2. 四川省农业科学院土壤肥料研究所,成都 610066;
3. 中国农业科学院农业资源和农业区划研究所,北京 100081
2. Soil and Fertilizer Research Institute, Sichuan Academy of Agricultural Sciences, Chengdu 610066, China;
3. Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081, China
玉米是我国的三大粮食作物之一,从上世纪五十年代以来,种植面积和产量都在不断增加[1–3]。田间管理措施的改进和品种的遗传改良是玉米产量增加的决定性因素[4]。氮作为影响作物产量的最关键营养元素,氮肥的使用可以显著地影响玉米产量。自绿色革命以来,氮肥的产量和使用量都在逐年增加,中国是世界最大的氮肥生产国和使用国,2012年消费量占世界的30%以上[5],但是中国的氮肥利用率只有30%,远低于50%的世界同期平均水平[6]。氮肥的不合理施用造成了土壤酸化、温室气体排放增加等严重的环境问题[7–9]。如何提高作物氮效率,降低氮肥流失带来的环境风险是当前中国农业生产面临的巨大挑战[9]。
提高玉米氮效率是实现农业高产高效的重要措施。氮的利用在植物体内分吸收和再转移两部分。研究发现花前氮的再转移贡献了玉米籽粒含氮量的60%~70%[10–11]。玉米叶片中氮的吸收同化和再转移在灌浆期是同时发生的[12–13]。叶片中氮对籽粒的贡献大约占50%~90%,跟基因型密切相关[14–16]。叶片衰老时,叶片中的蛋白质发生降解,产生的氨基酸进一步转化,进行体内氮的再转移,为发育中的籽粒和其他库提供氮源。而叶片中超过70%的蛋白质位于叶绿体中[17–18]。在所有的叶绿体蛋白中,Rubisco是蛋白质水解和氮转移的主要蛋白[19]。降解发生时Rubisco以自噬体的形式从叶绿体转移到液泡中,最后在液泡中被蛋白酶水解成氨基酸进入植物体内的氮循环和再转移过程[20]。其中半胱氨酸蛋白酶作为蛋白水解酶的一大类,可在液泡或者胞液中降解Rubisco大亚基[21]。ZmSee2β是玉米叶片中协同衰老的蛋白酶–豆荚蛋白的基因,它在叶片氮的利用和分配中具有重要作用[22]。蛋白质降解产生的氮先转化为氨基酸,然后装载到韧皮部中进行长距离的运输[23–24]。
尽管目前对叶片衰老过程中生理机制的研究已经很多,但是在田间条件下这些过程与氮转移效率的关系还不清楚,尤其是对玉米氮转移效率分子机制的研究很少。XY335是一个高产氮转移高效的品种 [16],NE9是一个高产氮转移低效的过度绿熟品种。在本研究中比较了在田间不同氮水平处理下,XY335和NE9在灌浆期氮转移效率的差异和相关基因的表达,目的是为了明确基因型间氮转移效率出现差异的关键机制。
1 材料与方法 1.1 试验时间和地点田间试验于2010年6—10月,在山东省德州市陵县中国农科院德州实验站 (37.35°N、116.57°E) 进行。玉米生长季降雨量为416.6 m,基本满足玉米生长需要,没有灌水。土壤基本理化性质如下:土壤容重1.43 g/cm3、pH 8.30 (1∶2.5, g/v)、有机质 10.62 g/kg、有效磷 7.34 mg/kg、速效钾69.32 mg/kg、总氮0.83 g/kg。
1.2 试验设计供试品种先玉335(XY335) 是先锋公司在2000年培育的中度绿熟品种,在灌浆期有较高的氮转移效率[16];NE9是中国农业大学在2007年培育的玉米杂交种,由于其在成熟期的保绿度较高,具有较低的氮转移效率。在2010年6月23日播种,10月6日收获。
试验地设计是裂区设计,小区长6 m,宽2.75 m。种植密度为 60000 株/hm2。试验氮肥 (尿素) 施用量设3个施氮水平为 45 kg/hm2 (N45)、120 kg/hm2 (N120) 和240 kg/hm2 (N240),每个处理三次重复。磷肥 (P2O5) 施用量是150 kg/hm2,钾肥 (K2O) 施用量是150 kg/hm2,所有肥料播前一次施用。
1.3 测定项目与方法 1.3.1 叶面积和SPAD值测定连续选取5株长势一致的玉米,分别在吐丝期以及吐丝后7、14、28、35、42 d和成熟期测定绿叶面积。在吐丝期和灌浆期 (吐丝后28 d) 测定穗位叶的SPAD值。
1.3.2 植株养分测定在吐丝后14、28、42 d和成熟期,每小区取三株玉米混样,分为叶、茎 (包括叶鞘和雄穗) 和籽粒三部分,在75℃烘箱中烘至恒重,称干重,粉样,用凯氏定氮法测定样品氮浓度。
