2. 贵州省植物保护研究所,贵阳 550006;
3. 中国农业科学院农业资源与农业区划研究所,北京 100081
2. Guizhou Institute of Plant Protection, Guiyang 550006, China;
3. Institute of Agricultural Resources and Regional Planning, CAAS, Beijing 100081, China
木霉菌(Trichoderma spp)是自然界广泛分布的具有较高生防应用价值的真菌类群。木霉菌对许多作物具有较好的防病效果,2014年尹大川等研究表明,绿木霉 (Trichoderma virens) 对樟子松 (Pinus sylvestris) 的枯梢病 (Sphaeropsis sapinea)、杨树 (Populus L.) 的烂皮病 (Cytospora chrysosperma) 以及由立枯丝核菌 (Rhizoctonia solani) 和尖孢镰刀菌 (Fusarium oxysporum) 引起的苗木立枯病具有较好的防治效果[1];长枝木霉 (Trichoderma longibrachiatum) 对黄瓜枯萎病[Fusarium oxysporum (Schl.) f.sp cucumerinum Owen][2]、绿色木霉 (Trichoderma viride) 对香蕉枯萎病 (Fusarium oxysporum f.sp.cubense, Foc) 等都具有明显的防治作用[3]。木霉也能够促进植物的生长。哈茨木霉 (Trichoderma harzianum) 能够促进茄子 (Solanum melongena) 幼苗[4]、辣椒 (Capsicum annum)[5]和番茄 (Lycopersicon esculentum)[6]等植物生长,深绿木霉 (Trichoderma aureoviride) 对白三叶草 (Trifolium rephens) 等具有明显的促生作用[7]。目前的研究结果表明,木霉可能的促生机制包括:1) 木霉可产生植物生长调节剂,对外源植物激素起到双向调节的作用[8–10];2) 木霉能抑制或降低植物根际周围的有害菌群产生的有利于自身生长但对植物生长有抑制作用的化合物[11–12];3) 木霉能够产生有机酸、螯合剂、还原酶等,增加植物对养分的利用率从而促进植物的生长[13–14]。
近年来,农杆菌介导的遗传转化方法 (Agrobacterium tumefaciens-mediated transformation,ATMT) 被广泛用于真菌的菌种改良、基因功能鉴定和基因标记与克隆等研究[15],它具有转化效率高[16],转化受体广泛(如孢子、菌丝、子实体等真菌组织可作为转化受体[17]),T-DNA单拷贝随机插入,能够形成遗传稳定的转化子等特点[18–19]。由于不需要对样品进行复杂处理,通过绿色荧光蛋白 (GFP) 标记的菌株在活细胞状态下即可以进行实时观察,使其在植物与微生物相互作用的研究中得到广泛的应用,例如Luc等[20]利用GFP标记,观察发现一种内生细菌在水稻的根系表面和茎组织细胞等部位定殖;Nicole等[21]分别利用红色荧光蛋白 (RFP) 和GFP标记研究了丛枝菌根真菌 (AMF) Glomus intraradices在植物根组织中的定殖。
钩状木霉 (Trichoderma hamatum) ACCC31649是一株具有生防潜力的优良专利菌株 (专利号:ZL201310023319.1),前期研究表明该菌株具有显著促进黄瓜生长的特性。其它研究也表明钩状木霉能够产生内切几丁质酶从而有效地抑制辣椒白绢病 (Sclerotium rolfsii Sacc.)[22],木霉菌(Trichoderma parareesei T6)莽草酸途径影响木霉菌对植物抗性的诱导和植物耐盐性[23]。本研究选择GFP基因作为标记基因,利用ATMT方法建立钩状木霉的遗传转化体系,获得稳定遗传的GFP标记转化子,并研究野生型和GFP标记的钩状木霉在宿主组织中的侵染和定殖过程及对辣椒的促生作用,为进一步研究钩状木霉在辣椒根际土壤的存活、定殖及其与病原菌的互作提供重要的技术和手段。
1 材料与方法 1.1 试验菌株与根癌农杆菌质粒供试钩状木霉菌株 (T. hamatum'ACCC 31649) 由中国农业科学院农业微生物菌种保藏中心提供。根癌农杆菌GFP基因表达载体质粒EGFP-PSK2251[18]由美国宾夕法尼亚州立大学 (The Pennsylvania State University) Kang教授实验室构建,并同意授权中国科学院微生物所真菌学国家重点实验室刘杏忠研究员惠赠。
1.2 GFP基因的转化根癌农杆菌介导 (ATMT) 遗传转化方法参照Mullins et al.的ATMT介导的GFP转化方法进行[18]。
