Sr++SO42-→SrSO4 |
Cs and Sr |
Cs+: 99.9
Sr2+: 99.5 |
|
| [79] |
| Chemical precipitation |
Sr2++CO32-→SrCO3 |
Sr |
Sr2+: ≥97.0 |
|
| [80] |
|
| Na3Mn4Si10O26·3H2O |
Sr |
Sr2+: ≥98.0 |
Sr2+: 249.0 |
Sr2+:
6.6 ×106 (pH=6) |
[81] |
| Bioremediation |
Saccharina japonica |
Sr |
Sr2+: |
Sr2+: 5.5 |
| [82] |
| Bioremediation |
Sargassum horneri |
Sr |
Sr2+: |
Sr2+: 9.3 |
|
| Adsorption |
Hydrate pelletizing |
Cs and Sr |
Sr2+: 83.0
Cs+: 86.5 |
|
| [83] |
| Notes: The xx ppm in the bracket indicates "xx parts per million"adsorbate solution (Cs+) concentration. Qm is the maximum adsorption capacity. |
|
Tab. 2 Comparison of different nuclear wastewater treatment technologies
|
The Bengiat et al[84]and Tokunaga et al[85] methods, however, cannot remove Sr and Cs at the same time and are merely effective for one of these. Application of more than two methods to remove Sr and Cs will make the treatment process more complicated. Wu et al[86] pointed out that the hydraulic pellet co-precipitation microfiltration (HPC-MF) process is a chemical precipitation method in the application of Sr removal. When the concentration of seed crystal was 0.3 g/L, the sodium carbonate dose was 1 000 g/L, and ferric chloride dose was 10 g/L, and strontium could be effectively removed. Moreover, previous research has proposed the co-precipitation of stable and radioactive isotopes as a method to enhance process performance due to their similar chemical properties[87]. It is crucial to understand that a significant amount of radioactive slurry is inevitably produced because of chemical precipitation. As shown in Fig. 9, another approach for the removal of 137Cs involves co-precipitation with K4[Fe(CN)6] ·3H2O and BaSO4. The effectiveness of 137Cs removal during BaSO4 co-precipitation and K4[Fe(CN)6] ·3H2O processes can be significantly improved by increasing the Fe3+ concentration in the wastewater[79]. The optimal conditions for the removal of 137Cs and 60Co have been determined to be 0.001 M K4[Fe(CN)6] ·3H2O and 0.003 M Fe3+, respectively. For the removal of 90Sr, a [Ba2+] ∶[SO42-] ratio of 1 ∶2 with a [Ba2+] concentration of 0.06 M must be achieved. By following these conditions, the radioactivity of the wastewater in solution can be reduced from 37.7 to below 0.05 Bq/mL for 137Cs, and 0.37 to 0.02 Bq/mL for 90Sr. However, the problems of current applications are in the separation between solids and liquids after precipitation and in the disposal of solid wastes. Most studies have focused on the effectiveness of different chemical precipitations for the removal of single radionuclides or multi-nuclide removal.
3.2 Adsorption using different adsorbents
The process of physical adsorption involves the heavy metals diffusional movement into the carbon adsorbents' pores. The adsorbents' surface area and distribution of pore size significantly affect physical adsorption[88]. The adsorption mechanism is determined by adsorbents properties and heavy metals, as well as temperature, adsorbent amount, pH value, adsorption time, and initial metal ion concentration. Previous studies have examined the removal rate of Cs+ and Sr2+ using various adsorbents, including sodium manganese silicate (Tab. 2) Hydroxyapatite (HA)[89], titanium doped hydroxyapatite (Ti-HA)[90], TitanoSilicate (DTS)[77], bentonite, attapulgite, and zeolite[91-92] and hydrate pelletizing[90]. Shen et al[81] carried out a study on the production of sodium manganese silicate (SMSO) using the one-pot hydrothermal method. The findings revealed that SMSO exhibits a consistently high absorption capacity, with an Sr2+ removal rate of nearly 98% across a wide range of pH values (3-12). Furthermore, SMSO demonstrated effective removal of competing ions. The study highlights the potential application of SMSO in Sr2+ absorption[93]. The Fiskum et al[94] point out that application of spherical resorcinol-formaldehyde resin in the Cs removal did not result in a high absorption capacity, as this was inhibited by higher temperatures. After 17 process cycles, it was discovered that when the temperature rose from 25℃ to 45℃ there was a 17% decrease in the Cs capacity of the sRF resin [0.09 M K simulant][94]. A tin antimonate sorbent was created by Zhang et al[95] to remove strontium and cobalt ions.Nanoscale rutile crystals and phyrochlore structure comprise the sorbent. Tin antimonate exhibits a significant strontium-binding capability in the pH range of 2-12[95]. Rae et al[74] studied the biosorbents (crab carapace and spent distillery grain).The major processes for Sr2+ removal that were indicated by the characterization of biosorbents (crab carapace) both before and after Sr2+ sorption were ion-exchange and outer- sphere complexation.Removal of Sr2+ at concentrations that are relevant to industry from aqueous media (adsorption capacity: 3.92 mg/g). Schematic of adsorption method was displayed in Fig. 10.