1.3.3 产量测定在成熟期,每小区取两行玉米测产,统计玉米株数,计算单株粒重。
1.3.4 蛋白质和氨基酸浓度的测定在吐丝期和灌浆期上午9点至12点,用剪刀迅速取下穗位叶 (去掉叶脉),用锡箔纸包好放在液氮中用于测定蛋白质和氨基酸浓度以及提取RNA做基因表达分析。蛋白浓度的测定方法为考马斯亮蓝法,用牛血清蛋白作标准溶液[25]。总氨基酸的测定用茚三酮和醋酸锂,用亮氨酸作标准溶液[26]。
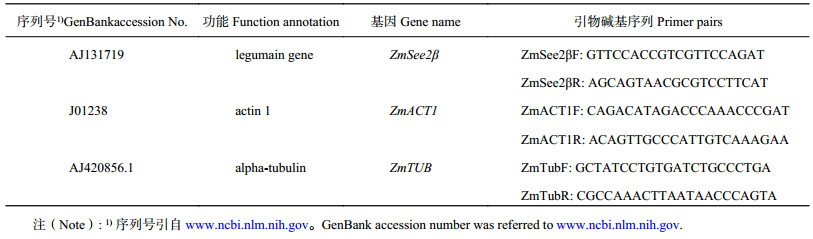
1.3.5 基因表达分析根据Gu 等[27] 2013年的方法,用Trizol总RNA 提取试剂盒 (Invitrogen) 提取玉米叶片RNA,将去除基因组DNA的RNA用ZmACT1内含子引物进行PCR验证,然后用反转录的cDNA进行q-PCR(7500 Real-Time PCR System,Applied Biosystems),ZmTUB作为内参基因。基因表达分析所用引物如表1所示。
| 表1 qRT-PCR所用引物列表 Table 1 Genes of maize (Zea mays) used for qRT-PCR |
 |
收获指数 (HI) = 籽粒产量/生物量 × 100%
氮收获指数 (NHI) = 籽粒含氮量/成熟期植株吸氮量 × 100%
氮的转移效率 (NRE) = (吐丝期吸氮量 – 成熟期秸秆氮残留)/吐丝期吸氮量 × 100%
每片叶的绿叶面积 = 叶长 × 叶宽 × 0.75
相对绿叶面积 = 某时期绿叶面积/吐丝期绿叶面积 × 100%
蛋白质降解率 = (吐丝期可溶性蛋白浓度 – 28DAS可溶性蛋白浓度)/吐丝期可溶性蛋白浓度 × 100%。
1.3.7 数据处理采用Excel 2007进行数据处理,用SAS软件进行方差分析和显著性分析,用SigmaPlot10.0软件作图。
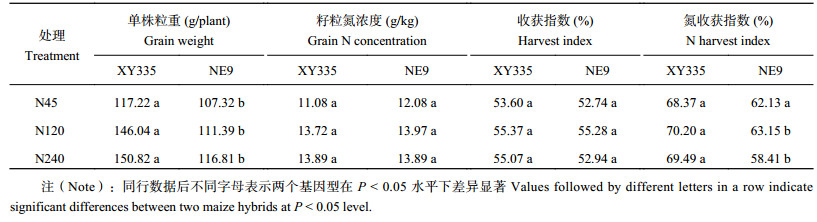
2 结果与分析 2.1 籽粒产量、籽粒氮浓度、收获指数和氮收获指数从表2可以看出,随施氮量的增加,品种XY335的籽粒产量较品种NE9增加更加显著。品种XY335的单株产量在三个氮水平下分别为117.22 g (N45)、146.04 g (N120) 和150.82 g (N240),显著高于同处理下的品种NE9。在N45水平下,品种XY335的产量比品种NE9高9.2%;在N120水平下,品种XY335的产量比品种NE9高31.1%;在N240品种下,品种XY335的产量比品种NE9高29.1%。籽粒氮浓度随施氮量的增加不断提高,但是品种XY335和品种NE9的籽粒氮浓度没有显著差异。在N45低氮水平下,除了籽粒产量,两个基因型在籽粒氮浓度、收获指数 (HI) 和氮收获指数 (NHI) 上没有差异。然而在N120和N240水平下,品种XY335的籽粒产量和NHI都要高于品种NE9。
| 表2 不同氮处理下两个基因型玉米的籽粒干重、籽粒氮浓度、收获指数和氮收获指数 Table 2 Grain weight, grain N concentration, harvest index and N harvest index of two maize hybrids grown under different N treatments |
 |
氮和基因型显著地影响玉米花后氮的吸收和花前氮的转移 (表3)。在吐丝期,品种XY335和品种NE9的吸氮量没有差异,而在成熟期品种XY335总的吸氮量显著高于品种NE9。品种NE9成熟期籽粒含氮量低于品种XY335,而茎叶含氮量高于品种XY335。在适宜供氮条件下 (N120),两个玉米品种的氮转移效率高于其他处理。相比较于品种NE9,品种XY335的氮转移效率较高主要是因为叶片氮转移效率的差异造成的,茎的氮转移效率没有差异。
| 表3 不同氮处理下两个基因型氮的吸收和转移 Table 3 Nitrogen accumulation and remobilization of two maize hybrids grown under different nitrogen treatments |
 |
