干旱、盐、低温等非生物胁迫严重限制了植物的生长发育。世界主要农作物每年有近50%的产量损失都与非生物胁迫有关[1]。了解植物非生物胁迫下的生理生化应答特征是评价作物对非生物胁迫抗性效果的重要指标。为了适应环境变化,植物形成了一系列防御机制以抵抗各种逆境伤害。其中,植物抗逆基因的转录调控 (transcription regulation) 对植物抵御逆境胁迫发挥着重要的调节作用。转录调控主要通过特定的转录因子与相应的顺式作用元件 (cis-acting element) 相互作用来实现。转录因子是指能够与基因启动子区域中顺式作用元件发生特异性相互作用的DNA结合蛋白,通过它们之间或与其它蛋白之间的互作,激活或抑制转录发挥调控作用。与植物抗逆相关的转录因子主要包括禽成髓细胞瘤病毒致癌基因同源物类 (v-myb avian myeloblastosis viral oncogene homolog, MYB)、碱性域亮氨酸拉链 (basic-domain leucine-zipper, bZIP)、乙烯应答元件组合蛋白/因子 (APELATA2/ethylene-responsive element binding proteins/factors, AP2/EREBP)、WRKY和NAC等五类。研究植物非生物胁迫相关转录因子的功能及其在植物非生物胁迫过程中的响应与调控机制,已成为研究者关注的热点。
1 非生物胁迫下植物的生理应答 1.1 干旱胁迫下植物生理生化的适应性变化干旱胁迫严重限制植物生长并降低作物的产量和品质[2]。由于干旱导致细胞周期蛋白依赖性激酶活性降低、细胞分裂缓慢,因此缺水情况下植株的生长速率显著降低,特别是苗期阶段[3]。此外,干旱通常引起植株叶面积、叶伸展速率及数量的下降。干旱胁迫下,植物叶片通常表现出不同程度的萎蔫甚至变黄现象[4],成熟植物能通过关闭叶片气孔及老叶的加速衰老和脱落,减少叶片蒸腾作用,以降低其造成的水分损失[5]。叶片气孔关闭的同时还降低了植物光合作用,使叶片中类胡萝卜素含量、总叶绿素含量明显下降[6]。另外,干旱胁迫下,植物细胞因失水萎缩可导致细胞膜的机械损伤。脱水的细胞因体积减小,使得细胞内溶物变粘稠,增加了蛋白质间互作的可能性,致使其聚集和变性[7]。植物体内活性氧 (ROS) 在干旱胁迫时的积累还可引起膜脂质的过氧化,丙二醛 (MDA) 作为质膜过氧化的最终产物,亦能造成细胞膜上蛋白质等的失活,破坏生物膜的结构及功能[4]。研究表明,干旱胁迫时植物抗氧化酶,如过氧化氢酶 (CAT)、过氧化物酶 (POD) 和超氧化物歧化酶 (SOD) 的活性以及渗透调节物质,如脯氨酸、谷氨酸及可溶性糖等的含量均有所增加[4]。溶质的积累在一定程度上降低了细胞的渗透势,可以提高植物抵御干旱的能力。植物中次生代谢物的含量也随着干旱胁迫程度发生变化,如小麦叶片中总黄酮的含量[8]及杨树中酚类化合物的浓度等[9]都表现出增加趋势。
1.2 盐胁迫下植物生理生化的适应性变化盐胁迫是造成几乎所有陆生植物生长和产量下降的主要胁迫因素之一[10]。其中,Na盐和Ca盐对植物伤害较严重,尤其Na盐的伤害更为普遍。盐胁迫不仅影响植物生长发育、光合作用及呼吸作用等重要的代谢过程,且对植物体内离子含量、酶活性、激素水平等均有影响。盐胁迫的初始阶段植物叶面积扩展速率降低,随着盐浓度的升高,叶面积减少,叶片相对含水量、水势、蒸腾速率降低,且植物茎、根的鲜重及干重亦有不同程度的下降[11]。高盐环境造成叶片气孔因缺水而关闭,使叶绿体类囊体膜膨胀、基粒消失且出现巨型淀粉粒,叶绿体结构遭破环。高盐胁迫还可导致植物中与光合作用相关的酶变性失活,降低叶片光合反应速率、影响同化产物合成[12]。通常,盐胁迫初期植物的呼吸作用增强,以产生抵御胁迫所需的能量,但随着胁迫强度的升高和时间的延长,植物呼吸强度呈现下降的趋势[13]。盐胁迫下的植物因经常处于“生理干旱”状态,使得体内ROS过度积累,干扰植物正常代谢、破坏体细胞膜完整性并增加质膜透性,导致植物体中抗氧化酶的活性随外界盐浓度的增加呈先上升后下降的趋势[12],无法持续维持较高水平,从而影响植物细胞的稳态[14]。研究发现,盐胁迫还能引起叶片硝酸还原酶活性 (NRA) 降低,造成含氮化合物代谢紊乱,影响植物总体新陈代谢水平[15]。另外,植物吸收矿质元素时,环境中过多的盐离子能与其相互竞争造成植物矿质营养胁迫。多种植物的盐胁迫实验表明,随处理中Na+ 和Cl- 含量的增加,Ca2+、K+和Mg2+ 的含量呈降低趋势,且高浓的Na+ 能严重阻碍植物对K+ 的吸收和运输[16]。同时,高盐能影响植物激素水平的分布,如脱落酸 (ABA) 能在渗透胁迫下积累,并参与修饰因盐胁迫上调的基因的表达[17]。
1.3 低温胁迫下植物生理生化的适应性变化低温对植物的伤害分为冷害 (0~15℃) 和冻害 (< 0℃)。低温胁迫下,植物叶片通常表现为萎缩、褪绿甚至坏死,且植物生长发育受到严重影响[5]。与干旱及盐胁迫类似,低温胁迫能减少植物对水分的吸收,破坏细胞膜完整性,使细胞丧失区室化[18],并干扰植物的呼吸作用、光合作用等过程。光合作用对低温较敏感[19],其对光合作用的抑制主要体现在两方面:一是,直接影响叶绿素合成及叶绿体结构,低温胁迫下,叶绿素a、b及类胡萝卜素含量降低,叶绿体基质、基粒片层松散,双层膜完整性被破坏[20];二是,通过减少叶片对CO2的吸收、阻碍光合产物运输、引起水分胁迫等[19]生理过程,降低光合速率。另外,低温胁迫下植物对氧的利用率降低,多余的氧在代谢过程中被转化为ROS。但是,植物可通过调节酶促防御体系来限制并清除体内过多的ROS,进而避免或减轻低温胁迫造成的伤害。此外,植物体中的可溶性蛋白、可溶性糖和游离脯氨酸等作为防冻剂和膜稳定剂,能参与细胞渗透调解,从而提高植物低温耐受性。