1.2.1 钩状木霉对潮霉素B敏感性测试 潮霉素B浓度梯度分别为15、25、50、75和100 μg/mL,不加潮霉素B的培养平板为对照处理,每个处理设5个重复,28℃暗培养,6 d后,观察记录结果,确定潮霉素B对野生菌株的合适敏感浓度。
1.2.2 钩状木霉细胞核数观察 荧光染料及配制:1)荧光染料1制备 称取DAPI (4′, 6-diamidine -2-phenylindole dihydrochloride, Roche),用适量无菌超纯水溶解调整浓度为50 μg/mL制备的母液,于–620℃下保存备用。使用时用无菌水稀释成1 μg/mL的染色液[24];2)荧光染料2制备 将1 g/L的荧光增白剂溶液卡氏白 (Calcoflour white,Sigma) 用无菌水稀释至终浓度为1 μg/mL使用[25];FAA固定液 (100 mL)为50%乙醇90 mL、醋酸5 mL、甲醛5 mL。
样品的荧光染色处理:染色方法参照并改进胡晓棣等的DAPI和Calcoflour white双重染色的方法[26]。将一盖玻片放于载玻片上,在盖玻片中央滴加一滴孢子悬液,微波炉加热30 sec (LG烧烤型WD800 (MG-5520M) 800 W),于超净台风干,再水洗2次,加FAA固定液 (或将待观察材料直接浸入FAA固定液中) 固定1~2 h,50%乙醇冲洗2次,加100 μL DAPI染色液 (1 μg/mL),室温黑暗放置30 min,加100 μL Calcoflour white染色液 (1 μg/mL),室温黑暗放置10 min,水冲洗2~3次。最后将盖玻片的观察材料的一面加微量水并避免气泡,反转倒置于载玻片上,指甲油封片。选择340~488 nm波长激发光与发射光进行荧光显微观察。
1.2.3 GFP基因的遗传转化 菌株ACCC31649孢子液制备:转接木霉菌株于PDA平板,28℃培养6天左右,用适量无菌水洗培养平板,经4层灭菌擦镜纸过滤掉菌丝,取2 mL用于血球计数板计算孢子数目,将孢子悬液浓度调到107 spores/mL。
根癌农杆菌菌株的活化和诱导培养:从–80℃取出,冻融,在50 ng/mL卡那霉素的YEB培养平板上划线,28℃暗培养2天,挑取单菌落接种于1 mL的MM液体培养基中,同时加入2 μL的25 μg/mL卡那霉素,28℃暗培养2天,取100 μL活化的农杆菌液于900 μL的诱导培养液 (IM) 中,同时加入2 μL的卡那霉素 (50 μg/mL) 和4 μL的0.1 mol/L乙酰丁香酮 (AS),28℃,140 r/min,黑暗条件下摇菌10 h,使农杆菌菌液OD600达到0.6左右。
MM培养液、IM诱导液及共生培养基配方及配制参照王梅娟等方法[27]进行。
根癌农杆菌与真菌共培养及遗传转化:取100 μL的农杆菌菌液和100 μL的孢子液均匀混合,涂布于共生培养平板[18]上,25℃,黑暗条件下培养60 h后,将共培养物用2 mL无菌水洗涤,取200 μL过滤液涂布于含有潮霉素B (50 μg/mL)、噻孢霉素 (30 μg/mL) 和利福平 (50 μg/mL) 的PDA平板上,风干后覆盖一层相同抗生素的PDA培养基[28]。每天观察检查,待平板上出现菌落时镜检,挑取有荧光的转化子于潮霉素B (50 μg/mL) PDA平板上培养。
1.2.4 遗传稳定转化子的筛选和PCR验证 将挑取的转化子在潮霉素B (50 μg/mL) PDA平板连续转接5代,再接种于不含潮霉素B的PDA平板上,培养5天左右 (长满平板并产孢) 进行单孢分离。体视镜视野下,用酒精灯灼烧的注射器针头挑取菌落菌丝于1mL灭菌水的1.5 mL EP管中,充分混匀,以挑取次数设置孢子浓度梯度,一般设挑一次和两次的两个梯度。再用灭菌的胶头滴管吸取孢子悬液滴于皿底划有网格线并加有链霉素 (50 μg/mL) 的2%的水琼脂培养基 (WA,2 g琼脂粉,100 mL蒸馏水) 平板上,室温静置培养15 h,在体视镜下,用注射器针头将视野中萌发的单个孢子挑取接种到潮霉素B (50 μg/mL) 的PDA平板,28℃,黑暗培养1~2天,镜检,保留强荧光的转化子,再重复4次上述单孢分离过程,筛选得到遗传稳定的GFP标记转化子。筛选保留10个遗传稳定的转化子。
将野生型菌株和转化子接种PDA平板上培养一周左右,刮取菌丝提取基因组DNA,以基因组DNA为模板和特异性引物Hmbf1 (5′-CTGTCGAGAAGTTTCTGATCG-3′) 和Hmbr1 (5′-CTGATAGAGTTGGTCAAGACC-3′) 扩增潮霉素标记基因片段进行验证[29]。扩增反应条件为95℃预变性3 min,95℃ 30 s,62℃ 45 s,72℃ 60 s,进行35个循环,72℃ 10 min。
1.2.5 钩状木霉的生物学特性观察测定 菌落及菌丝和孢子形态:在PDA平板上活化培养钩状木霉野生型菌株ACCC31649及10个筛选的稳定转化子,用打孔器取菌落边缘生长的菌丝块,接种于90 mm PDA平板中央,以野生型菌株ACCC31649为对照,28℃黑暗培养6天左右,记录其菌落形态,并制片,通过光学显微镜观察形态学特征。