如图1所示,吐丝后两个品种的相对绿叶面积在吐丝后28天开始降低,35天后绿叶面积下降速度加快。在缺氮条件下 (图1a、b、c) 品种XY335的相对绿叶面积开始降低的时间早于品种NE9。在三个氮水平下,叶片氮浓度在28天均开始显著降低,与绿叶面积的变化趋势一致。而在低氮条件下 (N45),品种XY335叶片中氮的输出早于品种NE9,吐丝期时两个品种的叶片氮浓度都在20 g/kg左右,到14天时品种XY335叶片氮浓度已经显著降低至15 g/kg (图1d、e、f) 而品种NE9没有变化,说明低氮下品种XY335更早启动氮转移。在灌浆时期,尽管两个基因型的叶片氮浓度变化趋势一致,但品种XY335叶片氮浓度一直低于品种NE9,大约低25%,在三个氮水平下表现一致。
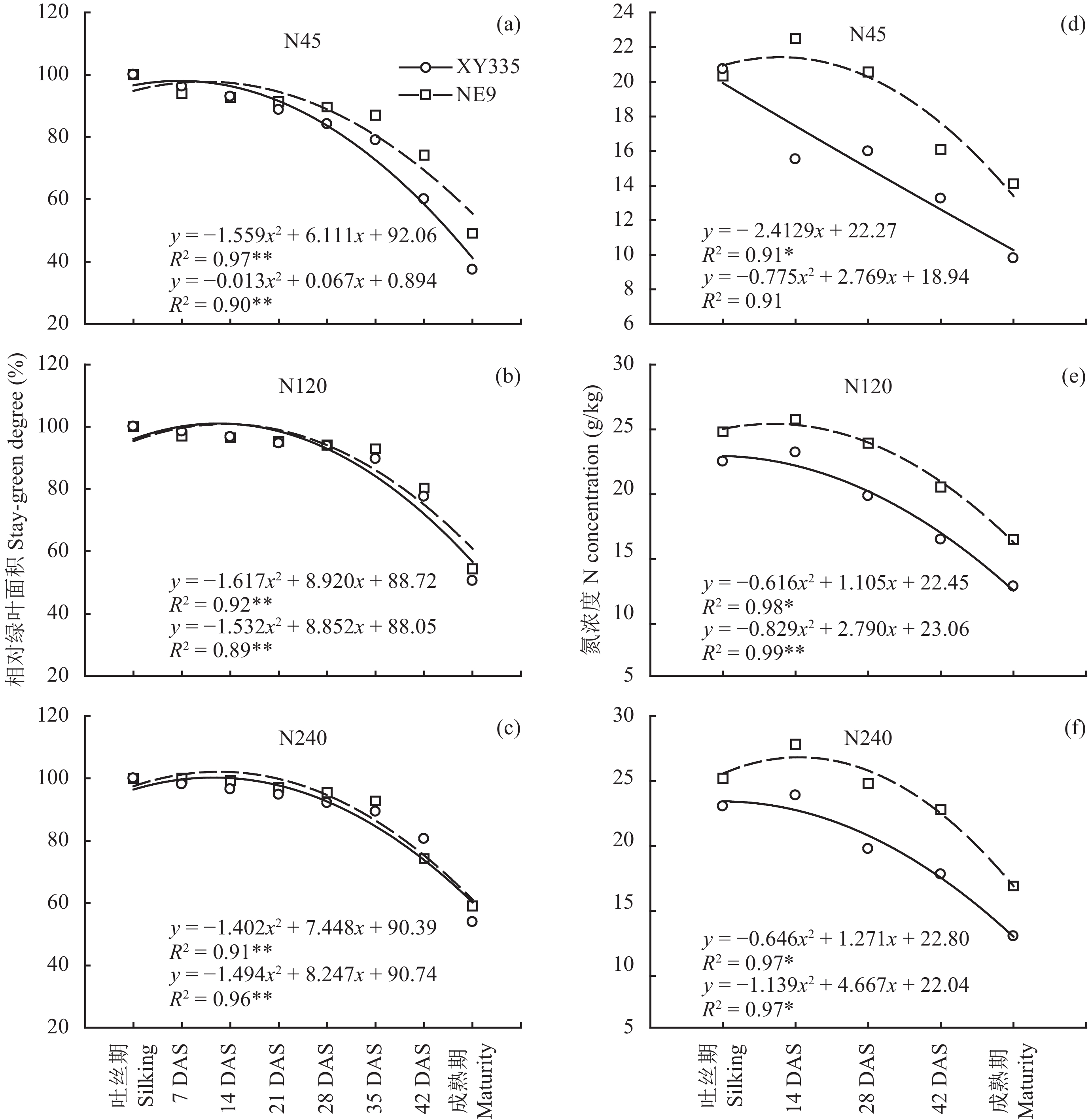 |
| 图1 两个基因型在三个氮水平下的相对绿叶面积和叶片氮浓度的动态变化 Fig. 1 Leaf stay-green degree and N concentration of two maize hybrids grown under three N treatments [注(Note):DAS—吐丝后天数 Days after silking. *—P < 0.05; **—P < 0.01.] |
如图2a所示,两个基因型随施氮量的减少,叶片SPAD值在降低,但是从吐丝期到灌浆期 (吐丝后28天),各处理的叶片SPAD值没有显著变化。处理间叶片中可溶性蛋白的浓度在吐丝期没有差异。但是在N45处理下,从吐丝期到灌浆期,品种XY335叶片可溶性蛋白的浓度降低33.2%,而NE9只降低16.7%。在N120处理下,品种XY335的叶片可溶性蛋白浓度降低8.5%,品种NE9降低2.3% (图2b)。在N240处理下,灌浆期时两个基因型间可溶性蛋白没有显著变化,表明在高氮投入的条件下,从吐丝期到28天可溶性蛋白的降解还没有开始。两个基因型叶片中游离氨基酸的浓度随施氮量的减少而降低,在吐丝期和灌浆期都呈现这种趋势 (图2c)。叶片中游离氨基酸的浓度从吐丝期到灌浆期有所增加,而在3个氮水平下品种XY335叶片中游离氨基酸的浓度增加的幅度大于品种NE9。ZmSee2β在叶片中具有协同衰老的作用。两个时期的ZmSee2β基因在品种XY335和品种NE9叶片中随施氮量的增加呈不断下调表达。而它的表达量从吐丝期到灌浆期呈增加趋势 (图2d)。在三个氮处理和两个时期,品种XY335中ZmSee2β的表达量都高于品种NE9。
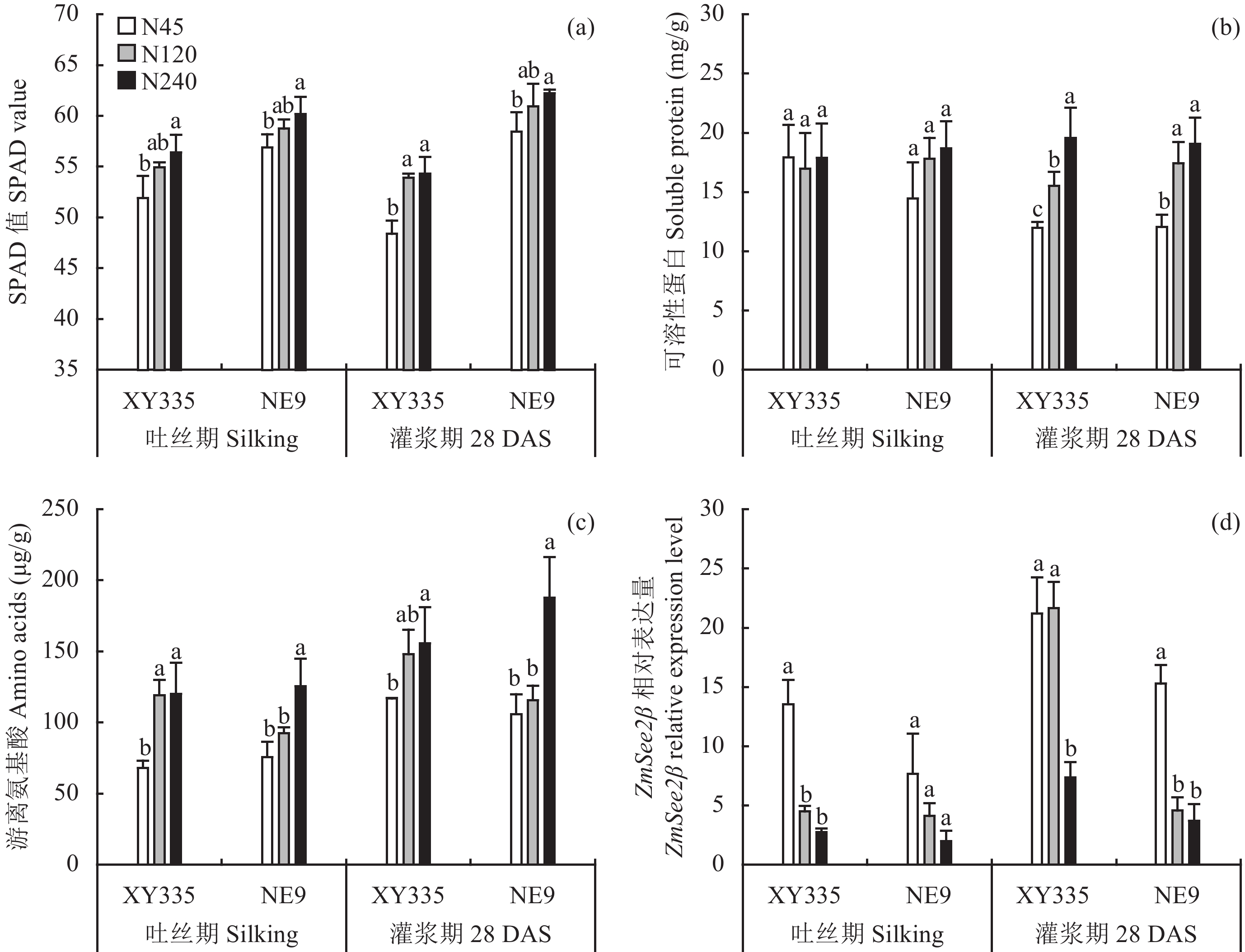 |
| 图2 两个基因型在三个氮水平下穗位叶SPAD值、可溶性蛋白浓度、游离氨基酸浓度和ZmSee2β的表达 Fig. 2 Concentration of the SPAD value and soluble protein and total free amino acids and expression of ZmSee2β in ear-leaves in two hybrids grown under three N treatments [注(Note):DAS—吐丝后天数 Days after silking. 柱状图代表平均值 ± 标准误差 (样本数n = 3) Bars indicate the mean ± SE (n = 3);柱上不同字母表示同一生育期不同处理在P < 0.05 水平下具有显著性差异 Different letters above the bars at the same stage indicate significant differences at P < 0.05 level between the N treatments.] |
前人研究表明,不同氮效率的品种在花后氮吸收和氮转移效率上存在显著差异,氮高效品种通常花后吸收氮素能力强,具有绿熟的特性[11, 16, 28–32]。相比而言,黄熟型 (成熟时绿叶数很少) 的玉米品种具有更高的氮转移效率和较低的花后氮吸收能力[16, 28–31]。这种类型品种灌浆期碳水化合物合成能力不足,所以通常产量较低。然而,过度持绿可能导致叶片氮转移效率过低,更多的氮素会残留在叶片中,因而导致籽粒含氮率下降[12]。国内外研究结果均表明,现代玉米品种随着绿熟型的增加,籽粒含氮率下降[4, 33–34]。因此,最优的氮高效品种应该是在保持开花后吸氮能力强的基础上,尽可能增加茎叶中氮素向籽粒的转运,实现吸收效率与氮转运效率的协调提高,从而保证产量提高的同时,籽粒含氮量得到稳定。