2 转录因子结构功能及在非生物胁迫下的应答调控 2.1 转录因子的结构与功能特征 2.1.1 MYB类转录因子的结构与功能特征MYB家族转录因子存在于所有真核生物中,由于具有保守的MYB结构域而得名。植物中MYB转录因子的共同特征是MYB结构域通常由1~4个不完全重复的R结构组成。根据R结构的数目和位置,可将MYB家族分为4个亚家族[21]:1)4R-MYB(包含4个重复的R1/R2),是最小的MYB亚家族。目前对该亚族成员的功能了解甚少,所发现的4R-MYB蛋白仅能在极少数植物 (拟南芥、葡萄和杨树) 中进行编码。2)3R-MYB(R1R2R3-MYB),在植物中其家族成员较少。拟南芥和水稻中仅5个左右基因编码3R-MYB蛋白。3)2R-MYB(R2R3-MYB),是植物中最大的一类MYB亚家族。水稻中有近90个2R-MYB亚家族成员,拟南芥中也有120个以上。2R-MYB蛋白能参与细胞分化、代谢调节、激素应答以及植物对逆境胁迫的响应等过程。4)R-MYB(MYB-related),是植物中第二大类MYB亚家族,它们既能调节植物生长发育也参与植物对逆境胁迫的响应[21–22]。
2.1.2 bZIP类转录因子的结构与功能特征bZIP是一类广泛分布于真核生物中以自身结构域命名的转录因子。bZIP结构域由碱性氨基酸域和亮氨酸拉链域构成。植物bZIP蛋白含约60~80个氨基酸,其N端包含由20个左右氨基酸残基构成的碱性结构域,该区域通过固定的N-x7-R/K-x9结构直接结合DNA,除决定DNA结合的特异性外,还起到核定位信号的作用[23]。另外,其N末端还具有酸性激活区,能以二聚体的形式结合DNA。bZIP转录因子的碱性结构域与亮氨酸拉链域紧密结合,亮氨酸拉链区域位于bZIP结构域C端,由7个或9个氨基酸组成一个重复单位,每个重复单位的第7(9) 位含有一个亮氨酸,此位置的亮氨酸也可被疏水性氨基酸代替。这种重复单位能形成两亲性α螺旋结构,该螺旋疏水侧的亮氨酸残基通过范德华力相互作用叠加成卷曲线圈,即亮氨酸拉链[24]。α螺旋结构参与bZIP蛋白与DNA结合前的二聚化,也能影响蛋白质二聚体的形成。植物bZIP类转录因子优先结合到具有ACGT核心序列的顺式作用元件,如G-box(CACGTG)、C-box(GACGTC)、A-box(ACGTA) 等[24]。
2.1.3 AP2/EREBP类转录因子的结构与功能特征AP2/EREBP类转录因子是植物特有的转录因子超家族。该家族成员具有约60个氨基酸残基组成的保守AP2/ERF结构域,该结构域能特异性结合DRE(dehydration-responsive element)/C-repeat顺式元件或GCC-box顺式元件。这两个顺式元件的共同核心序列为CCGNC,分别存在于受乙烯和脱水胁迫诱导表达的目标基因启动子序列中[25–26]。AP2/EREBP超家族可分为AP2、ERF(ethylene-responsive factor) 和DREB(dehydration-responsive element-binding protein) 等亚家族,ERF和DREB又可统称为EREBP家族。AP2亚族具有2个AP2结构域,可负责调控花、胚珠和种子的发育;EREBP亚族具有1个AP2结构域,主要参与植物生物和非生物胁迫应答反应[27]。
2.1.4 WRKY类转录因子的结构与功能特征WRKY转录因子是植物转录因子家族中较大的一类,也是最具特点的一类。WRKY家族成员因均含有与DNA结合的WRKY结构域而得名,该结构域由60个左右高度保守的氨基酸序列组成,在其N端具有WRKYGQK核心序列,C端包含一个锌指结构域C2H2(Cx4-5Cx22-23HxH) 和C2HC(Cx7Cx23HxC)[28–29]。根据WRKY结构域的数量和锌指结构域的类型,WRKY转录因子可分成3个亚家族:亚家族Ⅰ具有两个WRKY结构域和一个C2H2型锌指结构;亚家族Ⅱ具有一个WRKY结构域和一个C2H2型锌指结构;亚家族Ⅲ具有一个WRKY结构域和一个C2HC型锌指结构。受WRKY蛋白调控的基因启动子区中的TTGACT/C保守片段为W-box。W-box是目标基因与WRKY转录因子特异性结合的区域,目标基因与WRKY转录因子中的WRKY结构域和锌指结构域实现特异性结合,致使WRKY转录因子在植物响应非生物胁迫的过程中发挥重要作用[30]。
2.1.5 NAC类转录因子的结构与功能特征NAC转录因子是植物中特有的一类转录因子。因在矮牵牛NAM、拟南芥ATAF1/ATAF2和CUC2基因编码蛋白的N端,均发现一段高度保守的氨基酸序列,故以这三类基因的首字母将其命名为NAC结构域,包含NAC结构域的蛋白即NAC转录因子。NAC结构域一般由150~160个氨基酸组成,并分为5个亚结构域[31]。NAC蛋白单体可通过谷氨酸和精氨酸间的氢键或盐桥形成二聚体,二聚体所带的正电荷可与DNA结合[32]。NAC转录因子的C端为氨基酸序列具有高度多样性的转录调控区,能激活转录也能抑制转录。由于转录调控区的结构具有不稳定性,使得NAC蛋白能够与其他目标蛋白互作[33],从而使NAC转录因子在植物应答非生物胁迫过程中起到重要的调控作用。
2.2 转录因子对非生物胁迫的应答 2.2.1 转录因子对干旱胁迫的应答许多MYB家族成员均能参与植物对干旱胁迫的应答反应。Liao等[34]从前人研究的大豆基因中获得了156个MYB家族基因,利用酵母单杂交的方法筛选出了40个左右与逆境相关的基因,将这些基因转入拟南芥进行功能验证,发现转GmMYB177提高了拟南芥耐旱性。丁震乾等[35]在陆地棉中克隆了MYB转录因子基因GhRAX3,发现其表达在干旱胁迫0.5 h后即表现为显著上调,且48 h内持续高表达水平,而抑制GhRAX3的表达,加快了棉花植株的失水率、细胞质膜过氧化和细胞受损程度,降低了棉花对干旱胁迫的耐受性。目前发现的大多MYB类转录因子基因均表现为提高植物抗旱能力,但亦有例外,如Zhou等的研究指出,将JcMYB001转入拟南芥后,其过表达反而增加了拟南芥对干旱的敏感性[36]。水稻和白松树中过表达的AtbZIP60基因可以通过调节相关Ca2+-依赖性蛋白激酶基因来提高植物对干旱胁迫的耐受性[37]。玉米ZmbZIP72在拟南芥中的过表达提高了转基因株系抵御干旱的能力[38]。