Kim et al[77] observed that DTS maintains a high ability to adsorb Sr2+ and Cs+ under various conditions, including contaminated underground water, tap water, and seawater. Within tap water and groundwater contaminated systems, the Cs+ and Sr2+ KD values were discovered to outweigh 105 mL/g (% removal >99%), indicating the DTS displayed a well effectiveness in these ions simultaneous removement[77]. Li et al[91] conducted an analysis of six different adsorbents. The results ranked the adsorption efficiency of Sr as follows: zeolite, bentonite, attapulgite, montmorillonite, activated carbon, and kaolin. Zeolite had the maximum capacity for adsorption of 4.1 mg/g among them.
3.3 Membrane filtration
Membrane filtration has been extensively researched and applied in recent years, including the use of MOF (metal-organic framework)/graphene oxide, nanoribbon-based Nickel-MOF composites, and polyvinyl alcohol hydrogel membranes. The study conducted by Wang et al[96] demonstrated that a graphene oxide (GO) membrane's reaction to pH results in a high selectivity and permeability for monovalent salt ions, specifically Sr2+. The interaction between the NH3 and GO functional groups can be accelerated, and the decoration of GO with N-groups can be enhanced through the application of NH3 vapors in GO membrane N-functionalization[97]. This method prevents composite sheet aggregation[96]. Experimental results indicate that the Sr2+ recovery percentages range from 35% to 60% with multi-reusability, and the ideal Sr absorption capacity can reach 475 mg/g. Regardless of the exposure duration of NH3 and precursor GO membrane, the Sr absorption capacity can be enhanced twice by GO membranes with functionalized nitrogen groups. The synthesis of MOF/graphene oxide (GO) composite membranes involves the suction filtration of electrostatic self-assembly Ni-MOF (Ni-MOF nanobelts) and GO sheets. These membranes have freestanding forms in addition to interlaced structures[98]. Batch experiments conducted after a 24-h adsorption period showed that the membrane with a high Ni-MOF content had an optimal Sr2+ removing capacity, outperforming both bare GO and a membrane with a low level of Ni-MOF content (2 mg)[98]. The desalination and reverse osmosis membranes were assessed by Kim et al.[99] in the Cs, Co and Sr separation in seawater. For single nuclide, RO membrane rejection is greater than 93%, and for mixed nuclide separation, it is much higher than 98%. Although both membranes maintained good adsorption capacity in the experiments, the removal capacity of the membranes after multiple uses was not well evaluated. Some membrane technologies such as polyvinylidene fluoride (PVDF) membrane[100] showed wide applications for Cs+ and Sr2+ removal. However, the removal of Sr and Cs isotopes (radioactive hazardous solutions) was not supported by good experimental results. Schematic of membrane filtration was displayed in Fig. 10.