本研究中,中度绿熟品种XY335的籽粒产量高于品种NE9,但是籽粒氮浓度在两个品种间没有差异 (表2),说明品种XY335籽粒中积累了更多的氮素。与品种NE9相比,品种XY335不仅吐丝后氮素吸收能力强,而且同时具有更高的氮转移效率,尤其是体现在叶片中。吐丝后,品种XY335的叶片氮浓度的下降速度总是快于品种NE9(图1)。在三个氮处理水平下品种XY335叶片中的氮浓度一直低于品种NE9。因此,在品种XY335中体现了吐丝后期氮素高效吸收和氮素高效转运的协调,从而保证其籽粒氮素积累量较高 (图3)。在叶片氮素转运效率提高 (叶片氮浓度较低) 的条件下,如何保证叶片的光合作用,是一个重要的生理问题。Chen等研究发现品种XY335中上部叶片具有较高的光合氮利用效率、气孔导度和电子传递效率[35],保证了品种XY335在叶片氮素高效转运的同时,还能够保证较高的光合产物积累,达到高产。本研究中,虽然品种XY335叶片氮浓度及叶绿素 (SPAD值) 明显较低,但其叶面积减少速度与品种NE9很相近,只是在灌浆后期才明显低于品种NE9。这在一定程度上保证了其光合产物的持续供应。
玉米叶片衰老过程中的一个重要生理过程是氮素从同化过程向再转移过程的转变[36–37],从而为籽粒的生长提供大量的氮源。在叶片氮素再转移过程中,植物通过蛋白水解酶从衰老的叶片中释放被蛋白质束缚的氮[38]。叶片中的蛋白质 (主要是叶绿体蛋白,因为叶片中大约80%的氮位于叶绿体中,以蛋白质的形式存在) 被降解成天冬氨酸、谷氨酸、赖氨酸、芳香烃氨基酸和支链氨基酸等各种氨基酸[21],氨基酸通过一系列的转氨反应形成谷氨酸,谷氨酸进一步合成为谷氨酰胺和天冬酰胺被装载到韧皮部,其中谷氨酰胺是谷物中被优先向籽粒输出的氮化合物[36]。另外氮向籽粒中的装载受到韧皮部汁液中氨基酸浓度的调控[39–40]。Mu等[41]研究发现在田间条件下,低氮处理的玉米叶片中各个氮组分的转移均早于高氮处理 (Rubisco除外)。可溶性蛋白 (如 Rubisco 大亚基、PEPc 等) 的降解会导致叶片中游离氨基酸浓度的增加,这个过程在早衰的玉米品种中较持绿的玉米品种更加活跃,并伴随着谷氨酰胺合成酶1 (GS1) 活性的升高[30, 42]。本研究中,吐丝期开始后,叶片SPAD值开始保持稳定,但叶片氮浓度就开始下降,说明蛋白质分解已经开始。Yang等[43]利用15N的研究也表明,吐丝前积累在植株营养器官中的氮素,从吐丝期开始即开始发生转移。从吐丝期到灌浆期,N45和N120处理下叶片中蛋白质浓度显著下降,在品种XY335中下降得更为明显。相应地在这个时期叶片中游离氨基酸的浓度开始增加,在品种XY335中增加的幅度大于品种NE9。这些数据表明在N45和N120处理下,品种XY335中蛋白质的降解更加强烈 (图3)。蛋白质的降解受到酶的作用[15],在所有的叶绿体蛋白中,Rubisco是灌浆期衰老叶片中蛋白质水解和氮转移的主要蛋白[19]。自我吞噬降解途径可以以自噬体的形式将Rubisco从叶绿体转移到液泡中,最后在液泡中被肽链内切酶和外切酶水解成氨基酸进入随后的氮循环和再转移[20]。半胱氨酸蛋白酶作为蛋白水解酶的一大类,可在液泡或者胞液中降解Rubisco大亚基[21]。人们发现玉米半胱氨酸蛋白酶ZmSee2的基因有两个成员 (See2α和See2β,与半胱氨酸蛋白酶中的豆荚蛋白酶序列有很强的同源性) 编码与叶片衰老相关的蛋白酶[22]。其中See2β在氮从衰老叶片向发育中的籽粒进行再转移的过程中起着重要作用,突变体中ZmSee2β基因下调表达,呈现出持绿的表型,其叶片中叶绿素与蛋白质含量的比值在花后一直保持稳定,而野生型中则持续降低,由此证明ZmSee2β基因影响了蛋白质和叶绿素的降解以及氮的再转移[22]。本研究发现,品种XY335中ZmSee2β 基因的表达量显著高于品种NE9,尤其是在N45和N120 条件下 (图2d)。这种差异在吐丝期一开始就表现出差异。这与品种XY335叶片中蛋白质降解速度较快相一致。因此推测,ZmSee2β是控制玉米叶片中氮素转移效率的重要基因 (图3)。基于See2β基因在叶片氮转移和向籽粒进行氮输出时的重要作用,Zhang等[44]分析了49个玉米自交系的See2β基因序列,发现在几个关键自交系中有See2β基因的等位变异,这些等位变异有可能用于玉米氮高效的遗传改良。
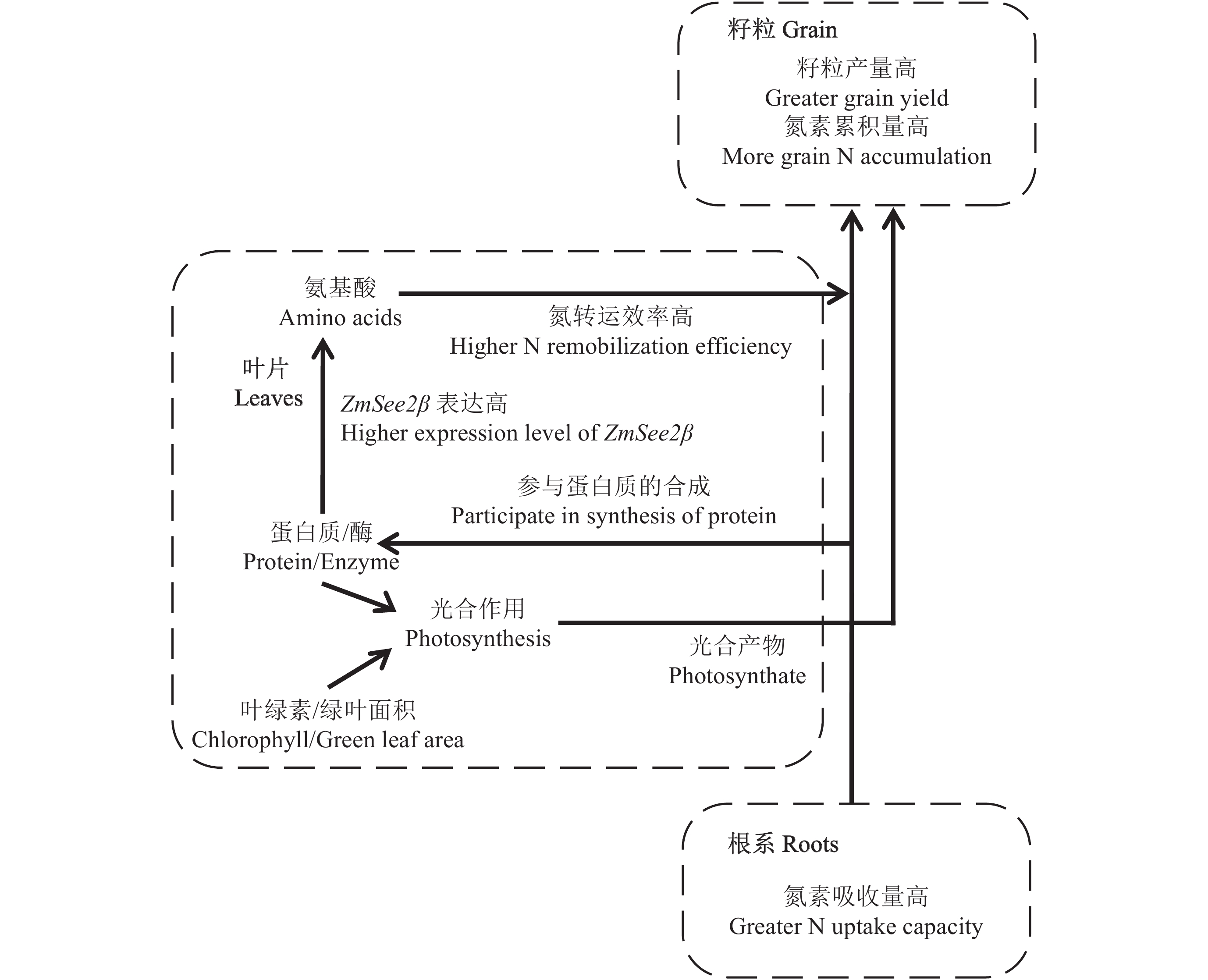 |