生长速率测定:以钩状木霉野生型菌株ACCC31649为对照处理,选择稳定转化子ACCC31649-GF21测定菌落的生长速率,每个处理5个重复,28℃,黑暗培养,采用十字交叉法测量菌落直径。
生物量的测定:参照陈美娟[30]的方法进行。以钩状木霉野生型菌株ACCC31649为对照处理,每个处理5次重复。在PDA平板 (Ф = 90 mm) 上铺一层灭菌的玻璃纸,将活化的菌株接种菌丝块 (Ф = 5 mm) 于玻璃纸中央,28℃,培养7 d,从玻璃纸上去除接种的菌丝块,刮取培养物,于电子天平上称量菌丝鲜重,然后在60℃烘箱中烘烤6 h,彻底去除水分,测量干重。
产孢量的测定:以钩状木霉野生型菌株ACCC31649为对照处理。将菌丝块接种到PDA平板上,28℃黑暗培养7 d,先加入3 mL灭菌蒸馏水,用灭菌涂布棒轻轻刮取培养基表面的菌丝,经4层擦镜纸过滤,再用1 mL灭菌蒸馏水冲洗过滤纸,收集分生孢子,用血球计数板测量分生孢子液浓度,每个处理5次重复。
1.3 野生型菌株与GFP标记菌株对辣椒促生作用挑选子粒饱满、大小均匀的辣椒种子 (中椒6号,中国农业科学院蔬菜花卉研究所选育),表面消毒 (3%NaClO,1 min;75%酒精,1 min)。将消毒后的种子均匀平铺在铺有灭菌纱布的保鲜盒中,覆盖一层灭菌纱布,加入适量 (纱布湿润即可) 的无菌水,密封、28℃黑暗下催芽。待种子冒白芽后播种于装有灭菌营养土的育苗钵中,长至四叶一心时进行木霉灌根接种处理。
将野生菌株ACCC31649于PDA平板上培养6 d左右,加无菌水制成分生孢子悬浮液,血球计数板计数调整浓度为1 × 10 7 spores/mL。取2 mL分生孢子液于120 mL PDB三角瓶中,28℃,150 r/min摇瓶培养6天,4层灭菌滤纸过滤,制备浓度为1 × 10 7 spores/mL孢子液。每株辣椒幼苗接种15 mL孢子液于根围,以无菌水为对照处理,每个处理设16个重复,于26℃、14 h光照/10 h黑暗交替培养30 d,测量辣椒根长、株高、鲜重、干重。计算菌株对辣椒幼苗的促生率[31]:
|
$\text{促生率} = \frac{{\text{生防菌处理组} - \text{对照处理组}}}{{\text{对照处理组}}} \times 100{\%}$
|
按照1.3的方法在辣椒幼苗根围接种GFP标记菌株ACCC31649-GF21,26℃,14 h光照/10 h黑暗交替培养,每隔24 h取辣椒根、茎和叶,徒手切片,制成水玻片,荧光显微镜下观察菌株在辣椒各组织中的定殖情况。
1.5 数据统计与分析数据采用DPS 7.05软件进行方差分析,并用LSD法比较各处理间的差异显著性 (P=0.05) 。
2 结果与分析 2.1 钩状木霉的遗传转化2.1.1 钩状木霉 ACCC31649对潮霉素B的敏感性 钩状木霉ACCC31649在不同浓度的潮霉素平板,28℃培养4 d。当潮霉素B浓度在50 μg/mL及以上时 (图1),ACCC31649菌丝不能生长。因此,本研究采用50 μg/mL的潮霉素B作为筛选转化子的适宜浓度。
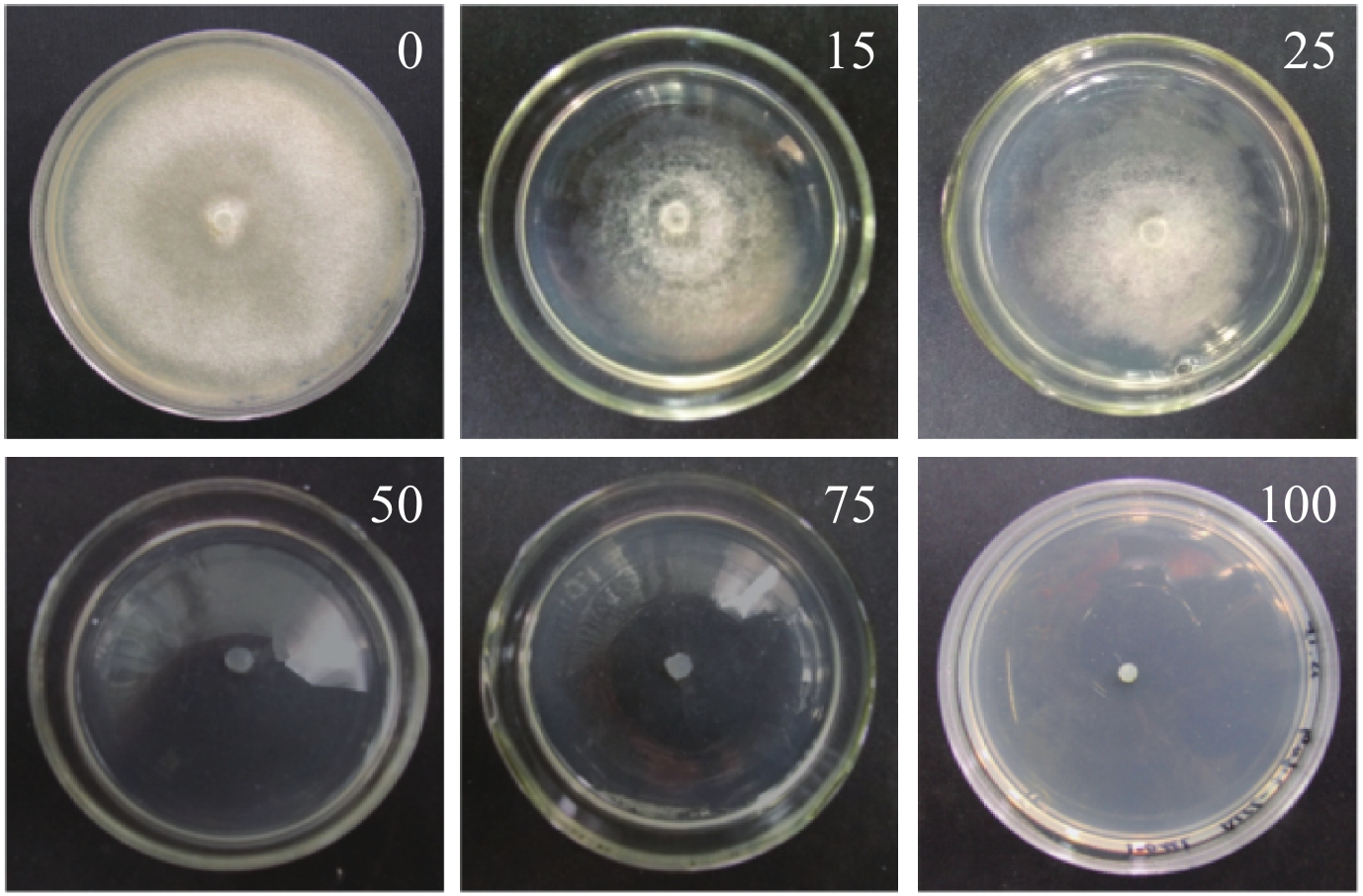 |
| 图1 不同潮霉素B浓度 (μg/mL) 对钩状木霉ACCC 31649的生长抑制 Fig. 1 Inhibition of different concentrations of hygromycin B (μg/mL) on mycelium growth of T. hamatum ACCC31649 |
2.1.2 细胞核染色及观察 通过DAPI染色液和Calcoflour white染色液对钩状木霉细胞核进行组合染色后,在345~488 nm发射波长范围进行荧光显微观察,由图2可清楚观察到钩状木霉分生孢子为单核细胞。
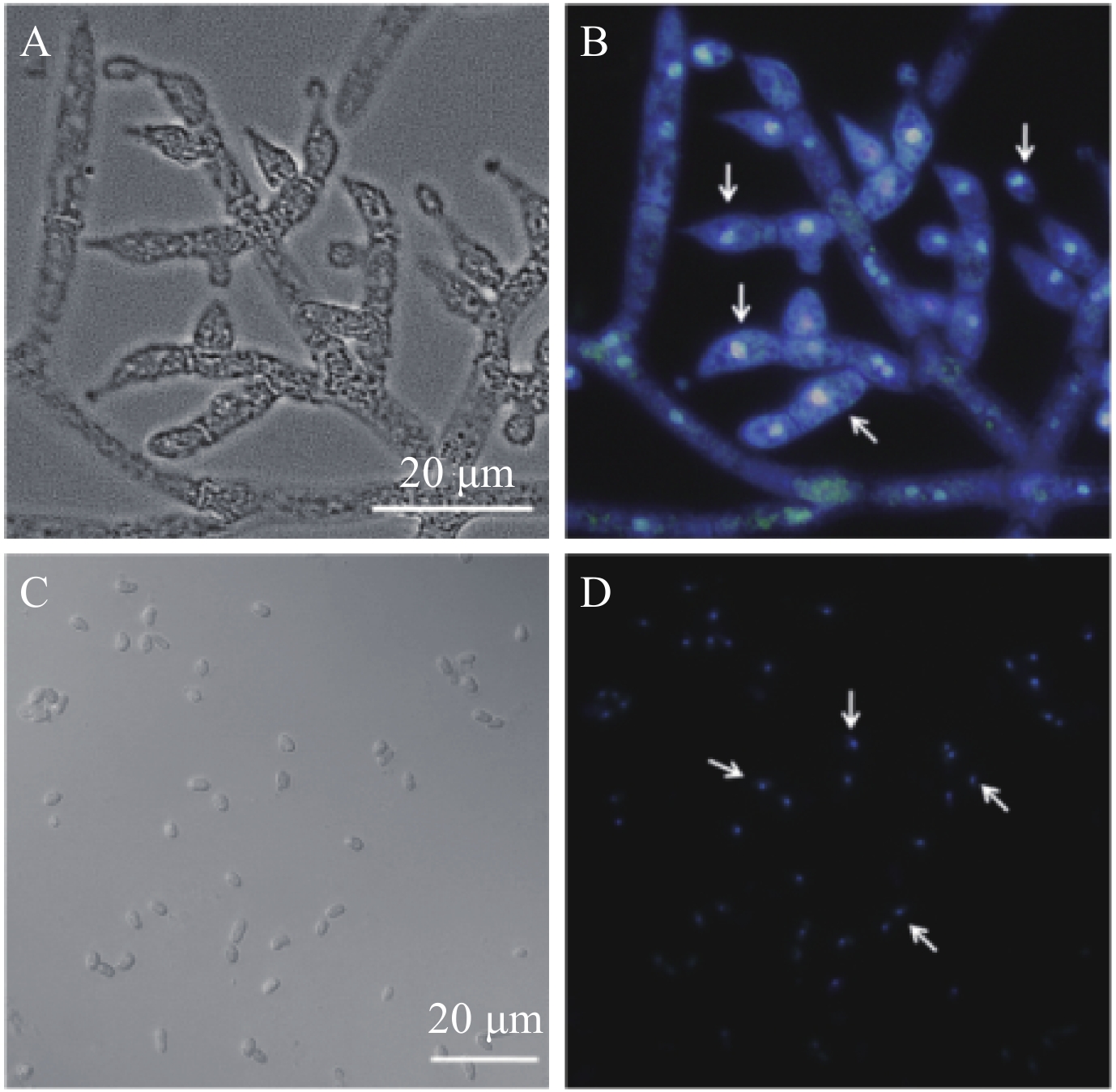 |
| 图2 ACCC31649-GF21在辣椒植株中的定殖 Fig. 2 Colonization of the ACCC31649-GF21 in the pepper plants [注(Note):A—明场下的野生型菌株 ACCC31649 的菌丝;B,D—DAPI 和卡氏白荧光染色图;C—明场下的野生型菌株 ACCC31649 的分生孢子;图中箭头示细胞核。A—Hyphae of T. hamatum ACCC31649 under bright field; B, D—DAPI and Calcoflour white stained epifluoresence microphotograph; C—Conidia of T. hamatum ACCC31649 under bright field; The arrows indicate nucleus.] |
