文章信息
- 谢锋, 朱芳, 刘峥嵘, 王红岩, 赵晓丹, 樊小刚
- Xie Feng, Zhu Fang, Liu Zhengrong, Wang Hongyan, Zhao Xiaodan, Fan Xiaogang
- 经皮穿刺治疗经内镜引流失败的恶性梗阻性黄疸
- Percutaneous Puncture to Treat Malignant Obstructive Jaundice in Patients Who Fail Endoscopic Drainage
- 中国医科大学学报, 2018, 47(2): 137-140
- Journal of China Medical University, 2018, 47(2): 137-140
-
文章历史
- 收稿日期:2017-10-23
- 网络出版时间:2018-01-08 10:54
2. 中国医科大学人民医院心功能科, 沈阳 110016;
3. 中国医科大学人民医院普外科, 沈阳 110016;
4. 沈阳出入境检验检疫局国际旅行卫生保健中心, 沈阳 110016
2. Department of Cardiac Function, The People's Hospital of China Medical University, Shenyang 110016, China;
3. Department of General Surgery, The People's Hospital of China Medical University, Shenyang 110016, China;
4. Shenyang Entry-exit Inspection and Quarantine Bureau International Travel Health Care Center, Shenyang 110016, China
梗阻性黄疸是胆管、胆囊、胰头、十二指肠壶腹周围恶性肿瘤及肝门区淋巴结转移瘤最常见的并发症。对于不可切除的病变,减黄治疗至关重要。经内镜减黄[1]及经皮穿刺减黄为其主要的两大途径[2]。根据情况不同,往往采取以下2种模式,一种是打通梗阻的胆管,使胆汁能够沿正常途径流入肠道;当无法打通梗阻的胆管时,采取外引流的方法,使胆汁排到体外,也可以达到减黄的目的。一些患者首先采用内镜的方法进行减黄,但有时胆总管与十二指肠的角度较小[1]或病变位于肝门处等原因,经内镜减黄疗效不佳,此时再根据病变的情况选择不同的经皮穿刺方法来进行减黄。本研究回顾性分析经内镜减黄失败的梗阻性黄疸患者采取经皮穿刺方式减黄的疗效。
1 材料与方法 1.1 一般资料回顾性分析2015年8月至2017年7月行经内镜减黄失败后在我院行经皮穿刺减黄的梗阻性黄疸患者共17例。其中,男7例,女10例,平均年龄(67±21)岁。
1.2 手术选择及参数对比肝内胆管穿刺成功且导丝能够通过狭窄段的首选支架植入术,肝内胆管穿刺成功但导丝不能通过狭窄段的选择经皮经肝胆管穿刺引流术(percutaneous transhepatic cholangiodrainage,PTCD),肝内胆管穿刺失败的选择经皮胆囊穿刺引流术。梗阻位于肝门区或胆汁存在感染的采取两步法,先行PTCD,1~2周后再行支架植入术,成功的纳入支架植入组,见图 1,不成功的纳入PTCD组。一些患者由于胆囊代偿功能较好,胆囊明显增大,而肝内胆管扩张不明显,则采用经皮胆囊穿刺引流术。PTCD及经皮胆囊穿刺引流术均属于置管组,见图 2。比较全部患者术前1 d及术后1周的血清总胆红素及谷丙转氨酶的变化情况。支架组和置管组再比较总胆红素和谷丙转氨酶下降效果及患者生存期的差别。
|
| 图 1 支架植入组CT定位像 Fig.1 Computed tomography localizing image in the stent group |
|
| 图 2 置管组CT定位像 Fig.2 Computed tomography localizing image in the tube group |
1.3 手术相关处理
手术由从事介入工作的影像科医生完成。手术适应证为总胆红素 > 70 mmol/L且直接胆红素/间接胆红素 > 0.5。术前30 min预防性应用抗生素,局部麻醉采用2%利多卡因,术中将地佐辛10 mg加入250 mL生理盐水中持续缓慢静脉滴入(30滴/min)。穿刺采用美国COOK公司21G引流套管针,导丝采用日本泰尔茂公司0.035英寸的泥鳅导丝,引流管采用美国波士顿科学公司8F猪尾引流管,金属支架采用美国Bard公司LUMINEXX金属支架(直径8 mm、10 mm,长度6 cm、8 cm)。
1.4 统计学分析采用SPSS 17.0进行统计学分析。全部患者手术前、后血清总胆红素及谷丙转氨酶的对比采用配对t检验。2组总胆红素及谷丙转氨酶下降程度的比较采用独立样本t检验。患者生存期的比较采用Kaplan-Meier生存分析。P < 0.05为差异有统计学意义。
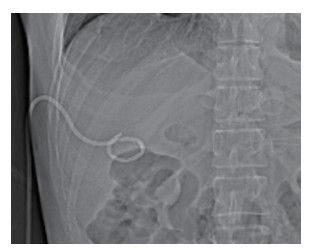
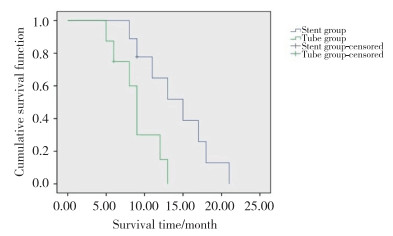
2 结果17例患者均手术成功,没有出现并发症。有2例患者梗阻位于肝门区,采用两步法放置支架;另有2例患者由于胆汁存在感染,也采用两步法放置支架,以上4例患者支架均放置成功。总体17例患者总胆红素从术前的(317.41±159.95)μmol/L下降为术后的(189.71±114.05)μmol/L,差异有统计学意义(P < 0.05)。总体患者谷丙转氨酶从(330.53±137.06)U/L下降为(229.94±99.50)U/L,差异有统计学意义(P < 0.05),见表 1。2组在总胆红素和谷丙转氨酶下降程度方面均无统计学差异(P > 0.05),见表 2。在延长患者生存期方面,支架组为(14.20±1.56)个月,优于置管组[(9.03±1.05)个月],差异有统计学意义(P < 0.05),见图 3。
| Therapeutic parameters | Before operation | After operation | P |
| Total serum bilirubin(μmol/L) | 317.41±159.95 | 189.71±114.05 | < 0.001 |