另外,Tu等发现葡萄VlbZIP36基因在拟南芥中的过表达能减轻干旱胁迫下的细胞损伤和水分流失,使其在种子萌发、幼苗和成熟阶段都显示出较强的耐脱水性[39]。干旱胁迫下DREB基因的过表达使得转基因小麦叶片仍保持绿色而野生型叶片失绿,恢复浇水10天后,转基因株系存活率显著提高,说明DREB转录因子能有效提高小麦耐旱性[40]。Chen等报道GmDREB2受干旱胁迫诱导,可激活转基因拟南芥中下游基因的表达,提高转基因株系对干旱的耐受性[41]。Jiang等发现番茄SlDREB1基因可受干旱的强烈诱导,将其转入拟南芥后,转基因拟南芥耐旱能力明显提高[26]。Yang等指出干旱胁迫可持续诱导柑橘CitERF的表达进而增加其抗旱能力[42]。Wang等报道了10个WRKY基因,发现将TaWRKY44转入烟草后其脯氨酸含量、可溶性糖含量、抗氧化酶活性均高于野生型,且MDA和H2O2含量显著降低,说明TaWRKY44的表达增强了转基因烟草对干旱的耐受性[43]。Niu等指出小麦TaWRKY2蛋白和TaWRKY19蛋白可通过与基因启动子的直接结合或间接机制来调节下游基因,进而提高小麦抗旱能力,且转TaWRKY2和TaWRKY19基因的拟南芥表现出较强的耐旱性[44]。玉米的ZmNAC55基因受干旱胁迫诱导,且ZmNAC111基因在玉米中的过表达能提高转基因植株的水分利用率,使干旱应答基因的表达上调,从而提高植物耐旱性[45–46]。Zhao等报道了MlNAC9过表达的转基因拟南芥中抗氧化酶活性提高、MDA含量显著降低,且干旱胁迫应答基因的表达在MlNAC9过表达株系中显著增加,说明MlNAC9过表达提高了拟南芥对干旱的耐受性[47]。
2.2.2 转录因子对盐胁迫的应答研究发现,AtMYB41、AtMYB20、GmMYB76、GmMYB92、GmMYB177等MYB类转录因子基因均受盐胁迫诱导[21, 34],且转入GmMYB76和GmMYB177的转基因拟南芥在高盐处理下的存活率显著高于野生型[34]。Chen等报道,盐胁迫下花生中4个R2R3-MYB基因,AhMYB1、AhMYB2、AhMYB6和AhMYB7的表达显著上升;4个MYB-related基因,AhMYB12、AhMYB18、AhMYB28和AhMYB30的mRNA丰度增加[48]。这说明花生中至少有8个MYB基因受到盐胁迫的诱导。Liu等指出,拟南芥AtbZIP17在盐胁迫下转录水平增加,并通过蛋白水解作用使其N端进入核内发挥转录激活作用,诱导下游逆境响应基因AtRD29A和AtRD20的表达,从而提高植株抗盐性[49]。Xiang等的研究表明,水稻OsbZIP23过表达的转基因水稻耐盐性显著提高,敲除该基因的突变体植株对盐耐受性明显降低,而将OsbZIP23转化到突变体后,其抗盐性得到恢复,说明OsbZIP23在植物响应盐胁迫中发挥重要作用[50]。大豆GmDREB2基因在转基因拟南芥中的过表达提高了转基因株系对高盐胁迫的抵御能力,且不影响拟南芥的生长周期[41]。水稻OsDREB1F基因、OsDREB2A基因均受高盐胁迫诱导,二者的转基因株系都表现出较强抗盐能力[51–52],且水稻OsDREB1A在拟南芥中的过表达诱导了拟南芥DREB1A基因的表达,从而提高了植株耐盐性[53]。Yao等发现杨树ERF76基因在盐胁迫下表达上调,转ERF76基因烟草的种子发芽率、株高、根长、鲜重以及脯氨酸含量、SOD活性等生理生化指标均高于野生型,说明杨树ERF76在转基因烟草的抗盐性中起关键的调控作用[54]。盐胁迫下,小麦TaWRKY44的表达水平显著上升,且转TaWRKY44基因的烟草提高了对盐胁迫的耐受性[43]。Qin等认为小麦TaWRKY93为盐诱导转录因子基因,TaWRKY93过表达的转基因拟南芥主根及侧根长度、脯氨酸含量、相对含水量、存活率等在盐胁迫环境下明显提高[55]。Zhou等指出,与野生型相比,在盐胁迫下陆地棉GhWRKY34在拟南芥中的过表达使其有较高的发芽率、根长和叶绿素含量,证明转GhWRKY34拟南芥的耐盐性更强[56];而Jia等则报道陆地棉GhWRKY68基因降低了转基因烟草的抗盐能力[57],这说明WRKY转录因子在植物应对盐胁迫中既能发挥正调控功能也能发挥负调控功能。Mohammed等[58]研究发现,盐胁迫下硬粒小麦的TtNAC-B60、TtNAC-A7、TtNAC-B35、TtNAC-B27和TtNAC-A51均受盐胁迫诱导并表现出上调趋势,小麦中的TaNAC29基因也参与了植物对盐胁迫的响应与信号通路,并能增强植株抗氧化物酶活性、减少H2O2积累和膜损伤[59]。Zhao等[47]发现MlNAC9过表达的转基因拟南芥能通过ABA依赖途径提高抵御盐胁迫的能力。而Wei等[60]对甜瓜NAC转录因子家族全基因组的研究则表明,虽然盐胁迫下的12h内CmNAC14的表达持续增加,但CmNAC14过表达的转基因拟南芥却增加了对盐胁迫的敏感性。
2.2.3 转录因子对低温胁迫的应答Gopal等鉴定了大白菜中475个MYB基因,发现与正常条件相比,低温胁迫下R2R3-MYB亚家族成员:BrMYB2、BrMYB13、BrMYB77、BrMYB81、BrMYB88、BrMYB121、BrMYB166、BrMYB169和BrMYB217的表达成倍上调;MYB-related亚家族成员:BrMYB1R25、BrMYB1R44、BrMYB1R70、BrMYB1R77、BrMYB1R171和BrMYB1R178也表现出上调[61]。研究发现,水稻的OsMYB30也受低温胁迫诱导,但OsMYB30在水稻中的过表达降低了水稻的低温耐受性,而OsMYB30敲除突变体水稻反而具有较好的抵御低温胁迫的能力[62]。Hwang等通过对芜菁bZIP转录因子的研究指出,Bra000256、Bra003320、Bra004689、Bra011648、Bra020735和Bra023540在低温胁迫下转录水平显著上调,它们编码的蛋白质可能参与植株对冷胁迫的响应[63]。甘蓝bZIP转录因子基因Bol008071、Bol033132和Bol042729受低温胁迫诱导而升高,表明其可能在植物响应低温胁迫时发挥重要作用[64]。Lee等分析了马铃薯中乙烯应答元件组合蛋白,发现冷诱导的StEREBP1基因过表达可诱导含有GCC-box顺式元件的基因表达,参与逆境响应[25],说明StEREBP1通过对GCC-box顺式元件的转录调节,在逆境胁迫中发挥作用。