2.1.3 钩状木霉转化子的筛选 将选择性培养基上长出的单孢菌落挑出接种于含潮霉素B (50 μg/mL) 的PDA培养基上,5天后荧光检测并挑取边缘菌丝接种于含潮霉素B的PDA培养基上,这样重复操作5次初步得到GFP标记的转化子。从上述转化子中挑选出10个具有强绿色荧光的转化子,每一个转化子分别进行5代的单孢分离培养,最终保留10个能够稳定遗传的GFP标记转化子 (图3)。
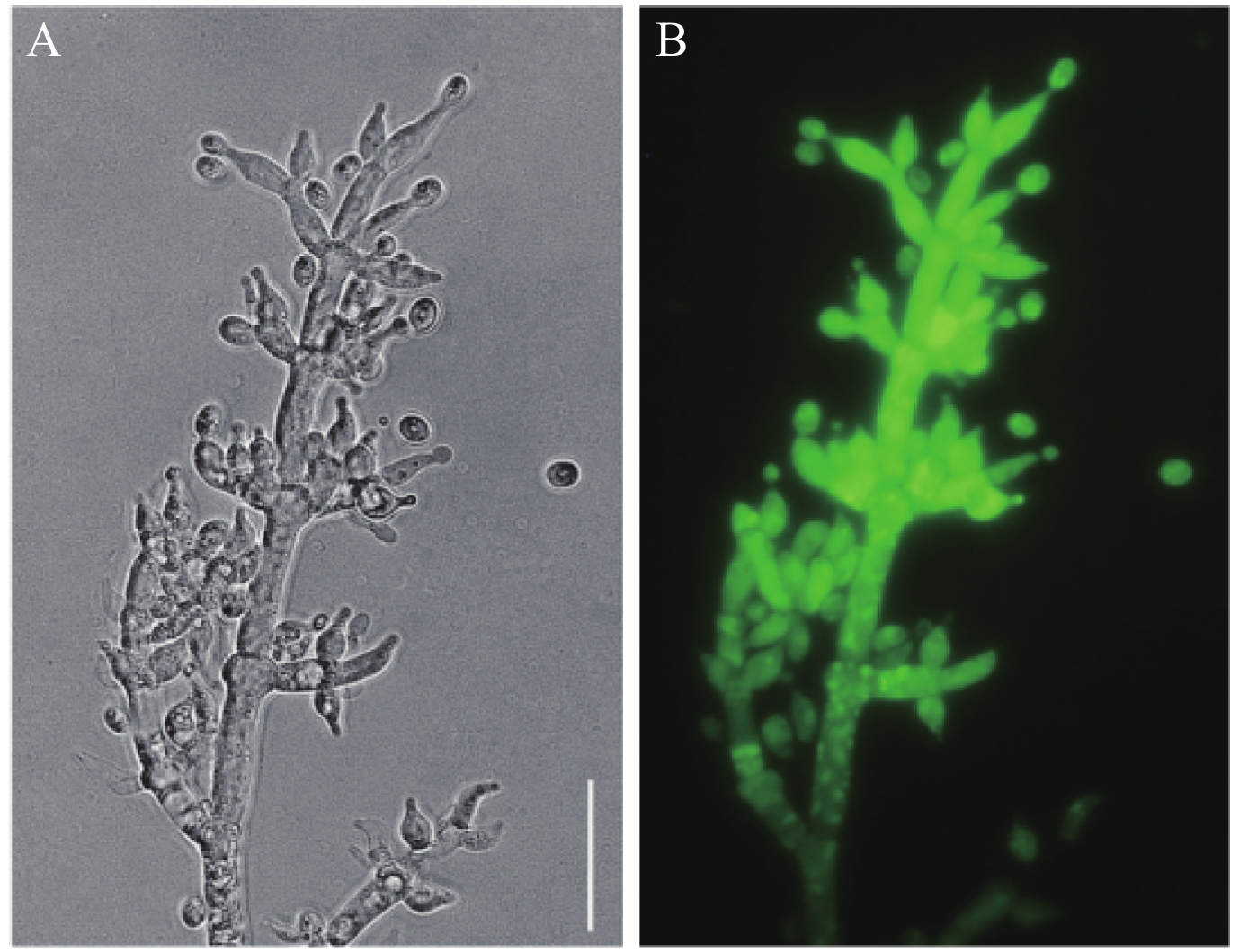 |
| 图3 钩状木霉ACCC31649-GF21的GFP荧光蛋白表达 (标尺 25 μm) Fig. 3 Expression of GFP fluorescent proteins in T. hamatum ACCC31649-GF21 (bar 25 μm) [注(Note):A— 明场下的GFP标记菌株ACCC31649-GF21的菌丝和分生孢子; B—紫外激发光下的GFP标记菌株ACCC31649-GF21的菌丝和分生孢子。A— Hyphae and conidia of T. hamatum ACCC31649-GF21 under bright field; B— Hyphae and conidia of T. hamatum ACCC31649-GF21 under fluorescence microscope.] |
转化子PCR验证:分别选取2个GFP标记转化子和野生型菌株的基因组DNA做为模板,特异性引物Hmbf1和Hmbr1扩增潮霉素标记基因片段进行验证。扩增产物凝胶电泳结果,其条带在750 bp左右 (图4),与预期目标片段大小一致。
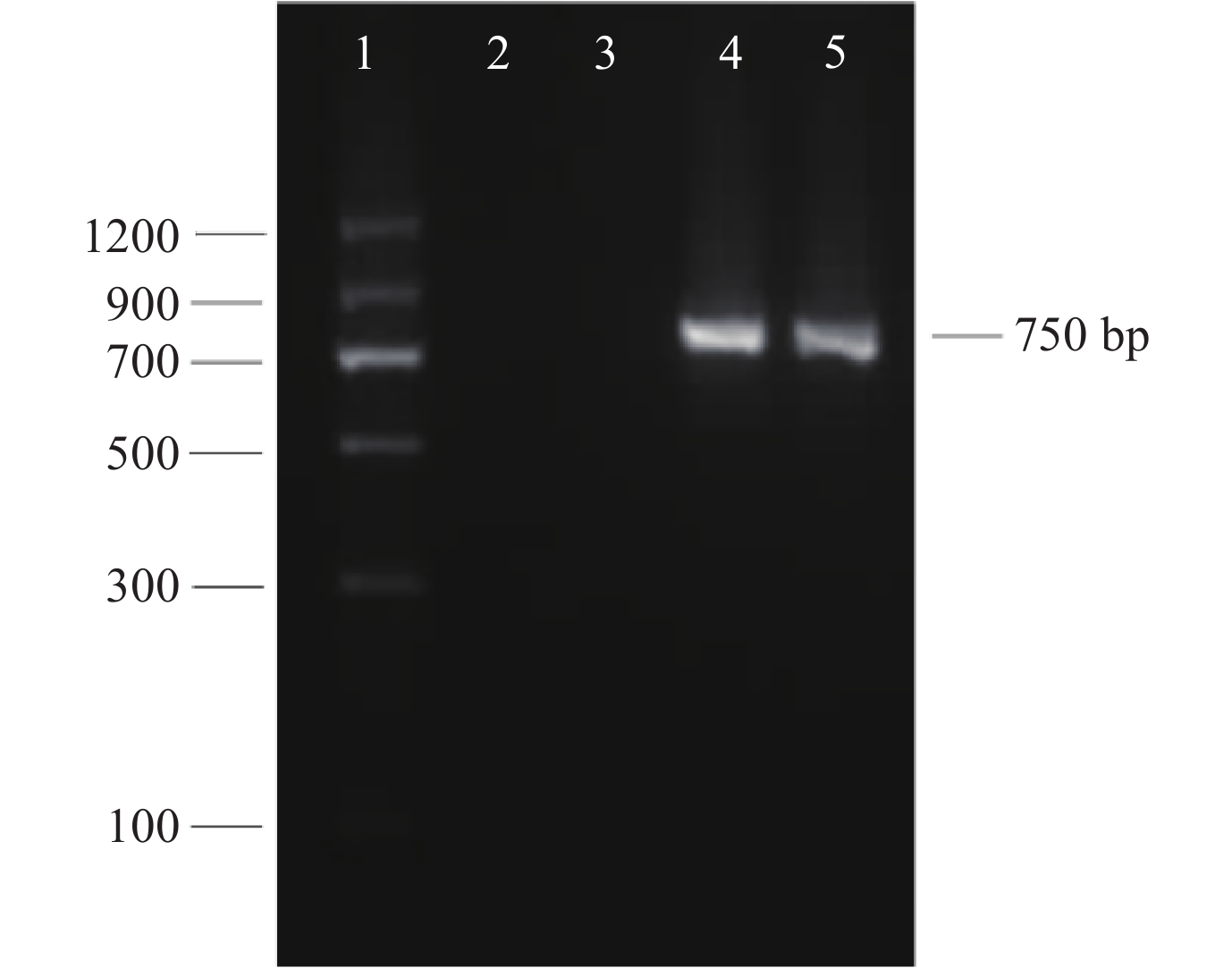 |
| 图4 钩状木霉ACCC31649-GF21的潮霉素标记基因PCR检测 Fig. 4 PCR detection of Hyg B gene of T. hamatum ACCC31649-GF21 [注(Note):泳道1~5依次为MarkerⅡ(DL1200, 天根生物),阴性对照,钩状木霉ACCC 31649, ACCC 31649-GF21-1, ACCC 31649-GF21-2。Lane 1–5: Marker Ⅱ DL1200 (TIANGEN Biotech), Negative control, T. hamatum ACCC 31649, T. hamatum ACCC 31649-GF21-1, T. hamatum ACCC 31649-GF21-2.] |
野生型钩状木霉ACCC31649菌落多为基内菌丝,少量气生菌丝,且气生菌丝呈卷毛状,白色至灰色,分生孢子产生于致密的垫状或者半球形的产孢簇中,产孢簇表面呈绒毛状,初期为白色,渐变为暗绿色,背面暗灰色;ACCC 31649-GF21菌落基内菌丝发达,白色至黄绿色,背面无色至灰色。野生型与GFP标记GF21菌株形态特征相比较,两者菌落边缘规则整齐,菌丝直径1~7 μm,分生孢子都为椭圆形,大小 (3.9~5.6) μm × (2.8~4.0) μm,尖端阔圆形,基部变细或者稍微呈尖形;气生菌丝上稀疏地产生分生孢子梗,在分生孢子梗尖端下部产生1~2个轮生瓶梗,瓶梗从基部向上至顶尖逐渐缢缩,中间部位有时候稍微膨大 ( 图5)。
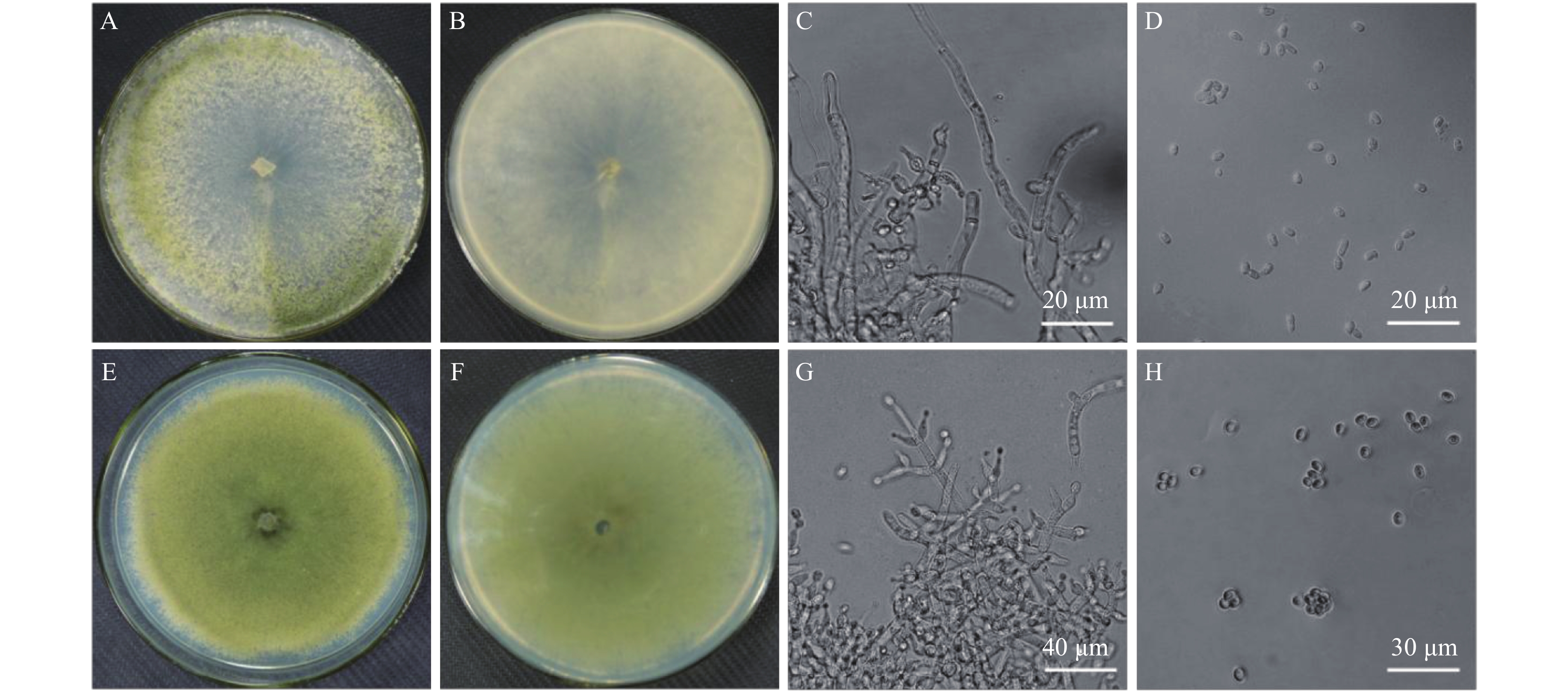 |
| 图5 钩状木霉ACCC31649与ACCC31649-GF21的形态特征 Fig. 5 The morphology of T. hamatum ACCC31649 and T. hamatum ACCC31649-GF21 [注(Note): A、B、C、D— 野生型菌株; E、F、G、H — GFP 标记菌株 ACCC31649-GF21。A、B、C、D—ACCC31649 T. hamatum ACCC31649; E、F、G、H— T. hamatum ACCC31649-GF21.] |
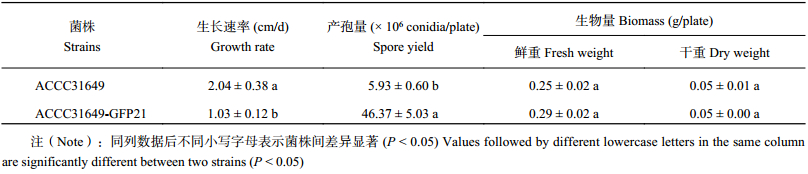
通过对标记前后菌株的培养特征进行显微观察和测定,在菌丝大小、产孢结构及孢子形态等方面,转化子与野生型无明显差异,而在相同培养条件下,GFP标记菌株ACCC 31649-GF21菌落的生长速率比野生型变慢了,而ACCC31649-GF21产孢量与野生型相比却增加了,但在菌落的鲜重和干重等生物量方面,ACCC31649-GF21与野生型菌株基本一致 (表1)。
| 表1 钩状木霉野生菌株和转化子生长速率、产孢量及生物量的比较 Table 1 Comparison of growth rate, biomass and conidiation of T. hamatum ACCC31649 and T. hamatum ACCC31649-GF21 |
