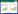文章信息
- 林莉, 殷典贺, 韩丹, 侯刚, 尹燕, 康健, 王秋月
- LIN Li, YIN Dianhe, HAN Dan, HOU Gang, YIN Yan, KANG Jian, WANG Qiuyue
- 熊果酸对香烟烟雾诱导大鼠肺气肿及氧化应激的影响
- Effects of Ursolic Acid on Cigarette Smoke-induced Emphysema and Oxidative Stress in Rats
- 中国医科大学学报, 2018, 47(10): 876-880
- Journal of China Medical University, 2018, 47(10): 876-880
-
文章历史
- 收稿日期:2018-03-13
- 网络出版时间:2018-09-27 9:23
慢性阻塞性肺疾病(简称慢阻肺)是一种可以预防和治疗的常见疾病,其特征是持续的呼吸道症状和气流受限,气流受限呈进行性发展,伴有气道和肺对有害颗粒或气体所致的慢性炎症反应的增加[1]。慢阻肺的主要病理改变之一是肺气肿,吸烟是慢阻肺发病的主要危险因素,大约90%的慢阻肺是吸烟导致[2]。除了炎症,吸烟引起的氧化应激在慢阻肺的发生、发展中也起到重要作用,大量研究[3]表明,慢阻肺患者机体氧化应激水平增加,同时,氧化应激还可以加重慢阻肺患者的肺部炎症反应。熊果酸又名乌索酸、乌苏酸,广泛分布于自然界许多植物中,已有研究[4]结果表明,熊果酸具有抗炎、抑制肿瘤细胞增殖、延缓动脉粥样硬化形成、改善糖尿病患者胰岛细胞功能等药理作用。新近研究发现,熊果酸除了上述作用外,还可以抗氧化及自由基清除活性,但熊果酸对香烟烟雾所致肺气肿及氧化应激的影响目前尚无文献报道,本研究初步探讨了熊果酸对烟熏所致大鼠肺气肿及氧化应激的影响。
1 材料与方法 1.1 材料 1.1.1 实验动物雄性SPF级Wistar大鼠(中国医科大学实验动物中心)24只,12周龄,平均体质量(180±20)g,实验室恒温(24±2)℃,相对湿度40%。
1.1.2 主要试剂熊果酸(天津马克生物技术有限公司),超氧化物歧化酶(superoxide dismutase,SOD)试剂盒、过氧化氢酶(catalase,CAT)试剂盒、(glutathione peroxidase,GSH-Px)试剂盒、丙二醛(malonaldehyde,MDA)试剂盒(南京建成生物工程研究所),8-异前列腺素F2α(8-iso-prostaglandinF2α,8-iso-PGF2α)ELISA试剂盒(美国RD公司进口分装),8-羟基脱氧鸟苷(8-hydroxy-2deoxyguanosine,8-OHdG)抗体(英国Abcam生物技术公司),二抗(中杉金桥生物技术公司)。
1.2 实验方法 1.2.1 动物模型的制备和分组采用随机数字表法将大鼠随机分对照组、模型组和熊果酸组,每组8只。对照组:标准饲养大鼠12周,每日放入自制玻璃箱(0.8 m×0.6 m×0.6 m,顶部有2 cm×2 cm的通气孔)内1 h(上、下午各30 min,间隔4~6 h),不进行熏烟,下午每次放入前半小时用生理盐水10mL/(kg·d)灌胃;模型组:每日将大鼠放入烟熏箱中用实验用香烟(红金龙香烟,焦油量12 mg,烟碱量1.1 mg)熏烟1 h(上、下午各30 min,间隔4~6 h),每次12支香烟,每周6 d,连续12周,每天下午熏烟前半小时用生理盐水10 mL/(kg·d)灌胃;熊果酸组:每天下午烟熏前半小时给大鼠用熊果酸10 mL/(kg·d)灌胃,其余条件同熏烟组。
1.2.2 样品的采集与处理实验12周后采用放血法处死大鼠。留取眼血,凝固后4 ℃离心(3 000 r/min,10 min),分离血清,-20 ℃保存备用。取左肺常规多聚甲醛固定、脱水、石蜡包埋、切片(厚5 μm),苏木素-伊红(HE)染色病理切片,显微镜下观察大鼠肺气肿情况。
1.2.3 肺气肿评分测量肺泡平均内衬间隔(mean linear intercept,MLI),在MetaMorph/UIC/US病理图像分析系统视野正中划“十”字交叉,计数通过该十字的肺泡间隔数(numbers,ns),测得十字线总长度(length,L),MLI=L/Ns,其数值反应肺泡平均直径;计数平均肺泡数(mean alveolar numbers,MAN),测MetaMorph/UIC/US病理图像分析系统下每个视野面积,计数该视野的肺泡数目,MAN =该视野肺泡数目/该视野面积,其数值反映平均肺泡密度。
1.2.4 免疫组化法检测肺组织8-OHdG表达石蜡切片脱蜡至水。3% H2O2室温孵育5 min灭活内源性过氧化物酶。PBS浸洗5 min/次,共3次。10% BSA室温孵育30 min。8-OHdG一抗(1: 100)于4 ℃冰箱中孵育过夜,后室温复温45 min。PBS浸洗5 min/次,共3次。山羊抗兔二抗(1: 500),37 ℃孵育30 min。PBS浸泡5 min/次,共3次。显色剂显色3~15 min(DAB),显微镜下观察,自来水充分冲洗终止反应,苏木素复染,脱水,透明,封片,显微镜观察,拍照。
1.2.5 氧化-抗氧化指标的测定采用黄嘌呤氧化酶法测定SOD的活性;采用比色法测定CAT和GSH-Px的活性;采用ELISA法测定8-iso-PGF2α的浓度;采用TBA法测定MDA的浓度。所有操作均严格按照试剂说明书进行。