3.4 Ion exchange
Ion exchange is a process used to decontaminate radioactive wastewater by employing inorganic and organic exchange materials, as well as composites. In their study, Zhu et al[101] utilized cadmium selenidostannate, [CH3NH3]3[NH4]3Cd4Sn3Se13 ·3H2O (CdSnSe-1), to accomplish ion exchange and capture Sr2+ and Cs+ using mono-lacunary super tetrahedral clusters (a microporous Cd-Sn-Se). The results demonstrated effective removal/adsorption of Cs+ and Sr2+ from slightly contaminated nuclear wastewater, with their levels substantially reduced. The superhigh exchange capacities were recorded as 371.4 mg/g for Cs+ and 128.4 mg/g for Sr2+. Li et al[102] studied potential materials ([Me2NH2]6In10S18 and [MeNH3]5.5[Me2NH2]0.5In10S18 ·7H2O), which are three-dimensional (3D) cluster-based microporous metal sulfides to remove the Cs and Sr in wastewater. The removal rate was 95.7% and 96.2% after two absorption cycles. Inorganic sorbents such as titanosilicates and hexacyanoferrates AkMn[Fe(CN)6] have been utilized in ion exchange due to their chemical and radiation stability[103]. The Behrens et al[104] evaluated the Sr and Cs Na4Ti9O20 ·xH2O with underground water and aqueous waste scenario all the ion exchanger removal of 89Sr outweigh 97%, and the removal of 137Cs outweigh 97%. However, there was a lack of the removal of 90Sr assessment and the experiments may require an assessment of in a scenario with solution full of competitive ions. Amesh et al[105] found the titanate based compound, sodium iron titanate (NaFeTiO) ion exchange processes for the Cs and Sr removing. It was discovered that strontium absorption capacity was 233 mg/g and that KD values of Sr rose up to pH 6. On NaFeTiO, the rate of caesium and strontium ion exchange was fast during the first 200 minutes of equilibration, and equilibrium was thereafter established.
3.5 Bioremediation
Utilizing microorganisms or plants to get rid of toxins from the environment at a reduced cost is known as bioremediation, and it can be expanded to remove contaminants from soil, air, and water[106]. Through the use of green plants, phytoremediation transfers, absorbs, or changes contaminants so they are safe for the environment[107-108]. A series of studies showed the possibility of the application of algae organism and bacteria inbioremediation[109-110]. The two kinds of brown algea Saccharina japonica and Sargassum horneri can be an potential effective biosorbents for absorbing superfluous Sr2+ in seawater[82]. S. japonica and S.horneri had Sr2+ concentrations of 5 534 mg/kg and 9 320 mg/kg, respectively. These were >90 and 230 times the Sr2+ concentration in seawater (7.4±0.4 mg/L), respectively. Recent research has pointed out that the Gloeomargarita lithophora with the function of intracellular calcium carbonates forming has a high absorption (radionuclide removal 99%) rate of 90Sr and 226Ra in solution and 90Sr activity declined by 45.3 kBq/L in 1 h[109].
4 Soil and water conservation technologies
4.1 Technologies in soil radionuclides (Cs and Sr)
The migration of pollutants, including Sr and Cs, into deeper soil profiles can lead to water and soil erosion, which can be mitigated through soil and water conservation measures (SWCM). Various chemical and physical methods, such as interpolyelectrolyte complexes (IPCS)[110-112], flue gas desulfurization gypsum[113], inorganic coagulants[114], divalent and monovalent ion washing agents[115], and ordinary Portland cement-based binders[116], are widely applied in soil and water conservation programs. According to Zezin et al[112] IPCs effectively reduced wind erosion, resulting in soil removal of < 0.5 mg/min under wind speeds of < 40 m/s. The stability of the IPCs, also known as IPCs binders, was promoted by the cooperative character of multisite electrostatic complexation with polyelectrolyte counterparts, which was related to the splitting up of polyelectrolyte counterparts[112]. The vicinity of the Chernobyl catastrophe has seen extensive usage of IPCs[112], which developed a structured top layer that solidified after drying and turned plastic after additional wetting. This is due to water's potent plasticizing impact on IPCs, which allowed for some swelling but not water dissolution[117]. The process involves extracting radionuclides from contaminated finely divided soil fractions with different densities[112]. Mikheikin et al[111] found that IPCs allow for the recovery and concentration of up to 95% of the radionuclides into a tiny volume (10%-15% of the original one)[111]. For inorganic coagulants, K+ and Ca2+ solutions represented a high outweigh of the 0.075 M EDTA and 0.05 M phosphoric acid, which both lowered the efficiency of Sr and Cs removal rate below the 40%. According to Wan et al[113], removal rates of Sr and Cs were found to be 68.2% and 81.3% in Ca2+ and K+ solutions (produced from dissolving CaCl2 and KCl in distilled water), respectively. However, the change of soil chemical and physical properties before and after washing by the K+ and Ca2+ solutions requires an assessment to make a comprehensive comparison with the traditional inorganic coagulant[113].