 |
通过取样切片荧光显微观察,在接种ACCC31649-GF21后的24 h,GF21的菌丝和孢子聚集在根表皮 (图6A、B);48 h后,GFP标记菌株在主根维管束中生长定殖 (图6C);接种3 d,菌丝穿透皮层细胞在主根韧皮部定殖 (图6D),同时在辣椒的茎部木质部 (图6E) 也观察到菌株的定殖;接种5 d,定殖至茎表皮 (图6F) 和根茎连接处 (图6G) 及茎部气孔 (图6H);25 d后,菌丝到达辣椒植株的第一片真叶的叶柄 (图6I、J) 和叶片 (图6M、L) 组织中。从30天到70天后,辣椒组织中仍然可以观察到绿色荧光菌丝。上述结果表明,通过灌根接种,标记的钩状木霉菌株能够从辣椒根部维管束自下向上定殖,首先在辣椒幼苗的侧根周围富集、吸附,后侵入侧根表层,进入维管束和韧皮部,沿维管束向上经主根、根茎交界、茎和叶定殖生长,但最后都未能够定殖到辣椒植株的顶端。
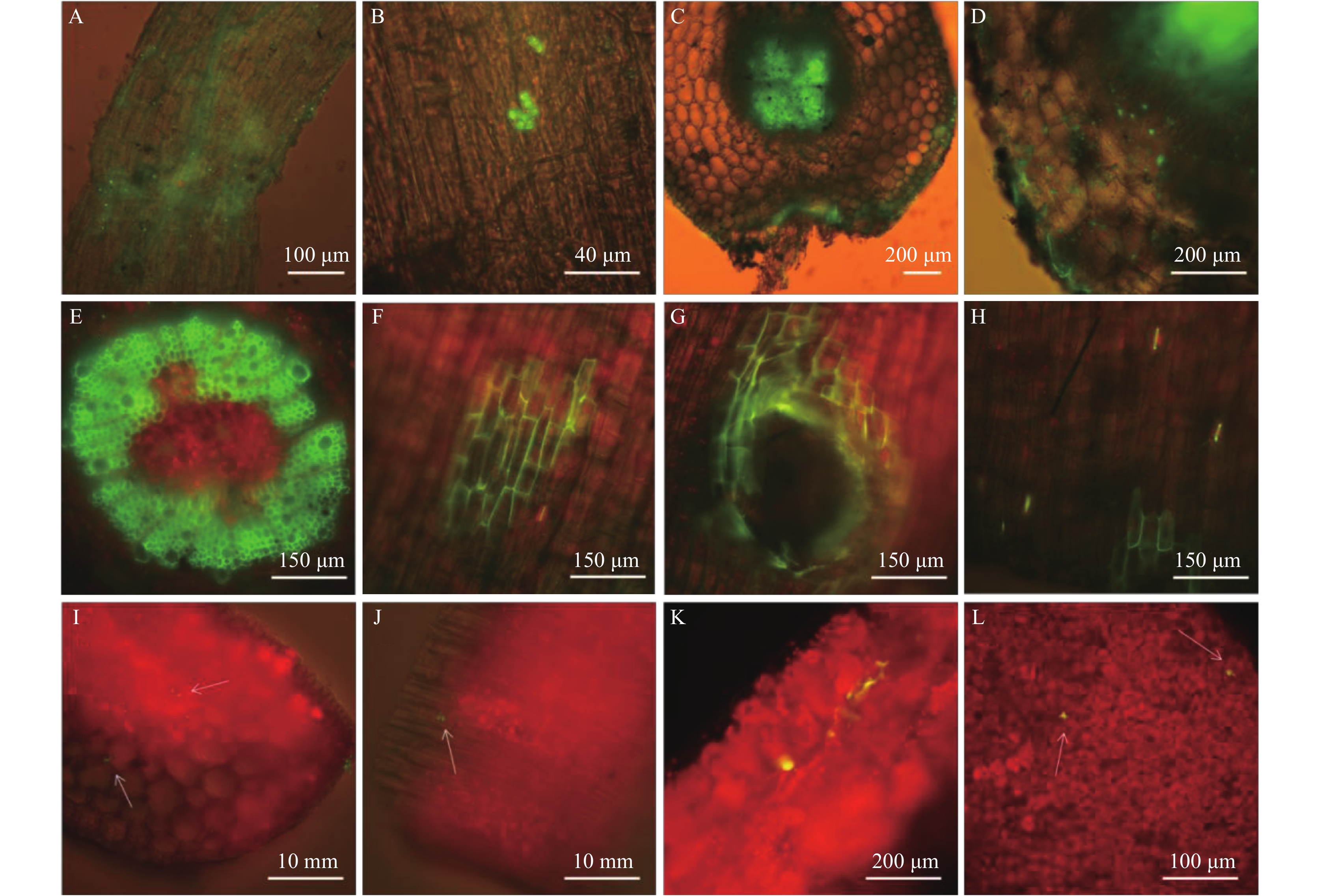 |
| 图6 ACCC31649-GF21在辣椒植株中的定殖 Fig. 6 Colonization of the ACCC31649-GF21 in the pepper plants [注(Note):A— 标记菌株的菌丝和分生孢子在辣椒侧根富集; B— 分生孢子吸附在辣椒根表皮; C— 标记菌株定殖于辣椒主根维管束中; D— 标记菌株菌丝穿透皮层细胞进入主韧皮部; E— 标记菌株在辣椒茎部木质部中定殖; F— 标记菌株向上定殖到达茎表皮; G— 根茎交接处聚集大量菌丝H— 菌丝定殖至辣椒茎部气孔; I、J— 菌丝到达辣椒叶柄中定殖; K、L— 标记菌株在辣椒叶片定殖; 绿色荧光的钩状木霉在图中呈现绿色或黄绿色,红色为辣椒组织自发荧光。A— The hyphae and conidia of labeled fungus clustered on the branch roots of the pepper; B— The conidia formed on the surface of the roots; C— The labeled fungus grew into the vascular bundle of taproot; D— The labeled fungus penetrated into the phloem of taproot; E— The labeled fungus penetrated into hadromestome of the stems; F— The labeled fungus colonized on the surface of the stems; G— The labeled fungus colonized into the junctions between roots and stems; H— The labeled fungus colonized into the stomata of stems; I, J— The labeled fungus colonized into the petioles; K, L— The labeled fungus grew in the tissues of leaves; The labeled T. hamatum presented in green or yellow-green, while the tissues of pepper presented in red by auto fluorescence.] |
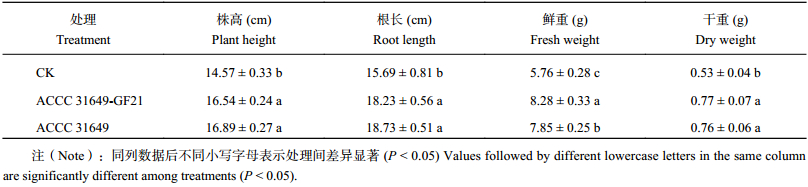
接种钩状木霉30天后,辣椒幼苗的株高、根长及植株的干重和鲜重等数据统计结果表明,经野生型菌株处理后,辣椒的株高比对照增加15.9%,根长增加19.4%,鲜重增加36.3%,干重增加43.4%,野生型菌株对辣椒幼苗具有显著的促生作用;而经转化子GF21处理的辣椒幼苗,辣椒的株高比对照增加13.5%,根长增加16.2%,鲜重增加43.8%,干重增加45.3%,转化子同样表现出显著的促生作用 (图7、表2)。上述结果表明,转化子GF21和野生型菌株都能够明显促进辣椒幼苗的生长。
 |
| 图7 钩状木霉对辣椒幼苗生长的影响 Fig. 7 Effects of the T. hamatum on the growth of the pepper seedlings |
| 表2 钩状木霉对辣椒幼苗生长的影响 Table 2 Effects of the T. hamatum on the growth of the pepper seedlings |
 |