1.3 统计学分析采用SPSS 16.0软件进行分析,数据以x±s表示,2组均数比较用t检验,多组均数比较用单因素方差分析,组间两两比较用SNK-q检验,P < 0.05为差异有统计学意义。
2 结果 2.1 大鼠肺组织病理学改变及肺气肿严重程度比较对照组大鼠肺组织及气道壁结构清晰、气道上皮排列整齐,肺泡大小、分布基本均匀、肺泡腔内无渗出液。与对照组比较,模型组大鼠肺组织体积增大,表面不平,光镜下病理学观察示肺泡腔扩大、部分肺泡间隔断裂、肺泡腔融合、肺气肿形成,气道上皮排列紊乱、部分脱落、局部上皮增生、管壁增厚、管腔变小、周围炎症细胞浸润并伴有细支气管管壁平滑肌增生、管壁平滑肌部分断裂、杯状细胞增生等改变。熊果酸组肺组织体积轻度增大,表面尚平整,光镜下病理学观察示肺泡腔呈局部轻度扩大,肺泡间隔略增宽,少量炎症细胞浸润,可见局部轻度肺气肿形成(图 1)。模型组MLI明显高于对照组和熊果酸组,MAN明显低于对照组和熊果酸组,熊果酸组MLI和MAN与对照组相比,差异无统计学意义,见表 1。
|
| A, control group; B, model group; C, ursolic acid group. 图 1 肺组织切片HE染色×20 Fig.1 HE staining of lung tissues of rats ×20 |
| Group | MLI(μm) | MAN(mm-2) |
| Control | 28.29±2.041) | 319.91±11.731) |
| Model | 56.26±3.69 | 154.71±16.72 |
| Ursolic acid | 31.70±2.621) | 300.37±21.091) |
| F | 228.10 | 226.87 |
| P | < 0.01 | < 0.01 |
| Compared with model group,1)P < 0.01. MLI,mean linear intercept;MAN,mean alveolar numbers. | ||
2.2 各组大鼠血清中SOD、CAT、GSH-Px活性和8-iso-PGF2α含量的比较
模型组大鼠血清中的SOD、CAT、GSH-Px活性明显低于对照组,而熊果酸组血清SOD、CAT、GSH-Px活性明显高于模型组,与对照组相比无统计学差异;模型组大鼠血清中8-iso-PGF2α含量明显高于对照组、低于熊果酸组,而熊果酸组与对照组比较差异不明显,见表 2。
| Group | SOD(U/mL) | CAT(U/mL) | GSH-Px(μmol/L) | 8-iso-PGF2α(pg/mL) |
| Control | 229.52±10.371) | 21.16±6.491) | 769.32±175.651) | 9.15±1.511) |
| Model | 217.10±11.33 | 15.38±3.82 | 542.84±170.37 | 11.12±1.58 |
| Ursolic acid | 231.67±10.051) | 21.91±3.381) | 787.05±244.621) | 8.92±2.451) |
| F | 4.42 | 4.51 | 3.72 | 3.55 |
| P | < 0.05 | < 0.05 | < 0.05 | < 0.05 |
| Compared with model group,1)P < 0.05. | ||||
2.3 各组大鼠肺组织匀浆上清液中MDA的表达
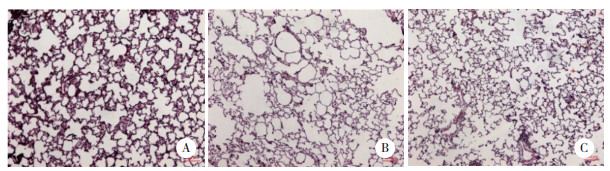
模型组大鼠肺组织匀浆上清液的MDA表达明显较对照组升高,而给予熊果酸干预后大鼠的肺组织匀浆上清液中MDA的表达明显下调,见图 2。
|
| *P < 0.05 compared with model group. 图 2 各组别大鼠肺组织匀浆上清液中MDA的表达(x±s,n = 8) Fig.2 Expression of MDA in the supernatant of lung tissue homogenate of rats from each group (x±s, n = 8) |
2.4 各组大鼠肺组织8-OHdG含量的比较
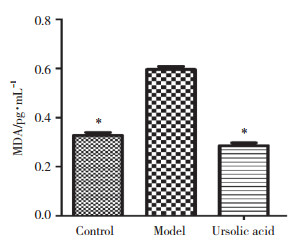
模型组大鼠肺组织8-OHdG表达与对照组比较明显增加,而给予熊果酸干预后8-OHdG表达明显降低,而熊果酸组与对照组比较差异不明显,见图 3。
|
| A, immunohistochemistry was used to analyze the expression of 8-OHdG in lung tissue cells; B, OD value of 8-OHdG expression. *P < 0.05 compared with model group. 图 3 各组大鼠肺组织8-OHdG的表达(n = 8) Fig.3 Expression of 8-OHdG in lung tissue of rats from each group (n = 8) |
3 讨论
氧化应激是指机体活性氧的生成过多和(或)抗氧化能力降低,氧化和抗氧化系统平衡紊乱,从而导致损伤的病理过程。机体内抗氧化酶是一类在机体组织细胞内具有极高的相对特异性、协同构成阻止活性氧自由基损伤机体防御体系的重要酶,主要包括SOD、CAT、GSH-Px等。内源性抗氧化酶在细胞内表达增加可以减轻机体的氧化损伤[5]。8-iso-PGF2α是经自由基催化不饱和脂肪酸脂质过氧化后的终末产物,8-OHdG为氧化应激导致DNA损伤指标,两者产生与机体氧化应激损伤关系极为密切,是评价氧化应激和脂质过氧化反应最有效的生物指标[6-7]。