| Alanine aminotransferase (U/L) | 330.53±137.06 | 229.94±99.50 | < 0.001 |
| Therapeutic parameters | Stent group | Tube group | P |
| Drop in the total serum bilirubin value (μmol/L) | 124.33±68.39 | 131.50±60.55 | 0.962 |
| Drop in the serum alanine aminotransferase value (U/L) | 114.67±71.29 | 84.75±46.82 | 0.057 |
| Survival time (month) | 14.20±1.56 | 9.03±1.05 | 0.015 |
|
| 图 3 支架组与置管组生存期对比 Fig.3 Comparison of the survival time between the stent and the tube group |
3 讨论
梗阻性黄疸是胆管、胆囊、胰头、十二指肠壶腹周围恶性肿瘤及肝门区淋巴结转移瘤常见的并发症。它不仅会影响患者肝脏功能,还可通过肝肾综合征损害肾脏功能[3],机械性梗阻也可造成肝内血管阻塞、门脉高压或肝后性腹腔积液[4],甚至对患者的心、肺功能造成损害[5],最终引发多器官功能衰竭,导致患者死亡。对于不可切除的肿瘤,采用某种姑息性的方法来降低患者的胆红素水平,不但可以改善患者生存质量、延长患者生存期,同时也为患者进行下一步治疗(放射治疗、免疫治疗和生物治疗等)争取机会[6-7]。在临床工作中,减黄的途径通常有2种[8]:经内镜自下而上逆行减黄和经皮穿刺顺行减黄。一些患者首先选择全麻下经内镜减黄,但部分患者由于肿瘤位置或解剖结构等原因,经内镜减黄失败,则不得不采用经皮穿刺的方法[9]。经内镜途径的优势在于全程全麻下进行,患者无痛苦,但由于逆行通过狭窄段,成功率较低,胰腺炎等并发症的发生率较高[10]。经肝穿刺途径的优势在于按照生理管道的正常解剖结构进行,顺行通过狭窄段,成功率较高,缺点在于需要肝内胆管明显扩张才能穿刺成功,局麻操作过程中患者意识清醒,开通过程中会感受到疼痛[11]。
血清总胆红素和谷丙转氨酶是反映肝脏功能恢复情况的重要指标[12]。在本研究中,患者总胆红素从术前的(317.41±159.95)μmol/L下降为术后的(189.71±114.05)μmol/L,差异有统计学意义,说明对于内镜减黄失败的梗阻性黄疸患者,经皮穿刺减黄依然可以取得较好的疗效。在降低总胆红素方面,支架组和置管组效果相近,无统计学意义,但置管组降低的程度略高于支架组,这一结果与之前关于肝门部胆管癌的研究[13]相类似。在谷丙转氨酶下降方面,2组也无统计学差异,但支架组下降的均值多于置管组。这可能说明在术后短期肝细胞功能恢复方面,支架组优于置管组。在患者生存期方面,支架组优于置管组,差异有统计学意义(P < 0.05)。这可能是由于植入支架后,胆汁沿正常的通道进入肠道,参与消化,不但会帮助肝细胞恢复功能,而且在保持机体功能完整性方面,有着明显的优势[14];同时,相对于外引流,内支架治疗可提高患者舒适度,降低患者心理压力[15]。因此支架组的长期预后明显好于置管组。
综上所述,经皮穿刺治疗经内镜引流失败的恶性梗阻性黄疸疗效确切;相对于留置引流管,放置支架患者生存期更长。
| [1] |
MATSUMOTO K, TAKEDA Y, ONOYAMA T, et al. Endoscopic treatment for distal malignant biliary obstruction[J]. Ann Transl Med, 2017, 5(8): 190. DOI:10.21037/atm.2017.02.22 |
| [2] |
YARMOHAMMADI H, COVEY AM. Percutaneous biliary interventions and complications in malignant bile duct obstruction[J]. Chin Clin Oncol, 2016, 5(5): 68. DOI:10.21037/cco.2016.10.07 |
| [3] |
DE SIMONE W, CRAFA F, NOVIELLO A, et al. Bilirubin removal with coupled plasma filtration and adsorption in patients affected by hilar cholangiocarcinoma[J]. G Ital Nefrol, 2017, 34(11/12): pii 2017-vol6. |
| [4] |
IMAMURA N, NANASHIMA A, TSUCHIMOCHI Y, et al. Intrahepatic portal vein thrombosis due to postoperative biliary obstruction successfully treated by a partial thrombectomy combined with thrombolytic drug therapy[J]. Int J Surg Case Rep, 2017, 42: 20-23. DOI:10.1016/j.ijscr.2017.11.047 |
| [5] |
AHN S, LEE YS, LIM KS, et al. Malignant biliary obstructions can we predict immediate postprocedural cholangitis after percutaneous biliary drainage?[J]. Support Care Cancer, 2013, 21(8): 2321-2326. DOI:10.1007/s00520-013-1796-5 |
| [6] |