Niu等发现黄花苜蓿MfDREB1和MfDREB1s基因在低温处理1 h后的表达水平均上调且6 h时达到峰值[65],推测二者是黄花苜蓿抵御低温的重要影响因子。小麦TaWRKY19能激活Cor6.6 (cold-regulated gene) 的表达并能结合其启动子,使转TaWRKY19的拟南芥表现出较好的低温耐受力[44]。水稻OsWRKY71可以通过调节下游靶基因增强转基因植株的耐寒性[66]。Wang等研究了葡萄基因组中59个VvWRKYs,发现有22个受冷胁迫诱导上调且VvWRKY55的涨幅最大[67]。黄瓜中CsWRKY46基因在寒冷胁迫下的表达上调,且其过表达的转基因拟南芥在低温胁迫下的幼苗存活率明显提高[68]。番茄SlNAC1过表达可以减少ROS积累并使CBF1的表达上调,从而提高植株对低温的适应能力[69],且Li等发现番茄中新型转录因子基因SlNAM1过表达的转基因烟草能减轻细胞膜在寒冷环境下的氧化损伤[70]。Zhao等将MlNAC9转入拟南芥后,发现转基因株系中低温胁迫应答基因的表达和ROS的清除能力增强[47]。
部分转录因子在干旱、高盐和低温胁迫下的应答调控见表1。
| 表1 干旱、高盐及低温胁迫下部分转录因子的应答调控 Table 1 Response of some transcription factors in drought, high-salt and low temperature stresses |
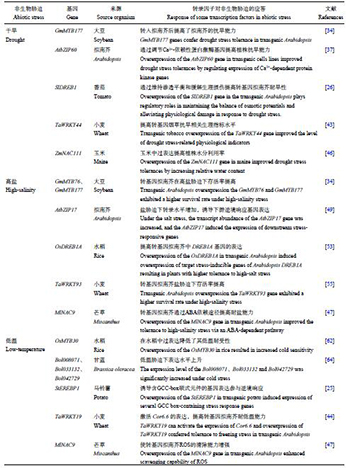 |
植物抗逆能力的提高与转录因子调控逆境相关的功能基因表达密不可分。基因通过不同信号途径进行特异性表达是不同基因启动子中顺式作用元件存在差异的结果,这些元件与相应的转录因子结合从而激活或改变转录效率。已发现的一系列顺式作用元件包括:GCC-box、G-box、C-box、W-box、DRE、MRE、ABRE、ERE等。不同转录因子中保守的DNA结合域能与下游基因的顺式作用元件结合并相互作用,以实现对基因表达的调控,其转录调控域可同其他转录因子或DNA作用以提高或抑制基因表达调控的效率和灵活性。
ABA作为逆境胁迫响应的内源激素,在植物应对干旱、盐等胁迫中发挥重要作用[71]。植物响应干旱胁迫的信号转导途径包括ABA依赖途径和非ABA依赖途径。ABA依赖途径中,许多ABA诱导基因的启动子区都含有ABRE(ABA-responsive cis-acting elements) 顺式作用元件,如小麦Em、拟南芥RD29B的启动子区等[72]。bZIP转录因子中的ABF(ABA-responsive element binding factors)/AREB(ABA responsive element binding protein) 家族,能与ABA依赖基因的ABRE元件结合并激活这些基因的表达。另外,MYB等转录因子中的成员,也能受ABA诱导表达。非ABA依赖途径中,在基因的启动子区含有DRE等其他核心元件,其作为蛋白识别位点受到相应因子的诱导。如AP2/EREBP类转录因子中的一个亚家族就能与DRE序列相结合,进而诱导相关基因的表达。植物对盐胁迫的响应机制主要通过Ca2+信号转导和SOS(salt overly sensitive) 信号途径。当植物遭受盐胁迫时,经Ca2+信号转导使SOS信号途径发挥作用,使其结合细胞质内过多的Ca2+,并通过离子转运功能在限制Na+进入细胞的同时,促进胞内过多的Na+外排[73]。植物对低温胁迫的抵御主要依靠信号转导途径来实现对低温诱导基因的表达调控。当植物受低温胁迫时,ICE-CBF-COR转录级联反应在感知上游低温信号和调控下游特异基因的表达中发挥重要作用[74]。根据CBF是否参与转录,此调控机制可分为CBF依赖途径和非CBF依赖途径。CBF依赖途径中,低温信号诱导激酶使ICE(inducer of CBF expression) 磷酸化,且与CBF(C-repeat Binding Factor) 启动子顺式作用元件结合,激活CBF基因的表达。有活性的CBF作为反式作用因子进而与COR(cold responsive) 的顺式作用元件结合诱导COR基因的表达,增强植物对低温的耐受性[75]。一些低温响应基因,如拟南芥同源结构域转录因子基因HOS9[76]、水稻OsMYB3R-2[77]基因不受CBF转录因子调控,而是通过非CBF依赖途径来提高植株的抗低温能力。
总的来说,植物受到外界环境 (干旱、高盐、低温等) 刺激后,会通过一系列抗逆胁迫信号的传导激活转录因子,使其与下游靶基因启动子区的相应顺式作用元件结合,调控靶基因表达,并通过基因产物的作用对内、外界信号作出调节,进而诱导相关功能基因的高表达,形成相应的基因产物以应答胁迫信号。
4 展望干旱、土壤盐渍化及低温胁迫严重影响农作物生产,而植物对干旱、高盐和低温的耐受性强弱通常受许多因子影响。转录因子作为调控基因表达的关键因素在植物抵抗非生物逆境胁迫方面发挥重要作用。一个转录因子能够调控多个与同类性状有关的基因表达,其通过增强某些关键调节因子的作用,促进抗逆基因资源发挥其功能,从根本上使植物的抗逆性得到改良。虽然现有研究已报道了许多与抗逆境相关的不同转录因子家族成员,但仍有大量转录因子家族成员的相关功能未得到验证,且目前对转录因子的研究侧重于单一逆境条件的调控,对其在不同信号途径相互作用的调控机制尚不十分明确。