目前,植物病害生物防治中应用较多的生防真菌主要有木霉菌[32]、芽孢杆菌[33–34]、丛枝菌根真菌[35–36]、毛壳菌[37]等。研究已经证明木霉菌能够通过溶菌、重寄生、竞争营养和空间来抑制病原真菌[38–39],同时还能够促进植物生长[40–41],从而增强植物的抗逆能力。本研究以GFP基因作为标记基因,利用ATMT方法建立了GFP基因的钩状木霉ACCC31649遗传转化体系。近年的研究表明,单核真菌通过ATMT介导的遗传转化子具有很高的遗传稳定性[42–43],因此,本研究通过DAPI对钩状木霉ACCC 31649分生孢子的细胞核进行染色,确定了钩状木霉ACCC31649清晰的单核细胞结构,经不断摸索并优化了转化条件,即当乙酰丁香酮 (AS) 浓度为200 mmol/L,诱导培养10 h,25℃黑暗共培养60 h时成功获得了稳定遗传的GFP标记菌株ACCC31649-GF21。转化子的遗传稳定性是进行生防菌定殖机理研究的重要条件[44],故对ACCC 31649-GF21进行扩增潮霉素抗性基因验证及真菌形态检测,结果表明该基因在转化子GF21中得到稳定、高效的表达;此外,对ACCC 31649-GF21的生长速率、产孢量和生物量测定结果表明,转化子GF21的生长速率变慢,但这种现象在其他菌株中也有出现,如车建美等用GFP标记青枯雷尔氏菌 (Ralstonia solanacearum)[45]及刘路宁[46]用GFP标记绿木霉 (Trichoderma virens) 的研究中,也出现了标记菌株生长变慢。但对菌株ACCC31649-GF21检测分析后发现,GFP标记菌株促生效果与野生型相比没有显著性差异。
通过GFP标记技术能够实时、动态地研究微生物的空间定位、微生物与环境及宿主之间的互作[47–48],如武坤毅等利用GFP标记技术成功观测了溶杆菌 (Lysobacter sp.) SNNU513在玉米 (Zea mays) 根部定殖规律[49];李亮亮等利用GFP标记短小芽孢杆菌 (Bacillus pumilu) 并观察到其在马尾松 (Pinus massoniana Lamb) 体内的定殖动态[50]。本研究证明了钩状木霉ACCC 31649能够通过菌丝和孢子在辣椒不同组织中定殖,可通过侧根、根毛和根表皮的自然孔口或气孔侵入辣椒植株根内,经皮层到达根维管束系统,在蒸腾拉力或其它因素 (如矿物质的运输等) 的作用下由地下根部向茎甚至叶等地上部迁移或扩散[51]。研究还发现,在辣椒植株的茎部维管束中未检测到ACCC31649-GF21定殖,所以标记菌株是通过茎部木质部进行自下而上的侵染,而不是通过茎部维管束。同时,接种生防钩状木霉ACCC31649对辣椒幼苗的株高、根长及生物量等方面具有显著的促生作用。本文的相关结果为进一步研究生防钩状木霉与辣椒病原菌及宿主植物之间的互作和生防作用奠定了重要基础。
致谢:本文在显微照相方面得到贵州省农业生物技术重点实验室谭玉梅、朱英两位老师和贵州省植物保护研究所吴石平老师的帮助和指导,在转化菌株和质粒方面得到中国科学院微生物所真菌学国家重点实验室刘杏忠研究员的帮助和指导,特此感谢!
| [1] |
尹大川, 邓勋, 宋瑞清, 等. 绿木霉 (Trichoderma virens) 对四种重要林木病原菌的抑制效果及抑菌机理
[J].
生态学志, 2014, 33(7): 1911–1919.
Yin D C, Deng X, Song R Q, et al. Inhibiting effect and mechanism of Trichoderma virens T43 on four major species of forest pathogen [J]. Chinese Journal of Ecology, 2014, 33(7): 1911–1919. |
| [2] |
谷祖敏, 毕卉. 长枝木霉THLJ菌株防治黄瓜枯萎病影响因素分析[J].
农药, 2016, 55(1): 58–60.
Gu Z M, Bi H. Influence factors of control efficacy against cucumber Fusarium wilt with Trichoderm longibrachiatum [J]. Agrochemicals, 2016, 55(1): 58–60. |
| [3] |
梁昌聪, 刘磊, 张建华, 等. 绿色木霉菌H06固体浅盘发酵工艺优化[J].
菌物学报, 2014, 33: 1313–1326.
Liang C C, Liu L, Zhang J H, et al. Optimization of shallow tray fermentation process of Trichoderma viride H06 [J]. Mycosystema, 2014, 33: 1313–1326. |
| [4] |
胡洁, 秦克伟, 蔡枫, 等. 哈茨木霉SQR-T037对茄子幼苗促生效应的研究[J].
安徽农业科学, 2012, 40(32): 15671–15673, 15694.
