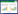文章信息
- 丁仁彧, 肇冬梅, 胡紫薇, 王亮, 李鑫, 孙旖旎, 章志丹, 马晓春
- DING Renyu, ZHAO Dongmei, HU Ziwei, WANG Liang, LI Xin, SUN Yini, ZHANG Zhidan, MA Xiaochun
- Rho激酶抑制剂通过抑制Toll样受体4和核因子κB信号通路缓解脂多糖诱导的肾损伤
- Rho-kinase Inhibitor Ameliorates Lipopolysaccharide-induced Kidney Injury by Inhibiting Toll-like Receptor 4 and Nuclear Factor-κB Signaling Pathway
- 中国医科大学学报, 2018, 47(1): 1-5
- Journal of China Medical University, 2018, 47(1): 1-5
-
文章历史
- 收稿日期:2017-04-28
- 网络出版时间:2017-12-20 14:17
急性肾损伤(acute kidney injury,AKI)是脓毒症常见的并发症,病死率高[1-2]。研究[3]表明,脓毒症AKI病理生理机制复杂,包括炎症反应、肾内血流动力学改变、内皮功能紊乱、微循环障碍以及肾小球内微血栓形成等,而炎症反应一般被认为是导致AKI的直接机制[4]。
脂多糖(lipopolysaccharide,LPS)是革兰阴性菌的细胞膜成分,是导致AKI的主要因素之一[5-6]。研究[7]表明,LPS通过激活Toll样受体4 (Toll like receptor 4,TLR4) /核因子κB (nuclear factor κB,NF-κB)信号转导通路,产生大量促炎性细胞因子包括肿瘤坏死因子α (tumor necrosis factor-α,TNF-α)、白细胞介素6 (interleukin-6,IL-6)、IL-1β,这些炎症介质在LPS诱导的肾损伤中起重要的作用。
Rho激酶(Rho kinase,ROCK)是影响细胞功能的关键信号蛋白,参与细胞骨架重构、细胞的黏附和迁移、活性氧簇(reactive oxygen species,ROS)的形成、细胞凋亡等[7-8]。本研究组在前期研究中发现ROCK抑制剂(法舒地尔)预处理能减少LPS诱导的炎症反应,减轻肺损伤,降低内毒素血症小鼠的死亡率[10]。然而,ROCK抑制剂对内毒素诱导肾损伤的影响及其分子生物学机制尚未阐明。本研究通过建立内毒素血症小鼠模型,检测肾损伤程度以及炎症相关指标,探讨ROCK特异性抑制剂Y-27632对内毒素血症小鼠肾损伤的影响及其分子生物学机制。
1 材料与方法 1.1 材料 1.1.1 主要试剂capase-3、TLR4、磷酸化NF-κBp65抗体(北京博奥森生物技术公司);LPS来源于大肠杆菌O55:B5 (Sigma-Aldrich公司);Y-27632 (美国Selleck Chemicals公司);尿素氮(urea nitrogen,BUN)、肌酐(creatinine,Cr)生化试剂盒(南京建成生物工程研究所);TIANScript cDNA第一链合成试剂盒、总RNA提取试剂盒(天根生化科技有限公司)。引物由生工生物工程(上海)有限公司合成。
1.1.2 实验动物分组及模型制备8~10周龄雄性C57BL/6小鼠(共24只),体质量20~25 g,购自中国医科大学动物部。将小鼠随机分为正常对照(control)组,LPS组,LPS+ Y-27632组,每组8只。腹腔内注射LPS (30 mg/kg)建立内毒素血症小鼠模型。于造模前18 h和1 h,给予LPS+Y-27632组ROCK特异性抑制剂Y-27632 (10 mg/kg),正常对照组和LPS组在相应时间点予以等量生理盐水腹腔注射。造模8 h后,麻醉小鼠,心脏采血并处死,留取血清和肾脏组织做进一步分析。
1.2 方法 1.2.1 血清BUN和Cr水平测定将血液离心10 min (4 ℃,3 000 r/min),提取上清液保存;根据试剂盒的说明书完成BUN和Cr测定。
1.2.2 肾组织病理观察取部分肾组织置10%甲醛溶液中,依次进行脱水、石蜡包埋、制切片(厚4 μm)、苏木精-伊红(HE)染色后,光镜下观察。