另外,目前尚未见干旱、高盐及低温胁迫下转录因子直接参与调控植物养分方面的报道。对于植物吸收氮、磷、钾等营养元素的分子机制研究,主要集中于转运蛋白及其相关基因的调节。如Duan等研究了氮充足和不施氮条件下,冬小麦根系中硝酸盐转运蛋白 (NRT) 基因TaNRT2.1、TaNRT2.2、TaNRT2.3、TaNRT1.1和TaNRT1.2和氨转运蛋白 (AMT)基因TaAMT1.1、TaAMT1.2、TaAMT2.1对土壤干旱胁迫的响应,发现氮充足情况下冬小麦对干旱胁迫更敏感,因为氮低效小麦品种中低亲和力的TaNRT1.1和TaNRT1.2基因表达受干旱胁迫诱导,而它们在氮高效小麦品种中的表达则受抑制,且干旱胁迫下,无论氮充足与否,高亲和力的TaNRT2.1基因表达均受抑制。另外TaAMT1.1和TaAMT2.1的表达也受干旱胁迫抑制[78]。Shen等[79]指出,高盐胁迫能显著诱导水稻高亲和力钾转运蛋白基因OsHAK21的表达。与野生型相比,OsHAK21功能遭破坏的水稻突变体对盐胁迫敏感,其芽和根中的K+积累较少且K+净吸收率显著降低,而Na+净吸收率增加。OsHAK21在K+摄取缺陷型酵母和拟南芥中的功能表征进一步证明了OsHAK21具有K+转运蛋白活性。说明OsHAK21在盐胁迫下对维持水稻体内Na+/K+动态平衡起重要作用[79]。对于磷转运蛋白而言,Song等提出水稻磷转运蛋白基因OsPT8在烟草中的过表达能提高转基因烟草对磷和硒的吸收,且增强了转基因烟草对低磷胁迫的耐受性[80]。董旭等对植物磷转运子PHT1家族的表达模式和功能等进行了总结,发现在拟南芥、水稻、大豆及茄科等植物中,多数PHT1家族成员受低磷信号调控且主要在根部表达,并行使相应的磷转运功能[81]。
在未来的研究中,应基于全基因组层面的科学分析,进一步发掘转录因子家族成员的相关功能,研究转录因子在植物多种信号途径相互作用中的调控机制,这对深入理解植物对干旱等非生物逆境应答及转录因子的调控过程具有科学价值,同时对逆境胁迫作物分子改良提供理论与材料支撑具有现实意义。
| [1] | Valliyodan B, Nguyen H T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants[J]. Current Opinion in Plant Biology, 2006, 9: 189–195. |
| [2] | Lu H D, Xue J Q, Guo D W. Efficacy of planting date adjustment as a cultivation strategy to cope with drought stress and increase rainfed maize yield and water-use efficiency[J]. Agricultural Water Management, 2017, 179: 227–235. |
| [3] | Schuppler U, He P H, John P C L, et al. Effects of water stress on cell division and cell-division-cycle-2-like cell-cycle kinase activity in wheat leaves[J]. Plant Physiology, 1998, 117: 667–678. |
| [4] | Wu X L, Jie Y, Aoxue L, et al. Drought stress and re-watering increase secondary metabolites and enzyme activity in Dendrobium moniliforme [J]. Industrial Crops and Products, 2016, 94: 385–393. |
| [5] | Shilpi M, Narendra T. Cold, salinity and drought stresses: An overview[J]. Archives of Biochemistry and Biophysics, 2005, 444: 139–158. |
| [6] | Bota J, Flexas J, Medrano H. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress?[J]. New Phytologist, 2010, 162: 671–681. |
| [7] | Hoekstra F A, Golovina E A, Buitink J. Mechanisms of plant desiccation tolerance[J]. Trends in Plant Science, 2001, 6(9): 431–438. |
| [8] | Ma D Y, Sun D X, Wang C Y, et al. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress[J]. Plant Physiology and Biochemistry, 2014, 80: 60–66. |
| [9] | Popović B M, Štajner D, Ždero-Pavlović R, et al. Water stress induces changes in polyphenol profile and antioxidant capacity in poplar plants (Populus spp.) [J]. Plant Physiology and Biochemistry, 2016, 105: 242–250. |
| [10] | Oksana S, Marian B, Marek Z, et al. Applying hyperspectral imaging to explore natural plant diversity towards improving salt stress tolerance[J]. Science of the Total Environment, 2017, 578: 90–99. |
| [11] |
王雨, 马立敏, 周睿颖, 等. 盐胁迫对5个产地菘蓝幼苗光合特性及抗逆指标的影响[J].