| 图3 玉米品种XY335花后氮素吸收与氮素转运的协调机制 Fig. 3 Coordination of efficient nitrogen uptake and nitrogen remobilization during post-silking stage of maize hybrid XY335 |
相对于绿熟品种NE9,品种XY335具有籽粒产量和籽粒氮素积累强的特点。这不仅由于吐丝后品种XY335具有较强的氮素吸收能力,而且因为品种XY335有更高的叶片氮转移效率。品种XY335叶片氮转移效率高可能是因为控制蛋白质降解的ZmSee2β基因表达能力强,促进了叶片中蛋白质的降解速度。
| [1] | Ci X K, Li M S, Liang X L, et al. Genetic contribution to advanced yield for maize hybrids released from 1970 to 2000 in China[J]. Crop Science, 2011, 51: 13–20. DOI:10.2135/cropsci2010.04.0207 |
| [2] | Ci X K, Li M S, Xu J S, et al. Trends of grain yield and plant traits in Chinese maize cultivars from the 1950s to the 2000s[J]. Euphytica, 2012, 185(3): 395–406. DOI:10.1007/s10681-011-0560-5 |
| [3] | Ciampitti I A, Vyn T J. Physiological perspectives of changes over time in maize yield dependency on nitrogen uptake and associated nitrogen efficiencies: A review[J]. Field Crop Research, 2012, 133: 48–67. DOI:10.1016/j.fcr.2012.03.008 |
| [4] | Chen X C, Chen F J, Chen Y L, et al. Modern maize hybrids in Northeast China exhibit increased yield potential and resource use efficiency despite adverse climate change[J]. Global Change Biology, 2013, 19: 923–936. DOI:10.1111/gcb.2013.19.issue-3 |
| [5] | Good A G, Beatty P H. Fertilizing nature: a tragedy of excess in the commons[J]. Plos Biology, 2011, 9(8): e1001124. DOI:10.1371/journal.pbio.1001124 |
| [6] | Fan M S, Shen J B, Yuan L X et al. Improving crop productivity and resource use efficiency to ensure food security and environmental quality in China[J]. Journal of Experimental Botany, 2012, 63: 13–24. DOI:10.1093/jxb/err248 |
| [7] | Guo J H, Liu X J, Zhang Y et al. Significant acidification in major Chinese croplands[J]. Science, 2010, 327(5968): 1008–1010. DOI:10.1126/science.1182570 |
| [8] | Liu X J, Zhang Y, Han W X et al. Enhanced nitrogen deposition over China[J]. Nature, 2013, 494(7438): 459–462. DOI:10.1038/nature11917 |