Additionally, phytoremediation and plant cover, which act as biobinders and stabilizers to accomplish removal and release the impact on soil contaminants, are also applied in Cs and Sr soil pollution treatment[118-119]. Recent research showed that two main species, Alstonia scholaris and Arabidopsis halleri, perform well in Cs and Sr treatment. A.halleri, a hyperaccumulator, can absorb substantial concentrations of several metals into its above-ground organs (e.g., leaves) without exhibiting noticeable toxicity[120]. It accumulated Sr more quickly than Cs, with transfer factors for Sr (>184) compared to Cs (>16). The findings showed a favorable association between the buildup of Cs and Sr and the transfer of K and Ca to leaves. Singh et al[121] pointed out that the absorption pattern of A.scholaris for calcium was 5 452.8-24 771.4 mg/kg DW (TF (transfer factor)=85.2-57.6), whereas the uptake pattern for sulfur was 1 307.4-8 705.7 mg/kg DW (TF=85.3-1.46). The improvement of plant cover effectively prevented soil erosion and change soil properties, which reduced the migration and diffusion of radionuclides (Cs and Sr) in the soil[121].
4.2 Water conservation technologies in catchment and basin
The nuclear leakage accident at Chernobyl led to the redistribution of pollutant 137Cs through soil and water erosion and fluvial processes, which was redistributed to downstream and surrounding areas[122-123]. Research indicated that soil loss from cultivated fields reduced the 137Cs inventories in catchment and basins, thereby limiting the migration and transformation of pollutants in surrounding areas[123]. The mobilized sediment from catchment slopes may reach the river system through the local relief and the complexity of the river and basin delivery system, affecting different pathways of the river system in Fukushima[123]. The Abukuma River valley, located in the interior, was less erosive to rainfall than coastal plains and mountain ranges, as indicated by studies in Fukushima[122-126]. Almost all the 137Cs (96.5%) of the Abukuma River were emitted into the ocean, occupying 12 TBq in total between June 2011 and August 2015. According to Taniguchi et al.[124], urban areas, farmland, and paddy fields were the primary sources of 137Cs fluvial transport, accounting for 85% of the discharging sources in 38% of the watershed areas. According to Yamashiki et al.[125-126], 84%-92% of the radiocesium in the basin moved and transferred through the river system as particles. The Abukuma basin, which was thought to be the most affected by the FDNPP accidents, showed high concentration radiocesium fluxes. The combination of ozone and tannic acid-based organic composite adsorbents may efficiently remove several radionuclides from rivers, throughout an extensive temperature and pH spectrum (1.9-7.6). The maximum absorption capacity of Cs and Sr achieved 1.8×10-3 mol/g and 5.6×10-4 mol/g, respectively[126]. Additionally, the complex absorbents for I- and IO3- represented a high level, exceeding more than tenfold magnitudes compared to other materials like Ag2O-T3NT (Titanate nanolamina with Ag2O nanocrystals) with 571 mg/g[127] and microporous zirconium silicate with 216 mg/g[128]. In Tab. 3[129-142], other materials also indicate a great level, such as Caustic-pretreated-yeast-poly(acrylic acid) (Cs: 229.5 mg/g; Sr: 166.8 mg/g)[129] and pre-activated clinoptilolite (Cs: 140 mg/g; Sr: 52 mg/g)[131]. However, there is a lack of river condition experiments to evaluate their performance.