南京农业大学学报, 2017, 40(3): 416–424.
Wang Y, Ma L M, Zhou R Y, et al. Effects of salt stress on photosynthetic characteristics and indexes of adverse circumstances-resistance of Isatis indigotica Fort. seedlings from five areas [J]. Journal of Nanjing Agricultural University, 2017, 40(3): 416–424. |
| [12] | Asish K P, Anath B D. Salt tolerance and salinity effects on plants: a review[J]. Ecotoxicology and Environmental Safety, 2005, 60: 324–349. |
| [13] | Manish P, Suprasanna P. Time course of physiological, biochemical, and gene expression changes under short-term salt stress in Brassica juncea L [J]. The Crop Journal, 2017, 5(3): 219–230. |
| [14] | Singh M, Kumar J, Singh S, et al. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review[J]. Reviews in Environmental Science and Bio/Technology, 2015, 14: 407–426. |
| [15] | Chokri Z, Micaela C, Ali F, et al. Water balance and N-metabolism in broccoli (Brassica oleracea L. var. Italica) plants depending on nitrogen source under salt stress and elevated CO2[J]. Science of the Total Environment, 2016, 571: 763–771. |
| [16] | Urich D, Aaron B S, Tomoaki H, et al. Plant salt-tolerance mechanisms[J]. Trends in Plant Science, 2014, 9(6): 371–379. |
| [17] | Christian Z, Geilfus C M, Karl H M, et al. The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance[J]. Journal of Plant Physiology, 2013, 170(2): 220–224. |
| [18] | Król A, Amarowicz R, Weidner S. The effects of cold stress on the phenolic compounds and antioxidant capacity of grapevine (Vitisvinifera L.) leaves [J]. Journal of Plant Physiology, 2015, 189: 97–104. |
| [19] | Fu J J, Roger N G, Xu Y F, et al. Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis following cold stress in Elymusnutans Griseb [J]. Journal of Photochemistry and Photo-biology B: Biology, 2016, 163: 30–39. |
| [20] | Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions[J]. Plant Physiology, 2006, 141: 391–396. |
| [21] | Li C, Ng K Y, Fan L M. MYB transcription factors, active players in abiotic stress signaling[J]. Environmental and Experimental Botany, 2015, 114: 80–91. |
| [22] | Katiyar A, Smita S, Lenka S K, et al. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis [J]. BMC Genomics, 2012, 13: 544–563. |
| [23] | Lee S C, Choi H W, Hwang I S, et al. Functional roles of the pepper pathogen induced bZIP transcription factor, CAbZIP1, in enhanced resistance to pathogen infection and environmental stresses [J]. Planta, 2006, 224(5): 1209–1225. |
| [24] | Jakoby M, Weisshaar B, Dröge-Laser W, et al. bZIP transcription factors in Arabidopsis [J]. Trends in Plant Science, 2002, 7(3): 106–111. |
| [25] | Lee H E, Shin D J, Park S R, et al. Ethylene responsive element binding protein 1 (StEREBP1) from Solanum tuberosum increases tolerance to abiotic stress in transgenic potato plants [J]. Biochemical and Biophysical Research Communications, 2007, 353: 863–868. |
| [26] | Jiang L L, Wang Y B, Zhang S H, et al. Tomato SlDREB1 gene conferred the transcriptional activation of drought-induced gene and an enhanced tolerance of the transgenic Arabidopsis to drought stress [J]. Plant Growth Regulation, 2017, 81: 131–145. |
| [27] |
李科友, 朱海兰. 植物非生物逆境胁迫DREB/CBF转录因子的研究进展[J].
林业科学, 2011, 47(1): 124–134.