慢阻肺的发生发展与炎症、氧化应激、蛋白酶和抗蛋白酶的失衡以及自主神经系统功能紊乱等密切相关。肺气肿和慢性支气管炎是慢阻肺主要的病理改变。氧化应激是加重慢阻肺患者肺部炎症的重要机制。吸烟被认为是导致慢阻肺氧化应激的主要危险因素之一[8-9]。WASEEM等[10]研究证明,吸烟者血浆中的抗氧化酶SOD、CAT、GPX的活性显著低于不吸烟者,MDA含量显著高于不吸烟者,因此提高慢阻肺患者机体内抗氧化酶活性可以降低肺组织的氧化应激性损伤。临床上也证明了应用抗氧化剂如N-乙酰半胱氨酸、羧甲司坦等可以降低慢阻肺反复加重的频率[11-13]。
熊果酸是广泛分布于自然界许多植物中的一种三萜类化合物,已证实在氧化应激相关的疾病中具有较好的抗氧化作用[14-16],可以增加抗氧化酶活性,减轻活性氧自由基引起的脂质、蛋白质、DNA等氧化应激性损伤。本研究结果显示,烟熏致肺气肿组大鼠肺气肿形成的同时,血清中酶性抗氧化物SOD、CAT、GSH-Px的活性明显降低,而血清中氧化应激指标8-iso-PGF2α的含量明显升高。肺组织中脂质过氧化指标MDA及氧化应激导致DNA损伤指标8-OHdG表达升高,上述结果均提示肺气肿大鼠氧化应激反应增强。熊果酸组大鼠烟熏前半小时给予熊果酸灌胃治疗,12周后观察到大鼠气道炎症及肺气肿的严重程度明显减轻,提示熊果酸可以明显降低香烟烟雾对大鼠肺组织的损伤,减轻肺气肿。本研究结果显示,熊果酸组大鼠血清中SOD、CAT、GSH-Px的活性较模型组明显升高,8-iso-PGF2α的含量明显降低,肺组织中MDA与8-OHdG表达降低,提示熊果酸对大鼠的保护作用机制可能与减轻烟雾所致氧化应激有关。熊果酸通过提高大鼠血清中抗氧化物酶的活性,降低脂质过氧化产物的含量,减轻烟雾对肺组织的损伤,只是其作用机制之一,可能熊果酸的抗炎作用也参与其中,这有待进一步研究。
本研究初步观察到熊果酸对烟熏所致肺气肿大鼠肺组织病理形态学及氧化应激指标的影响,为进一步研究熊果酸在慢阻肺中的作用及机制,进而为慢阻肺治疗药物的开发及研究奠定了基础。
| [1] |
KELSEN SG. The unfolded protein response in chronic obstructive pulmonary disease[J]. Ann Am Thorac Soc, 2016, 13(Suppl 2): S138-145. DOI:10.1513/AnnalsATS.201506-320KV |
| [2] |
HOOPER R, BURNEY P, VOLLMER W, et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project[J]. Eur Respir J, 2012, 39(6): 1343-1353. DOI:10.1183/09031936.00002711 |
| [3] |
THOMSON N. Targeting oxidant-dependent mechanisms for the treatment of respiratory diseases and their comorbidities[J]. Curr Opin Pharmacol, 2017, 40: 1-8. DOI:10.1016/j.coph.2017.11.013 |
| [4] |
MA J, DING J, XIAO Z, et al. Ursolic acid ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney by inhibiting the STAT3 and NF-κB activities[J]. Int Immunopharmacol, 2014, 21(2): 389-395. DOI:10.1016/j.intimp.2014.05.022 |
| [5] |
PAUDEL Y, ALI M, ADIL M, et al. "2-(4-Fluorobenzamido)-4-methylthiazole-5-carboxylic acid" a novel thiazole compound, ameliorates insulin sensitivity and hyperlipidaemia in streptozotocin-induced diabetic rats:plausible role of inflammatory and oxidative stress markers[J]. Biomed Pharmacother, 2017, 95: 1232-1241. DOI:10.1016/j.biopha.2017.09.014 |
| [6] |
HAZMAN Ö, BOZKURT M, FIDAN A, et al. The effect of boric acid and borax on oxidative stress, inflammation, ER stress and apoptosis in cisplatin toxication and nephrotoxicity developing as a result of toxication[J]. Inflammation, 2018, 41(3): 1032-1048. DOI:10.1007/s10753-018-0756-0 |
| [7] |