表 3(Tab. 3)

Tab. 3 Comparison of different soil and water conservation technologies
|
| Method |
Contamiants |
Mechanism or main
materials |
Scope of application |
Removal rate/% |
Qm/Flux/
Transfer |
Reference |
| Absorption |
Caustic-pretreated-yeast-poly(acrylic acid) |
Cs and Sr |
Interconnected superporous structure by a one-step solution polymerization of acrylic acid (AA) monomers in the emulsion |
Basin and catchment |
| Cs+: 229.5 mg/g
Sr2+: 166.8 mg/g |
[129] |
| Chemical precipitation |
Pre-activated clinoptilolite
(NaCl-Clinoptilolite at 300 ppm) |
Cs and Sr |
Pre-activation using the NaCl solution |
Basin and catchment |
Cs+: >99.6
Sr2+: 84.4-61.3 |
Cs+: 140.0 mg/g
Sr2+: 52.0 mg/g |
[130] |
|
| GO/PB-modified NF membrane(M10) |
Cs and Sr |
PB: Enhancing adsorption sites |
Underground water |
Cs+: >99.5
Sr2+: >97.5 |
Cs+: 1133.0 μg/g |
[131] |
| Membrane filtration |
High Rejection Seawater Reverse Osmosis Membrane (SW 30 HR) |
Cs and Sr |
GO: Improved the Donnan effect in polyamide (PA) layer
PEG: Promoted the uniform dispersion |
Underground water |
Cs+: 92-
96Sr2+: 99 |
Cs+ flux: 3.2-0.2 mmol/(m2·h) |
[131]
[132]
[133]
|
|
| Carbon nanotube membranes |
Cs and Sr |
Cs+-electrostatic interactions
Sr2+-inner-sphere complexation |
Underground water |
Sr2+: >96.6 |
Cs+: 0.81 mmol/g
Sr2+: 4.41 mmol/g |
[134]
[135]
[47]
|
| Physical treatment |
PBR with Ca, citrate and PO4- solution |
Sr |
Establishing an ongoing, diffuse calcium phosphate (apatite) barrier |
Underground water |
Sr2+: 88.7-86.2 |
| [136]
[137] |
| inorganic polymer resin Clevasol (PBR) |
Cs and Sr |
Clevasol-PVACC |
Underground water |
Cs+: 99.9
Sr2+: 99.8 |
Cs+: 3.3×107 mL/g
Sr2+: 2.1×107 mL/g |
[138] |
| Bioremediation |
Reducing the radionuclides flux to prevent the migration |
Cs |
Camellia japonica |
Soil (Forestry areas) |
Cs+ flux: 2 810.0 Bq/(m2 ·a) |
[139]
[140] |
| Cs |
Quercus serrata |
Soil (Forestry areas) |
Cs+ flux: 375.0 Bq/(m2 ·a) |
|
| Phytoextraction |
Cs and Sr |
Arabidopsis halleri |
Soil (Agricultural and forestry areas) |
Cs+ transfer factors: 16.5
Sr2+ transfer factors: 184.0 |
[140] |
| Cs |
Chromolaena
odorata |
Soil (Agricultural and forestry areas) |
Cs+: 73.7 |
[141] |
| Cs |
Calendula alata |
Basin and catchment |
Cs+: 46.3 |
[142] |
Chenopodium
album |
Cs+: 53.0 |
Amaranthus
chlorostachys |
Cs+: 50.3 |
| Notes: The Bq/(m2 ·a) refers to becquerels per square meter per year. The Qm indicates the maximum adsorption capacity for removing Cs and Sr. |
|
Tab. 3 Comparison of different soil and water conservation technologies
|
4.3 Soil and water conservation technologies in groundwater
The majority of ions in groundwater, including radionuclides, are retained via reverse osmosis (RO). The concentrated retentate stream (usually 10%-15% of the initial volume) from the RO system contained the radionuclides and common ions found in groundwater, requiring treatment before disposal[143]. A Prussian blue and graphene oxide modified nanofiltration membrane was fabricated to remove radionuclides Cs+ and Sr2+[131]. Effective rejection of caesium (Cs+, 99.5%) and strontium (Sr2+, 97.5%) was accomplished by the modified membrane. Even after treating naturally occurring surface water that contained a variety of inorganic salts and organic materials, its Cs+ rejection rate was still high (about 96%). Another membrane of carbon nanotube membranes with Ar/O2 plasma-treated, presented a high selectivity on Cs+ and Sr2+[47]. The membrane maintained selectivity for monovalent cations (perhaps due to electrostatic interactions) whereas divalent cations promoted the development of inner-sphere complexation[135]. According to Ali et al[135], the functionalized carbon nanotube membranes' distribution coefficients (KD) for divalent cations, such as Sr2+, were determined to be 4.4 mmol/g, whereas those for monovalent cations, such as Cs+, were 0.8 mmol/g. The highest Cs and Sr KD values were found in plasma-functionalized MWCNT (P-MWCNT) membrane, which reached 4.4 mmol/g and around 1.1 mmol/g in a real wastewater sample[135]. The seawater reverse osmosis membrane (SW 30) achieves a Cs+ removal rate between 92%-96% and a Sr2+ removal rate of 99%. The Cs+ and Sr2+ removal rates of the high rejection seawater reverse osmosis membrane (SW 30 HR) are above 96% and reach 99%, respectively[133].