Li K Y, Zhu H L. Research progress of DREB/CBF transcription factor in response to abiotic-stresses in plants[J]. Scientia Silvae Sinicae, 2011, 47(1): 124–134. |
| [28] | Kenichiro M, Shingo H, Hisae K S, et al. Role of conserved residues of the WRKY domain in the DNA-binding of tobacco WRKY family proteins[J]. Bioscience, Biotechnology and Biochemistry, 2001, 65(11): 2428–2436. |
| [29] | Yu G H, Jiang L L, Ma X F, et al. A soybean C2H2-type zinc finger gene GmZF1 enhanced cold tolerance in transgenic Arabidopsis [J]. PLoS One, 2014, 9(10): e109399. |
| [30] | Paul J R, Imre E S, Patricia R, et al. WRKY transcription factors[J]. Trends in Plant Science, 2010, 15(5): 247–258. |
| [31] | Kazuo N, Hironori T, Junya M, et al. NAC transcriptionfactors in plant abiotic stress responses[J]. Biochimica et Biophysica Acta(BBA)-Gene Regulatory Mechanisms, 2012, 1819: 97–103. |
| [32] | Puranik S, Bahadur R P, Srivastava P S, et al. Molecular cloning and characterization of a membrane associated NAC family gene, SiNAC from foxtail millet [Setariaitalica(L.) P. Beauv.] [J]. Molecular Biotechnology, 2011, 49(2): 138–150. |
| [33] | Kjaersgaard T, Jensen M K, Christiansen M W, et al. Senescence-associated barley NAC (NAM, ATAF1, 2, CUC) transcription factor interacts with radical-induced cell death 1 through a disordered regulatory domain[J]. The Journal of Biological Chemistry, 2011, 286(41): 35418–35429. |
| [34] | Liao Y, Zou F, Wang H W, et al. Soybean GmMYB76, GmMYB92, and GmMYB177 genes confer stress tolerance in transgenic Arabidopsis plants [J]. Cell Research, 2008, 18(10): 1047–1060. |
| [35] |
丁震乾, 陈天子, 刘廷利, 等. 棉花干旱诱导MYB类转录因子GhRAX3的功能分析
[J].
中国农业科学, 2015, 48(18): 3569–3579.
Ding Z Q, Chen T Z, Liu T L, et al. Function analysis of a drought stress induced MYB transcription factor GhRAX3 in cotton [J]. Scientia Agricultura Sinica, 2015, 48(18): 3569–3579. |
| [36] | Zhou C P, Chen Y B, Wu Z Y, et al. Genome-wide analysis of the MYB gene family in physic nut (Jatropha curcas L.) [J]. Gene, 2015, 572: 63–71. |
| [37] | Wei T, Michael P. Transcription factor AtbZIP60 regulates expression of Ca2+-dependent protein kinase genes in transgenic cells [J]. Molecular Biology Reports, 2013, 40(3): 2723–2732. |
| [38] | Ying S, Zhang D F, Fu J, et al. Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis [J]. Planta, 2012, 235: 253–266. |
| [39] | Tu M X, Wang X H, Feng T Y, et al. Expression of a grape (Vitisvinifera) bZIP transcription factor, VlbZIP36, in Arabidopsis thaliana confers tolerance of drought stress during seed germination and seedling establishment [J]. Plant Science, 2016, 252: 311–323. |
| [40] | Wang J W, Yang F P, Chen X Q, et al. Induced expression of DREB transcriptional factor and study on its physiological effects of drought tolerance in transgenic wheat[J]. Acta Genetica Sinica, 2006, 33(5): 468–476. |
| [41] | Chen M, Wang Q Y, Cheng X G, et al. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants [J]. Biochemical and Biophysical Research Communications, 2007, 353: 299–305. |
| [42] | Yang X Y, Xie J X, Lu X P, et al. Isolation of a citrus ethylene-responsive element binding factor gene and its expression in response to abiotic stress, girdling and shading[J]. Scientia Horticulturae, 2011, 127: 275–281. |
| [43] | Wang X T, Zeng J, Li Y, et al. Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances [J]. Frontiers in Plant Science, 2015, 6: 615–628. |
| [44] | Niu C F, Wei W, Zhou Q Y. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants [J]. Plant, Cell and Environment, 2012, 35(6): 1156–1170. |
| [45] | Mao H D, Yu L J, Han R, et al. ZmNAC55, a maize stress-responsive NAC transcription factor, confers drought resistance in transgenic Arabidopsis [J]. Plant Physiology and Biochemistry, 2016, 105: 55–66. |
| [46] | Mao H D, Wang H W, Liu S X, et al. A transposable element in a NAC gene is associated with drought tolerance in maize seedlings[J]. Nature Communications, 2015, 6: 8326–8339. |
| [47] | Zhao X, Yang X W, Pei S Q, et al. The Miscanthus NAC transcription factor MlNAC9 enhances abiotic stress tolerance in transgenic Arabidopsis [J]. Gene, 2016, 586: 158–169. |
| [48] | Chen N, Yang Q L, Pan L J, et al. Identification of 30 MYB transcription factor genes and analysis of their expression during abiotic stress in peanut (Arachis hypogaea L.) [J]. Gene, 2014, 533: 332–345. |
| [49] | Liu J X, Srivastava R, Howell S H. Stress-induced expression of an activated form of AtbZIP17 provides protection from salt stress in Arabidopsis [J]. Plant, Cell and Environment, 2008, 31(12): 1735–1743. |
| [50] | Xiang Y, Tang N, Du H, et al. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice [J]. Plant Physiology, 2008, 148: 1938–1952. |
| [51] | Wang Q Y, Guan Y C, Wu Y R, et al. 2008. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice [J]. Plant Molecular Biology, 2008, 67(6): 589–602. |
| [52] | Zhang X X, Tang Y J, Ma Q B, et al. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean [J]. PloS One, 2013, 8(12): e83011. |
| [53] | Dubouzet J G, Sakuma Y, Ito Y, et al. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression [J]. Plant Journal, 2003, 33(4): 751–763. |
| [54] | Yao W J, Wang L, Zhou B, et al. Over-expression of poplar transcription factor ERF76 gene confers salt tolerance in transgenic tobacco [J]. Journal of Plant Physiology, 2016, 198: 23–31. |