| [9] | Zhang F S, Chen X C, Vitousek P. An experiment for the world[J]. Nature, 2013, 497(7447): 33–35. DOI:10.1038/497033a |
| [10] | Gallais A, Coque M. Genetic variation and selection for nitrogen use efficiency in maize: A synthesis[J]. Maydica, 2005, 50(3–4): 531–547. |
| [11] |
米国华, 陈范骏, 张福锁. 作物养分高效的生理基础与遗传改良[M]. 北京: 中国农业大学出版社, 2012.
Mi G H, Chen F J, Zhang F S. Physiological basis and genetic improvement of nutrient use efficiency in crop [M]. Beijing: China Agricultural University Press, 2012. |
| [12] | Thomas H, Ougham H. The stay-green trait[J]. Journal of Experimental Botany, 2014, 65(14): 3889–3900. DOI:10.1093/jxb/eru037 |
| [13] | Avila-Ospina L, Moison M,Yoshimoto K, Masclaux-Daubresse C. Autophagy, plant senescence, and nutrient recycling[J]. Journal of Experimental Botany, 2014, 65(14): 3799–3811. DOI:10.1093/jxb/eru039 |
| [14] | Masclaux-Daubresse C, Quilleré I, Gallais A, Hirel B. The challenge of remobilisation in plant nitrogen economy. A survey of physio-agronomic and molecular approaches[J]. Annals of Applied Biology, 2001, 138(1): 69–81. DOI:10.1111/aab.2001.138.issue-1 |
| [15] | Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, et al. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture[J]. Annal of Botany, 2010, 105(7): 1141–1157. DOI:10.1093/aob/mcq028 |
| [16] | Chen Y L, Xiao C X, Chen X C, et al. Characterization of the plant traits contributed to high grain yield and high grain nitrogen concentration in maize[J]. Field Crop Research, 2014, 159: 1–9. DOI:10.1016/j.fcr.2014.01.002 |
| [17] | Lim P K, Kim H J, Nam H G. Leaf senescence[J]. Annual Review of Plant Biology, 2007, 58: 115–136. DOI:10.1146/annurev.arplant.57.032905.105316 |
| [18] | Kant S,Bi Y M, Rothstein S J. Understanding plant response to nitrogen limitation for the improvement of crop nitrogen use efficiency[J]. Journal of Experimental Botany, 2011, 62(4): 1499–1509. DOI:10.1093/jxb/erq297 |