Fig. 11 illustrates the process of PRB (Permeable Reactive Barriers) treatment, which involves vertically positioning a reactive media in line with the direction of groundwater flow to trap pollutants and radionuclides under natural hydraulic gradients[137]. The passive, in situ treatment of groundwater using PRB is a reasonably priced technique[144].Only the removal of 99Tc and 90Sr at scale demonstrated the effectiveness of PRBs, a less invasive alternative[137]. To intercept the pollutant plume beneath naturally occurring hydraulic gradients, a PRB must be positioned its reactive media perpendicular to the direction of groundwater flow[136]. The Torres et al[136] study pointed out that activity concentrations showed a slower secondary reduction (λ2) after a year. Although enabling groundwater to flow through the barrier, the reactive medium changed pollutants into an immobile or less dangerous form. The ability of microwave(MW) to remove Cs from GAC (granular activated carbon) was demonstrated by regeneration yield (δ=79%-110%) and WL weight loss (6.8% for 10 cycles) values in lab-scale data[144].
4.4 Soil and water conservation technologies in different land use
The study by Kitamura et al[145] assessed the soil erosion of 137Cs in crop fields using soil and water conservation technologies (SACT). Crop fields lose the most soil on average per unit area, with wasteland coming in second. The annual soil erosion of crop fields and forest areas per unit area reached 8 t/(hm2 ·a) and 0.6 t/(hm2 ·a), respectively, with 67% of the initial radiocesium remaining in forested landscapes[145]. Additionally, a series of engineering appliances were built after the Fukushima accident (e.g., groundwater bypass, bore rehabilitation, ice wall, and subdrain system) to isolate pollutants migration and expand[146-147].
4.4.1 Forestry land use
Forest areas are the primary source of Cs migration in eastern Fukushima. Although forest study areas only caused 24% of the total soil loss, the137Cs migration accounted for 64%. In Japan, alpine meadows and foothills were used as pastures[122]. Forest areas continued to represent more stable repositories of contaminants[137]. According to Onda et al[50] 2 600 km2 of forest received fallout >100 kBq/m2, accounting for the majority of FDNPP 137Cs fallout in the terrestrial environment. The Konoplev et al[122] study noted that conifers, which can better intercept and remove radioactive and aerosol particles, were relatively efficient in removing radionuclides such as 137Cs from forest land. Some studies indicated that the removal of woods and trees increased surface runoff[145, 148]. The study pointed out that the special species can maintained a higher soil hydraulic conductivity and prevent the surface runoff compared to the livestock grazed forest[148]. When livestock graze beneath trees, their ability to mitigate flooding was compromised[148]. Planting specific trees, which had a trait of low depositional fluxes of radionuclides, can limited the accumulation of Cs and Sr pollution in forest areas' soil. The cedar can be a potential species to prevent radionuclide spreading. The study by Feng et al[139] pointed out that the 137Cs flux generated from the oak (Quercus serrata) and cedar (Camellia japonica) stands was 2 810 and 375 Bq/(m2 ·a), respectively. The higher 137 Cs flux provided more air-source pollution[140]. The study results were similar to the previous discovery, which found that deciduous trees and grasslands were less effective interceptors and scavengers of radioactive and aerosol particles. Additionally, the Cs and Sr distributions inside the trees were different in the canopy, root, and leaves. The results revealed that 20%-40% of foliar 137Cs were located inside the leaf, while 60%-80% clung to the leaf surface[149]. Before the accident, the 137Cs/133Cs ratios within the sprouting leaves were significantly greater than those found in the soil extract and lower than those found in bulk precipitation and throughfall. During or shortly after radioactive fallout, the foliar uptake and subsequent translocation of 137Cs are significant contamination vectors in a variety of tree species[149]. The sedum can also be an option for the soil in the rhizosphere of Phyllostachys violascens. The growth and uptake of heavy metals by bamboo and sedum intercropping enhanced the overall extraction of heavy metals that were accessible[150].
The radionuclides were retained by clay mineral layers[151-152]. The primary determinant of the radiocesium interception potential was micaceous minerals[151]. For radioactive soil, hydrothermal treatment produced effective Cs desorption (96%). With an increase in treatment temperature, Mg2+ diffused into collapsed interlayers from the near-edge to the central area, easily substituting anhydrous Cs+ from these regions[152]. The Cs could be almost removed at 250℃[152].