| [55] | Qin Y X, Tian Y C, Liu X Z. A wheat salinity-induced WRKY transcription factor TaWRKY93 confers multiple abiotic stress tolerance in Arabidopsis thaliana [J]. Biochemical and Biophysical Research Communications, 2015, 464: 428–433. |
| [56] | Zhou L, Wang N N, Gong S Y, et al. Overexpression of a cotton (Gossypium hirsutum) WRKY gene, GhWRKY34, in Arabidopsis enhances salt-tolerance of the transgenic plants [J]. Plant Physiology and Biochemistry, 2015, 96: 311–320. |
| [57] | Jia H H, Wang C, Wang F, et al. GhWRKY68 reduces resistance to salt and drought in transgenic Nicotianaben thamiana [J]. PLoS One, 2015, 10(3): e0120646. |
| [58] | Mohammed N S, Dhawya M, Faiçal B. Identification and expression analysis of the NAC transcription factor family in durum wheat (Triticum turgidum L. ssp. durum) [J]. Plant Physiology and Biochemistry, 2017, 112: 117–128. |
| [59] | Xu Z Y, Gong B Z X, Wang C Y, et al. Wheat NAC transcription factor TaNAC29 is involved in response to salt stress [J]. Plant Physiology and Biochemistry, 2015, 96: 356–363. |
| [60] | Wei S W, Gao L W, Zhang Y D, et al. Genome-wide investigation of the NAC transcription factor family in melon (Cucumis melo L.) and their expression analysis under salt stress [J]. Plant Cell Reports, 2016, 35(9): 1827–1840. |
| [61] | Gopal S, Park J I, Ahmed N U, et al. Characterization and expression profiling of MYB transcription factors against stresses and during male organ development in Chinese cabbage (Brassica rapassp. pekinensis) [J]. Plant Physiology and Biochemistry, 2016, 104: 200–215. |
| [62] | Yan L, Mei Y, Dan H, et al. The OsMYB30 transcription factor suppresses cold tolerance by interacting with a JAZ protein and suppressing β-amylase expression [J]. Plant Physiology, 2017, 173(2): 1475–1491. |
| [63] | Hwang I, Jung H J, Park J I, et al. Transcriptome analysis of newly classified bZIP transcription factors of Brassica rapa in cold stress response [J]. Genomics, 2014, 104: 194–202. |
| [64] | Hwang I, Manoharan R K, Kang J G, et al. Genome-wide identification and characterization of bZIP transcription factors in Brassica oleracea under cold stress [J]. BioMed Research International, 2016, 2016: 1–18. |
| [65] | Niu Y D, Hu T M, Zhou Y G, et al. Isolation and characterization of two Medicago falcate AP2/EREBP family transcription factor cDNA, MfDREB1 and MfDREB1s [J]. Plant Physiology and Biochemistry, 2010, 48: 971–976. |
| [66] | Kim C Y, Vo K T X, Nguyen C D, et al. Functional analysis of a cold-responsive rice WRKY gene, OsWRKY71 [J]. Plant Biotechnology Reports, 2016, 10(1): 13–23. |
| [67] | Wang L, Zhu W, Fang L C, et al. Genome-wide identification of WRKY family genes and their response to cold stress in Vitis vinifera [J]. BMC Plant Biology, 2014, 14: 103–116. |
| [68] | Zhang Y, Yu H J, Yang X Y, et al. CsWRKY46, a WRKY transcription factor from cucumber, confers cold resistance in transgenic-plant by regulating a set of cold-stress responsive genes in an ABA-dependent manner [J]. Plant Physiology and Biochemistry, 2016, 108: 478–487. |
| [69] | Ma N N, Zuo Y Q, Liang X Q, et al. The multiple stress-responsive transcription factor SlNAC1 improves the chilling tolerance of tomato [J]. Physiologia Plantarum, 2013, 149(4): 474–486. |
| [70] | Li X D, Zhuang K Y, Liu Z M, et al. Overexpression of a novel NAC-type tomato transcription factor, SlNAM1, enhances the chilling stress tolerance of transgenic tobacco [J]. Journal of Plant Physiology, 2016, 204: 54–65. |
| [71] | Zhu J K. Abiotic stress signaling and responses in plants[J]. Cell, 2016, 176: 313–324. |
| [72] | Guiltinan M J. A plant leucine zipper protein that recognizes an abscisic acid response element[J]. Science, 1990, 250(4978): 267–271. |
| [73] | Shi H Z, Ishitani M, Kim C, et al. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+antiporter [J]. Proceedings of the National Academy of Science of the United States, 2000, 97(12): 6896–6901. |
| [74] | Chinnusamy V, Zhu J H, Zhu J K. Cold stress regulation of gene expression in plants[J]. Trends Plant Science, 2007, 12(10): 444–451. |
| [75] | Lee B H, Henderson D A, Zhu J K. The Arabidopsis cold-responsive transcriptome and its regulation by ICE1 [J]. The Plant Cell, 2005, 17(11): 3155–3175. |
| [76] | Zhu J H, Shi H Z, Lee B H, et al. An Arabidopsis homeodomain transcription factorgene, HOS9, mediates cold tolerance through a CBF-independent pathway [J]. Proceedings of the National Academy of Sciences of the United States, 2004, 101(26): 9873–9878. |
| [77] | Dai X Y, Xu Y Y, Ma Q B, et al. Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis [J]. Plant Physiology, 2007, 143: 1739–1751. |
| [78] | Duan J F, Hui T, Gao Y J. Expression of nitrogen transporter genes in roots of winter wheat (Triticum aestivum L.) in response to soil drought with contrasting nitrogen supplies [J]. Crop and Pasture Science, 2017, 67(2): 128–136. |
| [79] | Shen Y, Shen L K, Shen Z X, et al. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice[J]. Plant, Cell and Environment, 2015, 38(12): 2766–2779. |
| [80] | Song Z P, Shao H F, Huang H G, et al. Overexpression of the phosphate transporter gene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum [J]. Environmental and Experimental Botany, 2017, 137: 158–165. |
| [81] |
董旭, 王雪, 石磊, 等. 植物磷转运子PHT1家族研究进展[J].
植物营养与肥料学报, 2017, 23(3): 799–810.
Dong X, Wang X, Shi L, et al. Advances in plant PHT1 phosphate transporter family research[J]. Journal of Plant Nutrition and Fertilizer, 2017, 23(3): 799–810. |
 2017, Vol. 23
2017, Vol. 23  doi:
doi: 