LOCATELLI M, FERRANTE C, CARRADORI S, et al. Optimization of aqueous extraction and biological activity of harpagophytum procumbens root on EX vivo rat colon inflammatory model[J]. Phytother Res, 2017, 31(6): 937-944. DOI:10.1002/ptr.5821 |
| [8] |
MACHADO D, CAMPOS K, DA SILVA N, et al. The administration of surfactant decreased oxidative stress in lungs of mice exposed to cigarette smoke[J]. Int Immunopharmacol, 2018, 54: 275-279. DOI:10.1016/j.intimp.2017.11.023 |
| [9] |
ARIMILLI S, SCHMIDT E, DAMRATOSKI B, et al. Role of oxidative stress in the suppression of immune responses in peripheral blood mononuclear cells exposed to combustible tobacco product preparation[J]. Inflammation, 2017, 40(5): 1622-1630. DOI:10.1007/s10753-017-0602-9 |
| [10] |
WASEEM S, MOBARAK M, ISLAM N, et al. Comparative study of pulmonary functions and oxidative stress in smokers and non-smokers[J]. Indian J Physiol Pharmacol, 2012, 56(4): 345-352. |
| [11] |
ZENG Z, YANG D, HUANG X, et al. Effect of carbocisteine on patients with COPD:a systematic review and meta-analysis[J]. Int J Chron Obstruct Pulmon Dis, 2017, 12: 2277-2283. DOI:10.2147/COPD.S140603 |
| [12] |
CAZZOLA M, ROGLIANI P, CALZETTA L, et al. Impact of mucolytic agents on COPD exacerbations:a pair-wise and network meta-analysis[J]. COPD, 2017, 14(5): 552-563. DOI:10.1080/15412555.2017.1347918 |
| [13] |
FOWDAR K, CHEN H, HE Z, et al. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease:a meta-analysis and systematic review[J]. Heart Lung, 2017, 46(2): 120-128. DOI:10.1016/j.hrtlng.2016.12.004 |
| [14] |
MA J, DING J, ZHANG L, et al. Ursolic acid protects mouse liver against CCl4-induced oxidative stress and inflammation by the MAPK/NF-κB pathway[J]. Environ Toxicol Pharmacol, 2014, 37(3): 975-983. DOI:10.1016/j.etap.2014.03.011 |
| [15] |
CHEN J, KO K. Ursolic-acid-enriched herba cynomorii extract protects against oxidant injury in H9c2 cells and rat myocardium by increasing mitochondrial atp generation capacity and enhancing cellular glutathione redox cycling, possibly through mitochondrial uncoupling[J]. Evid Based Complement Alternat Med, 2013, 2013: 924128. DOI:10.1155/2013/924128 |
| [16] |
LIOBIKAS J, MAJIENE D, TRUMBECKAITE S, et al. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria[J]. J Nat Prod, 2011, 74(7): 1640-1644. DOI:10.1165/rcmb.2007-0221OC |
 2018, Vol. 47
2018, Vol. 47