4.4.2 Agricultural land use
The plant's root system plays an important role in increasing the pollutants and plants contact area, accelerating absorption procedure. Meanwhile, the function of remaining soil stabilization, curbing erosion, and reducing water permeation can be contributed by extensive plant root systems[150]. The majority of sediment (μ 76, σ 14%) was supplied by fluvisols, an alluvial soil type that was commonly used for paddy fields, according to the results of geochemical modelling[153]. For the agricultural land use areas, phytoremediation sustainable strategies to prevent the nuclear spreading by the potential soil and conservation problems. In polluted soil, phytoextraction effectively eliminates contaminants like radionuclides and heavy metals[154]. Heavy metal radionuclides in the soil can be taken up by roots and moved to parts or shoots that can be harvested. This process includes metal mobilization, uptake, compartmentation, and chelation[111, 155]. The A.halleri, Vetiveria zizanoides, Helianthus annuus, and Amaranthus plants are the main species in 137Cs and 90Sr phytoextraction research and common exist in farmland areas[120, 141, 156-157]. According to Burger et al[120], A.halleri is a hyperaccumulator plant species. It can absorb a lot of different radionuclide metals into its above-ground organs[120].The highest transfer factors of Cs and Sr achieved around 16.5 and 184 under conditions of Cs concentration(0.02 mM) and Sr concentration (0.01 mM). The study pointed out that toxic effects will impact the transfer factors and plant growth under different Cs and Sr concentrations[120]. H. annuus has a co-contribution with bacillus (Bacillus cereus) as a bioaugmentation for absorbing, fixing, and migrating soil radionuclides. B.cereus increased sunflower's Y (yritrium) and La (lanthanum) concentrations by a factor of 21 and 38.3. It also increased clover biomass for aerial portions by a factor of 3.7[158]. Calotropis gigantea also exhibited an ideal effect on radionuclide phytoextraction. The C. gigantea absorbed 90% of the metal under 90Sr(5×103 kBq/l) within 24 h. The removal rate increased by 97% after 168 h. The Cs removal rate reached 44% under 137Cs (5×103 kBq/l) solution after 168 h[155]. Kobayashi et al[159] examined the migration factors of thirteen species from Asteraceae, Fabaceae, and Poaceae in shallow and deeply cultivated fields. The research indicated that the 137Cs concentrations in shallowly cultivated fields were higher than those in deeply cultivated fields. In the deep field, 137Cs shallow field transfer factors ranged from 0.019 to 0.130. The transfer factors of 137Cs for plants cultivated in the deep field varied from 0.022 to 0.130[159]. According to Burger et al[120], Plantago major had a high potential for absorbing radionuclides Cs and Sr in leaves and roots. The leaves absorbed 47 mg/kg dry weight (DW) of Cs under 0.2 mM concentration of Cs. The DW of Sr absorption from leaves achieved 105.8 mg/kg with the condition of 100 mM Sr. Researchers have used P. major to migrate and remediate pesticides and insecticides. It showed significant potential for radioactive heavy metal remediation[160]. Previous studies showed that P. major has been applied to the migration and treatment of pesticides[161]. It also demonstrates great promise for application in radioactive heavy metal treatment.
Tillage system alternation and change are commonly used to reduce the transport and spread of sediment and pollution caused by soil erosion[162]. Different tillage systems also contributed to preventing radionuclide migration in crops[163-164]. Li et al[164] indicated that three different tillage systems (no tillage (NT), moldboard plow (MP), and rotary cultivation (RC)) impacted the vertical distribution of 137Cs and the transfer factor from soil to crops. The study results showed that under the NT and RC treatments, the amount of 137Cs in the soil decreased exponentially with depth. The concentration of 137Cs in crops increased due to the greater ExCs/exchangeable K ratio in the surface soil. Compared to NT, the transfer factor for soybean grain was significantly lower in MP and RC. The NT method has several environmental protection advantages, and induced soil inversion significantly reduces crop radiocesium pollution. It would be appropriate to reverse tillage in order to counteract the nuclear disaster[163-164]. However, NT is a way to quickly reduce the accumulation of Cs and Sr radioactive in the soil. This result is similar to the previous study in the River Wensum Demonstration Test Catchment[165].