HATZIDAKIS AA, TSETIS D, CHRYSOU E, et al. Nitinol stents for palliative treatment of malignant obstructive jaundice:should we stent the sphincter of Oddi in every case?[J]. Cardiovasc Intervent Radiol, 2001, 24(4): 245-248. DOI:10.1007/s00270-001-0030-x |
| [7] |
SAHAI P, KUMAR S. External radiotherapy and brachytherapy in the management of extrahepatic and intrahepatic cholangiocarcinoma:available evidence[J]. Br J Radiol, 2017, 90(1076): 20170061. DOI:10.1259/bjr.20170061 |
| [8] |
KONDO M, OKAMOTO E, TAKAGI K, et al. Insertion of percutaneous transhepatic biliary endoprosthesis for unresectable lower cholangiocellular carcinoma following cholangitis due to endoscopic biliary plastic stent obstruction[J]. Gan To Kagaku Ryoho, 2013, 40(12): 1777-1779. |
| [9] |
LI X, YU J, LIANG P, et al. Ultrasound-guided percutaneous microwave ablation assisted by three-dimensional visualization operative treatment planning system and percutaneous transhepatic cholangial drainage with intraductal chilled saline perfusion for larger hepatic hilum hepatocellular (D ≥ 3 cm):preliminary results[J]. Oncotarget, 2017, 8(45): 79742-79749. DOI:10.18632/oncotarget.19275 |
| [10] |
LANDI F, DE ANGELIS N, SEPULVEDA A, et al. Endoscopic treatment of anastomotic biliary stricture after adult deceased donor liver transplantation with multiple plastic stents versus self-expandable metal stents:a systematic review and meta-analysis[J]. Transpl Int, 2017. DOI:10.1111/tri.13089 |
| [11] |
HUNG HH, CHEN TS, TSENG HS, et al. Percutaneous transhepatic cholangiography and drainage is an effective rescue therapy for biliary complications in liver transplant recipients who fail endoscopic retrograde cholangiopancreatography[J]. J Chin Med Assoc, 2009, 72(8): 395-401. DOI:10.1016/S1726-4901(09)70395-8 |
| [12] |
BEDREAG OH, ROGOBETE AF, SANDESC D, et al. Modulation of the redox expression and inflammation response in the Critically Ⅲ polytrauma patient with thoracic injury. Statistical correlations between antioxidant therapy[J]. Glin Lab, 2016, 62(9): 1747-1759. DOI:10.7754/Clin.Lab.2016.160206 |
| [13] |
XU C, LV PH, HUANG XE, et al. Analysis of different ways of drainage for obstructive jaundice caused by hilar cholangiocarcinoma[J]. Asian Pac J Cancer Prev, 2014, 15(14): 5617-5620. DOI:10.7314/APJCP.2014.15.14.5617 |
| [14] |
FRANCESCHET I, ZANETTO A, FERRARESE A, et al. Therapeutic approaches for portal biliopathy:a systematic review[J]. World J Gastroenterol, 2016, 22(45): 9909-9920. DOI:10.3748/wjg.v22.i45.9909 |
| [15] |
WESTWOOD DA, FEMANDO C, CONNOR SJ. Internal-external percutaneous transhepatic biliary drainage for malignant biliary obstruction:a retrospective analysis[J]. J Med Imaging Radiat Oncol, 2010, 54(2): 108-110. DOI:10.1111/j.1754-9485.2010.02147.x |
 2018, Vol. 47
2018, Vol. 47