| [19] | Mae T, Thomas H, Gay A P, et al. Leaf development in lolium temulentum: Photosynthesis and photosynthetic proteins in leaves senescing under different irradiances[J]. Plant and Cell Physiology, 1993, 34(3): 391–399. |
| [20] | Ishida H, Yoshimoto K, Izumi M, et al. Mobilization of rubisco and stroma–localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process[J]. Plant Physiology, 2008, 148(1): 142–155. DOI:10.1104/pp.108.122770 |
| [21] | Liliana A O, Michael M, Kohki Y, Masclaux-Daubresse C. Autophagy, plant senescence, and nutrient recycling[J]. Journal of Experimental Botany, 2014, 65(14): 3799–3811. DOI:10.1093/jxb/eru039 |
| [22] | Donnison I S, Gay A P, Thomas H, et al. Modification of nitrogen remobilization, grain fill and leaf senescence in maize (Zea mays) by transposon insertional mutagenesis in a protease gene[J]. New Phytologist, 2007, 173: 481–494. DOI:10.1111/j.1469-8137.2006.01928.x |
| [23] | Hirel B, Thierry T, Lea P J, Dubois F. Improving nitrogen use efficiency in crops for sustainable agriculture[J]. Sustainability, 2011, 3: 1452–1485. DOI:10.3390/su3091452 |
| [24] | Gregersen P L, Culetic A, Boschian L, Krupinska K. Plant senescence and crop productivity[J]. Plant Molecular Biology, 2013, 82(6): 603–622. DOI:10.1007/s11103-013-0013-8 |
| [25] | Bradford M M. A rapid and sensitive method for the quantitation of microgram of protein utilizing the principle of protein-dye binding[J]. Analytical Biochemistry, 1976, 72: 248–254. DOI:10.1016/0003-2697(76)90527-3 |
| [26] | Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for ninhydrin reaction[J]. Journal of Biological Chemistry, 1968, 243: 6281–6283. |
| [27] | Gu R L, Duan F Y, An X, et al. Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize (Zea mays L.) [J]. Plant Cell Physiology, 2013, 54: 1515–1524. DOI:10.1093/pcp/pct099 |
| [28] |
米国华, 陈范骏, 春亮, 等. 玉米氮高效品种的生物学特征[J].