5 Discussion
The binding sites in the ion-exchange materials limited the effectiveness of Cs and Sr removal under Fukushima scenario. For practical consideration, membrance, membrane and co-precipitation technologies are relatively suitable methods for removing Cs and Sr from tanks. When using phytoremediation, it is necessary to consider how long radionuclides will live in the tank water environment, and the process takes longer than membrane and co-precipitation technologies. In terms of cost, certain membrane technologies can facilitate multiple-use filtration and yield superior removal outcomes. The co-precipitation method is highly effective but may be costlier compared to membrane filtration. For bioremediation, many factors, including precipitation, temperature, and human management, nevertheless restrict the long-term phytoremediation effects.
Soil and water conservation measures can prevent the migration and diffusion of various radioactive elements in soil and other landuses. For example, forest land management methods focus on selecting local tree species. A forest cleanup initiative applied this technology, covering 70% of the Fukushima-affected area's geographic expanse. To reduce pollutant fluxes both vertically and horizontally in the woodland, C. japonica is a suitable selection. Furthermore, a combination of multi-tree and grass species to achieve low pollutant flux in forestry areas can be used. However, there is little research in this area, so the construction of low-pollutant flux woodlands (including multiple trees and grasses) still requires further investigation. Soil and water conservation measures for forests and agricultural use focus on sustainability and environmentally friendly phytoremediation. The agricultural lands should consider some crops, such as H. annuus, to constantly remove Cs and Sr.
While analogous technologies, such as ground frozen shield, address groundwater contamination following the Fukushima nuclear accident, PRS and reverse osmosis technology can be utilized in the vicinity of the FDNPP and in specific zones of elevated groundwater contamination. This technology can also be utilized for addressing radioactive wastewater leaks and mitigating the dissemination of toxins resulting from severe weather events.
6 Conclusions
Treatment of radioactive nuclear wastewater encompasses various technologies while each offers unique advantages and challenges. Chemical precipitation, with its ability to form metal precipitates, can be enhanced through flocculation and the use of organic ligands for selective removal of contaminants. Multi-nuclide separation of solids and liquids after chemical precipitation is the main challenge for this technique in nuclear wastewater treatment. Previous studies point out that membranes and adsorption are the most developed technologies[135]. Membrane filtration has a complete design (like thermal-driven processes), but most of them are under lab scale, and membrane adsorption is limited to the number of times it can be used. Nanosorption and membrane filtration expensive cost limits large-scale application for Fukushima nuclear wastewater treatment, although some studies claim that the materials can be applied several times.
For the Fukushima accident, new technology using KFe[Fe(CN)6] and BaSO4 co-precipitation can be applied in nuclear wastewater treatment, based on the above analysis. The technology fills the gaps of 90Sr and 137Cs rapid co-removing from complex acidic wastewater, Sr and Cs concentrations can be reduced by an order of magnitude. Nanosorption and membrane filtering can be employed to remediate specific casks in the Fukushima storage site containing high concentration of Sr and Cs. This mitigates the potential of radioactive wastewater leaking from the casks and its release into the ocean. The plants like C. japonica and H. annuuscan may be applied in agricultural areas treatment. Soil and water conservation strategies are recommended to impede the dispersion of migrating Strontium and Cesium across various settings and media.
This article addresses the deficiency in research about integrated treatment technologies for Sr and Cs in Fukushima. It proposes employing phytoremediation, soil and water conservation, and related technologies to address nuclear wastewater treatment and the dispersion of radionuclides (Sr and Cs) in Fukushima. However, this article lacks estimates of the costs of individual technologies' application of Cs and Sr. Calculations of maximum adsorption capacity and KD values for different technologies are incomplete due to lacking relevant data.
Future research should focus on considering the application of improvement in radioactive elements removal efficiency. The future study will concentrate on minimising costs and enhancing the practical application of co-precipitation technology. Soil and water conservation strategies in forestry regions should establish various vegetation systems to mitigate pollution fluxes. Although there are many reports that the tritium content in the tanks exceeds the limit and the Japanese government has implemented a tritium dilution program, the residues of other radioactive heavy metals may still be harmful to the environment. Therefore, further removal of radioactive Cs, Sr, and other metal elements from the tanks is necessary.
 2024, Vol. 22
2024, Vol. 22 