Hu J, Qin K W, Cai F, et al. Study on growth-promoting effect of Trichoderma harzianum strain SQR-T037 on eggplant seedlings [J]. Journal of Anhui Agricultural Sciences, 2012, 40(32): 15671–15673, 15694. DOI:10.3969/j.issn.0517-6611.2012.32.037 |
| [5] |
胡琼. 哈茨木霉TC对辣椒促生效果的研究[J].
安徽农业科学, 2013, 41(21): 8941–8943.
Hu Q. Study on promotion effects of Trichoderma TC on pepper (Capsicum annum Linn.) growth [J]. Journal of Anhui Agricultural Sciences, 2013, 41(21): 8941–8943. DOI:10.3969/j.issn.0517-6611.2013.21.048 |
| [6] |
李栎, 肖曼, 高新征, 等. 哈茨木霉TL-1促进植物生长及病害防治效果初报[J].
广东农业科学, 2012, 22: 91–94.
Li L, Xiao M, Gao X Z, et al. Primary study on growth-promoting and biological control effects of Trichoderma harzianum TL-1 [J]. Guangdong Agricultural Sciences, 2012, 22: 91–94. DOI:10.3969/j.issn.1004-874X.2012.02.034 |
| [7] |
张树武, 徐秉良, 程玲娟, 等. 深绿木霉对白三叶草促生作用及生理生化特性的影响[J].
草业学报, 2015, 24(2): 161–167.
Zhang S W, Xu B L, Cheng L J, et al. Effects of Trichoderma aureoviride fermentation on the growth and physiological characteristics of Trifolium repens [J]. Acta Prataculturae Sinica, 2015, 24(2): 161–167. DOI:10.11686/cyxb20150218 |
| [8] | Harman G, Howell C R, Viterbo A, et al. Trichoderma species opportunistic, avirulent plant symbionts [J]. Nature, 2004, 2: 43–56. |
| [9] | Hexon A C C, Lourdes M R, Carlos C P, et al. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through and auxin-dependent mechanism in Arabidopsis [J]. Plant Physiology, 2009, 149: 1579–1592. DOI:10.1104/pp.108.130369 |
| [10] | Valerie G, Hani A, Russell J, et al. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA) [J]. Soil Biology and Biochemistry, 2007, 39: 1968–1977. DOI:10.1016/j.soilbio.2007.02.015 |
| [11] | Ezzi M I, Lynch J M. Cyanide catabolizing enzymes in Trichoderma spp. [J]. Enzyme and Microbial Technology, 2002, 31: 1042–1047. DOI:10.1016/S0141-0229(02)00238-7 |
| [12] | Adams P, De-Leij F A A M, Lynch J M. Trichoderma harzianum Rifai 1295-22 mediates growth promotion of crack willow (Salix fragilis) saplings in both clean and metal-contaminated soil [J]. Microbial Ecology, 2007, 54: 306–313. DOI:10.1007/s00248-006-9203-0 |
| [13] | Altomare C, Norvell W A, Björkman T, et al. Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22 [J]. Applied Environmental Microbiology, 1999, 65: 2926–2933. |
| [14] | Adams P, Lynch J M, De-Leij F A A M. Desorption of zinc by extracellularly produced metabolites of Trichoderma harzianum, Trichoderma reesei and Coriolus versicolor [J]. Applied Microbiology, 2007, 103(6): 2240–2247. DOI:10.1111/jam.2007.103.issue-6 |
| [15] |
李国田, 杨合同, 周红姿. 根癌农杆菌介导的木霉插入转化及其应用[J].
山东科学, 2006, 19(6): 24–30.
Li G T, Yang H T, Zhou H Z. Agrobacterium tumefaciens mediated transformation and its application in Trichoderma spp [J]. Shandong Science, 2006, 19(6): 24–30. |
| [16] | De Groot M J A, Bundok P, Hooykaas P P J, et al. Agrobacterium -tumefaciens mediated transformation of filamentous fungi [J]. Nature Biotechnology, 1998, 16(9): 839–842. DOI:10.1038/nbt0998-839 |
| [17] | Bundock P, Den Dulk-Ras A, Beijersbergen A, et al. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae [J]. The EMBO Journal, 1995, 14(13): 3206–3214. |
| [18] | Mullins E D, Chen X, Romaine P, et al. Agrobacterium mediated-transformation of Fusarium oxysporum: An efficient tool for insertional mutagenesis and gene transfer [J]. Phytopathology, 2001, 91: 173–180. DOI:10.1094/PHYTO.2001.91.2.173 |
| [19] | Tanaka S, Kondo N, Naito S. Isolation of pathogenicity and gregatin-deficient mutants of Phialophora gregata f. sp. adzukicola through Agrobacterium tumefaciens-mediated transformation [J]. Journal of General Plant Pathology, 2007, 73(4): 242–249. DOI:10.1007/s10327-007-0021-0 |
| [20] | Luc F M, Rouws C H S G, et al. Monitoring the colonization of sugarcane and rice plants by the endophytic diazotrophic bacterium Gluconacetobacter diazotrophicus marked with gfp and gusA reporter genes [J]. Letters in Applied Microbiology, 2010, 51: 325–330. DOI:10.1111/lam.2010.51.issue-3 |
| [21] | Nicole H, Natalia R. Expression of the fluorescence markers DsRed and GFP fused to nuclear localization signal in the arbussular mycorrhizal fungus Glomus intraradices [J]. New Phytologist, 2008, 177: 537–548. |
| [22] |
李俊, 谢伟国, 程鹏, 等. 抗辣椒白绢病致病菌的钩状木霉中几丁质酶诱导表达分析[J].
陕西农业科学, 2016, 62(5): 12–14.
Li J, Xie W G, Cheng P, et al. Analysis on viride chitinase of Trichoderma hamatum for Sclerotium rolfsii [J]. Shaanxi Journal of Agricultural Sciences, 2016, 62(5): 12–14. |
| [23] | Rubio M B, Quijadn N M, Perez E, et al. Identfying beneficial qualities of Trichoderma parareeset for plants [J]. Applied and Environmental Mictobiology, 2014, 80(6): 1864–1873. DOI:10.1128/AEM.03375-13 |
| [24] | Liesche J, Ziomkiewicz I, Schulz A. Super-resolution imaging with Pontamine Fast Scarlet 4BS enables direct visualization of cellulose orientation and cell connection architecture in onion epidermis cells[J]. BMC Plant Biology, 2013, 13(2): 285–288. |
| [25] | Thomas J, Ingerfeld M, Nair H, et al. Pontamine fast scarlet 4B: a new fluorescent dye for visualising cell wall organisation in radiata pine tracheids[J]. Wood Science and Technology, 2013, 47(1): 59–75. DOI:10.1007/s00226-012-0483-x |
| [26] |
胡晓棣, 李熠, 任蜀豫, 等. 冬虫夏草、蛹虫草菌丝隔膜和细胞核荧光染色[J].
菌物学报, 2016, 35(9): 1099–1105.
Hu X L, Li Y, Ren S Y, et al. Fluorescent staining of septa and nuclei in Ophiocordyceps sinensis and Cordyceps militaris [J]. Mycosystema, 2016, 35(9): 1099–1105. |
| [27] |
王梅娟, 李坡, 吴敏, 等. 玉米大斑病菌ATMT突变体库的构建及其分析[J].
中国农业科学, 2012, 45(12): 2384–2392.
Wang M J, Li P, Wu M, et al. Constuction and evaluation of ATMT mutant library of Setosphaeria turcica [J]. Scientia Agricultura Sinica, 2012, 45(12): 2384–2392. DOI:10.3864/j.issn.0578-1752.2012.12.006 |
| [28] | Zeilinger S. Gene disruption in Trichoderma atroviride via Agrobacterium-mediated transformation [J]. Current Genetics, 2003, 45: 54–60. |
| [29] | Crespo-Sempere A, Lopez-Perez M, Martinez-Culebras P V, et al. Development of a green fluorescent tagged strain of Aspergillus carbonarius to monitor fungal colonization in grapes [J]. International Journal of Food Microbiology, 2011, 148(2): 135–140. DOI:10.1016/j.ijfoodmicro.2011.05.021 |
| [30] |
陈美娟. 希金斯剌盘孢Ch-STE7和Ch-SEC1基因的功能分析[D]. 武汉: 华中农业大学硕士学位论文, 2014.
Chen M J. Functional analysis of Ch-STE7 and Ch-SEC1 in Colletotrichum higginsianum[D]. Wuhan: MS Thesis of Huazhong Agricultural University, 2014. |
| [31] |
乔俊卿, 陈志谊, 梁雪杰, 等. 枯草芽孢杆菌Bs916防治番茄青枯病[J].