植物营养与肥料学报, 2007, 13(1): 155–159.
Mi G H, Chen F J, Chun L, et al. Biological characteristics of nitrogen efficient maize genotypes[J]. Plant Nutrition and Fertilizer Science, 2007, 13(1): 155–159. DOI:10.3321/j.issn:1008-505X.2007.01.026 |
| [29] |
米国华. 论作物养分效率及其遗传改良[J].
植物营养与肥料学报, 2017, 23(6): 1525–1535.
Mi G H. Nutrient use efficiency in crops and its genetic improvement[J]. Journal of Plant Nutrition and Fertilizer, 2017, 23(6): 1525–1535. |
| [30] | Martin A, Belastegui-Macadam X, Quillere I, et al. Nitrogen management and senescence in two maize hybrids differing in the persistence of leaf greenness: agronomic, physiological and molecular aspects[J]. New Phytologist, 2005, 167: 483–492. DOI:10.1111/j.1469-8137.2005.01430.x |
| [31] | Rajcan I, Tollenaar M. Source: link ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling[J]. Field Crop Research, 1999, 60: 255–265. DOI:10.1016/S0378-4290(98)00143-9 |
| [32] |
春亮, 陈范骏, 张福锁, 等. 不同氮效率玉米杂交种的根系生长、氮素吸收与产量形成[J].
植物营养与肥料学报, 2005, 11(5): 615–619.
Chun L, Chen F J, Zhang F S, et al. Root growth, nitrogen uptake and yield formation of hybrid maize with different N efficiency[J]. Plant Nutrition and Fertilizer Science, 2005, 11(5): 615–619. DOI:10.3321/j.issn:1008-505X.2005.05.008 |
| [33] | Duvick D N. The contribution of breeding to yield advances in maize[J]. Advances in Agronomy, 2005, 86: 83–145. DOI:10.1016/S0065-2113(05)86002-X |
| [34] | Ciampitti I A, Vyn T J. Grain nitrogen source changes over time in maize: A review[J]. Crop Science, 2013, 53(2): 366–377. DOI:10.2135/cropsci2012.07.0439 |
| [35] | Chen Y L, Wu D L, Mu X H, et al. Vertical distribution of photosynthetic nitrogen use efficiency and its response to nitrogen in field-grown maize[J]. Crop Science, 2016, 56: 397–407. DOI:10.2135/cropsci2015.03.0170 |
| [36] | Masclaux-Daubresse C, Reisdorf-Cren M, Orsel M. Leaf nitrogen remobilisation for plant development and grain filling[J]. Plant Biology, 2008, 10(Suppl. 1): 23–26. |
| [37] | Kumudini S. Trials and tribulations: a review of the role of assimilate supply in soybean genetic yield improvement[J]. Field Crops Research, 2002, 75(2–3): 211–222. |
| [38] | Fischer A. The complex regulation of senescence[J]. Critical Reviews in Plant Sciences, 2012, 31(2): 124–147. DOI:10.1080/07352689.2011.616065 |
| [39] | Lohaus G, Moellers C. Phloem transport of amino acids in two Brassica napus L. genotypes and one B. carinata genotype in relation to their seed protein content [J]. Planta, 2000, 211(6): 833–840. DOI:10.1007/s004250000349 |
| [40] | Tilsner J, Kassner N, Struck C, Lohaus G. Amino acid contents and transport in oilseed rape (Brassica napus L.) under different nitrogen conditions [J]. Planta, 2005, 221(3): 328–338. DOI:10.1007/s00425-004-1446-8 |
| [41] | Mu X H, Chen Q W, Chen F J, et al. Within-leaf nitrogen allocation in adaptation to low nitrogen supply in maize during grain-filling stage[J]. Frontiers in Plant Science, 2016, 7: 699. |
| [42] | Orsel M, Moison M, Clouet V, et al. Sixteen cytosolic glutamine synthetase genes identified in the Brassica napus L. genome are differentially regulated depending on nitrogen regimes and leaf senescence [J]. Journal of Experimental Botany, 2014, 65(14): 3927–3947. DOI:10.1093/jxb/eru041 |
| [43] | Yang L, Guo S, Chen Q W, et al. Use of the stable nitrogen isotope to reveal the source–sink regulation of nitrogen uptake and remobilization during grain filling phase in maize[J]. PLoS ONE, 2016, 11(9): e0162201. DOI:10.1371/journal.pone.0162201 |
| [44] | Zhang J J, He M, Liu Y H, et al. Sequence polymorphism characteristics in the See2b gene from maize key inbred lines and derived lines in China [J]. Biochemical Genetics, 2012, 50: 508–519. DOI:10.1007/s10528-012-9495-3 |
 2018, Vol. 24
2018, Vol. 24  doi:
doi: 