中国生物防治学报, 2016, 32(2): 229–234.
Qiao J Q, Chen Z Y, Liang X J, et al. Biocontrol efficacy on tomato bacterial wilt by Bacillus subtilis Bs916 [J]. Chinese Journal of Biological Control, 2016, 32(2): 229–234. |
| [32] |
吴琳, 黄华平, 杨腊英, 等. 拮抗香蕉枯萎病镰刀菌木霉菌株的分离筛选[J].
热带作物学报, 2010, 31(1): 106–110.
Wu L, Huang H P, Yang L Y, et al. Screening of Trichoderma spp. antagonizing Fusarium oxysporum, f. sp. cubense Owen [J]. Chinese Journal of Tropical Crops, 2010, 31(1): 106–110. |
| [33] |
张晓云, 李宝庆, 郭庆港, 等. 生防枯草芽孢杆菌CAB-1抑菌蛋白产生条件及其稳定性研究[J].
中国农业科技导报, 2011, 13(2): 59–64.
Zhang X Y, Li B Q, Guo Q G, et al. Optimization of antifungal protein production by Bacillus subtilis strain CAB-1 and its stability analysis [J]. Journal of Agricultural Science and Technology, 2011, 13(2): 59–64. |
| [34] |
薛鹏琦, 刘芳, 乔俊卿, 等. 油菜菌核病生防芽孢杆菌的分离鉴定及其脂肽化合物分析[J].
植物保护学报, 2011, 38(2): 127–132.
Xue P Q, Liu F, Qiao J Q, et al. Screening of Bacillus strains with high inhibition on rape Sclerotinia disease and its lipopeptide compounds detection [J]. Acta Phytophylasica Sinica, 2011, 38(2): 127–132. |
| [35] | Ashok S, Keerti D, Deepak V, et al. Interactions between arbuscular mycorrhizae and Fusarium oxysporum f. sp. Ciceris: effects on fungal development, seedling growth and wilt disease suppression in Cicer arietinum L [J]. Archives of Phytopathology and Plant Protection, 2015, 48(3): 240–252. DOI:10.1080/03235408.2014.884831 |
| [36] | Li Y J, Liu Z L, Hou H Y, et al. Arbuscular mycorrhizal fungi-enhanced resistance against Phytophthora sojae infection on soybean leaves is mediated by a network involving hydrogen peroxide, jasmonic acid, and the metabolism of carbon and nitrogen [J]. Acta Physiologiae Plantarum, 2013, 35(12): 3465–3475. DOI:10.1007/s11738-013-1382-y |
| [37] |
印容, 高慧娟, 赵秀云. 球毛壳菌及其产生的鞘氨醇对油菜的室内生防作用[J].
华中农业大学学报, 2016, 35(5): 58–62.
Yin R, Gao H J, Zhao X Y. Biocontrol effect of Chaetomium globosum and sphingosine on Plasmodiaphora brassicae [J]. Journal of Huazhong Agricultural University, 2016, 35(5): 58–62. |
| [38] | Chen J L, Sun S Z, Miao C P, et al. Endophytic Trichoderma gamsii YIM PH30019: a promising biocontrol agent with hyperosmolar, mycoparasitism, and antagonistic activities of induced volatile organic compounds on root-rot pathogenic fungi of Panax notoginseng [J]. Journal of Ginseng Research, 2016, 40(4): 315–324. DOI:10.1016/j.jgr.2015.09.006 |
| [39] | Bisen K, Keswani C, Mishra S, et al. Unrealized potential of seed biopriming for versatile agriculture[A]. Rakshit A, Singh H B, Sen, A. Nutrient use efficiency: From basics to advances[M]. New Delhi: Springer, 2015: 193-206. |
| [40] | Contreras-Cornejo H A, Macias R L, Cortes P C, et al. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis [J]. Plant Physiology, 2009, 149: 1579–1592. DOI:10.1104/pp.108.130369 |
| [41] | Fan L L, Fu K H, Yu C J, et al. Construction and functional analysis of Trichoderma harzianum mutants that modulate maize resistance to the pathogen Curvularia lunata[J]. Journal of Environmental Science and Health(Part B), 2014, 49(8), 569-577. |
| [42] |
康振生, 李振歧, 商鸿生, 等. 植物病原真菌细胞核和隔膜的双重荧光染色技术[J].
真菌学报, 1993, 12(2): 174–186.
Kang Z S, Li Z Q, Shang H S, et al. On technique of double fluorescence staining for nucelei and septa of plant pathogenic fungi[J]. Acta Mycologica Sinica, 1993, 12(2): 174–186. |
| [43] |
毕朝位, 仇剑波, 周明国, 等. 禾谷镰孢菌Fusarium gram inearum Schwabe分生孢子萌发过程中的核相变化及有丝分裂过程观察
[J].
菌物学报, 2008, 27(6): 901–907.
Bi C W, Qiu J B, Zhou M G, et al. Mitotic division in germ tube of Fusarium graminearum Schwabe [J]. Mycosystema, 2008, 27(6): 901–907. |
| [44] |
张淑梅, 王玉霞, 李晶, 等. 基因标记枯草芽孢杆菌BS-68A在黄瓜上定殖[J].
生物技术, 2007, 16(4): 73–74.
Zhang S M, Wang Y X, Li J, et al. Colonization of a gene-marked Bacillus subtilis BS-68A in cucumber [J]. Biotechnology, 2007, 16(4): 73–74. |
| [45] |
车建美, 蓝江林, 刘波. 转绿色荧光蛋白基因的青枯雷尔氏菌生物学特性[J].
中国农业科学, 2008, 41(11): 3626–3635.
Che J M, Lan J L, Liu B. GFP tagging Ralstonia solanacearum with gfp/lux AB mini-Tn5 [J]. Scientia Agricultura Sinica, 2008, 41(11): 3626–3635. DOI:10.3864/j.issn.0578-1752.2008.11.024 |
| [46] |
刘路宁. 绿木霉 (Trichoderma virens) TY009菌株胶霉毒毒素分离纯化及绿色荧光蛋白标记研究[D]. 杭州: 浙江大学博士学位论文, 2008.
Liu L N. Isolation and purification of gliotoxin from Trichoderma virens strain TY009 and its GFP-labeling[D]. Hangzhou: PhD Dissertation of Zhejiang University, 2008. |
| [47] | Monteiro R A, Schmidt M A, de Baura V, et al. Early colonization pattern of maize (Zea mays L. Poales, Poaceae) roots by Herbaspirillum seropedicae (Burkholderiales, Oxalobacteraceae) [J]. Genetics and Molecular Biology, 2008, 31: 932–937. DOI:10.1590/S1415-47572008000500021 |
| [48] | Sarrocco S, Falaschi N, Vergara M, et al. Use of Fusarium oxysporum f. sp. Dianthi transformed with marker genes to follow colonization of carnation roots [J]. Journal of Plant Pathology, 2007, 89: 47–54. |
| [49] |
武坤毅, 王斐斐, 崔浪军, 等. 溶杆菌SNNU513基因gfp标记及在玉米根部定殖[J].
中国生物防治学报, 2014, 30(1): 134–142.
Wu K Y, Wang F F, Cui L J, et al. Colonization of gfp-tagged Lysobacter sp. SNNU513 on maize’s root [J]. Chinese Journal of Biological Control, 2014, 30(1): 134–142. |
| [50] |
李亮亮, 谈家金, 陈凤毛. GFP标记短小芽孢杆菌LYMC-3在马尾松体内的定殖[J].
华中农业大学学报, 2016, 35(6): 68–73.
Li L L, Tan J J, Chen F M. Colonization of GFP-tagged Bacillus pumilus strain LYMC-3 in masson pine [J]. Journal of Huazhong Agricultural University, 2016, 35(6): 68–73. |
| [51] |
沈新迁, 刘通, 胡晓璐, 等. 短小芽孢杆菌转座突变株的GFP标记及在水稻上的定殖[J].
中国农业科学, 2012, 45(24): 5024–5031.
Shen X Q, Liu T, Hu X L, et al. Labeling Bacillus pumillus with Green Fluorescent Protein (GFP) and its colonization in rice seedlings [J]. Scientia Agricultura Sinica, 2012, 45(24): 5024–5031. DOI:10.3864/j.issn.0578-1752.2012.24.007 |
 2017, Vol. 23
2017, Vol. 23  doi:
doi: 