1.2.3 肾脏组织免疫组化10%甲醛固定肾组织,石蜡包埋,制成切片(厚4 μm)。以二甲苯脱蜡、水化,用柠檬酸盐缓冲液(0.01 mol/L,pH6.0)冲洗。滴加抗caspase-3多克隆抗体(1:100稀释),于4 ℃孵育过夜。PBS彻底冲洗后,加入二抗,室温孵育30 min,滴加DAB (二氨基联苯胺)液,苏木精复染。梯度乙醇脱水干燥,二甲苯透明,中性树胶封固,晾干后观察。
1.2.4 肾组织TNF-α、IL-1β mRNA表达的检测利用总RNA提取试剂盒提取总RNA,反转录获得cDNA,行PCR检测。TNF-α forward primer序列为5’-TTCTACTGAACTTCGGGGTGAT-3’,TNF-α reverse primer序列为5’-CACTTGGTGGTTTGCTACGA-3’;IL-1β forward primer序列为5’-TTTGAAGTTGACGGACCCC-3’,IL-1β reverse primer序列为5’-ATCTCCACAGCCACAATGAGTG-3’;β-actin forward primer序列为5’-CTGTGCCCATCTACGAGGGCTAT-3’,β-actin reverse primer序列为5’-TTTGATGTCACGCACGATTTCC-3’。扩增条件如下:50 ℃ 2 min,95 ℃ 5 min;95 ℃ 10 s,60 ℃ 20 s,72 ℃ 30 s,共40个循环。
1.2.5 Western blotting检测取部分肾组织置于冰冷的匀浆缓冲液(20 mmol/L Tris-HCl,100 mmol/L NaCl,2.7 mg/mL肝素)中进行匀浆,12 000 r/min离心10 min后,收集上清液。BCA法测定蛋白浓度,上样,电泳,转膜。4 ℃封闭过夜。一抗、二抗室温孵育2 h。暗室内曝光,显像,扫描结果并进行图像分析,以平均光密度值为标准对各组TLR4和磷酸化NF-κBp65蛋白进行评定。
1.3 统计学分析采用SPSS 17.0软件进行统计学处理,计量资料以x±s表示,多组间比较采用方差分析,2组间比较采用t检验。P < 0.05为差异有统计学意义。
2 结果 2.1 ROCK抑制剂Y-27632预处理缓解LPS诱导的AKI如图 1所示,与对照组相比,LPS组小鼠肾组织内可见炎性细胞浸润,肾小管结构紊乱,部分刷状缘脱落(图 1B),而LPS+Y-27632组肾损伤程度较LPS组明显减轻(图 1C);LPS组小鼠血清中BUN和Cr水平较对照组明显升高(P < 0.001),LPS+Y-27632组较LPS组则显著降低(P < 0.01)。
|
| A to C, representative histologic features of liver from control (A), LPS (B) and LPS+ Y-27632 (C) group (×400);D to E, the serum BUN and Cr levels at 8 hours after LPS challenge. *P < 0.001 vs control group; #P < 0.01 and ## P < 0.001 vs LPS group. 图 1 ROCK抑制剂Y-27632预处理对LPS诱导肾损伤的影响 Fig.1 Effect of Rho kinase inhibitor Y-27632 pretreatment on acute kidney injury after LPS challenge |
2.2 ROCK抑制剂Y-27632预处理抑制内毒素血症小鼠肾脏凋亡蛋白caspase-3的表达
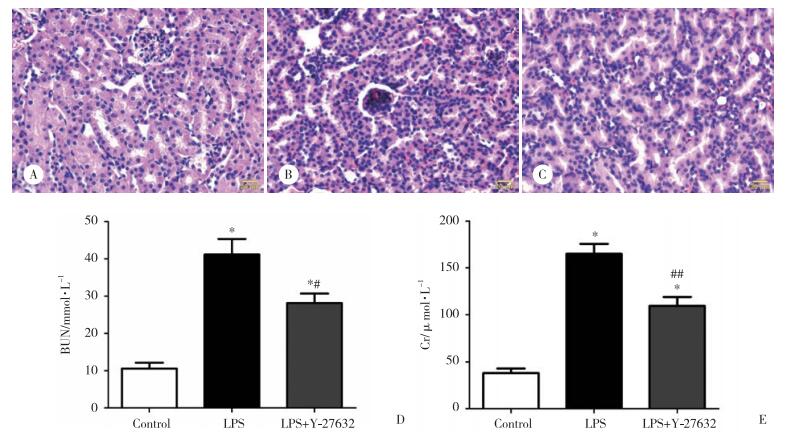
caspase-3是细胞凋亡的关键介质,因此,本研究用免疫组化法检测了3组小鼠肾组织中caspase-3蛋白的表达情况,如图 2所示,LPS组内毒素血症小鼠的肾脏中caspase-3表达显著增加;与LPS组相比,Y-27632预处理则明显减少了caspase-3的表达。
|
| A to C, IHC staining with caspase-3 antibody of kidney sections from control (A), LPS (B) and LPS+Y-27632 (C) group. 图 2 ROCK抑制剂Y-27632预处理对内毒素诱导的肾脏caspase-3蛋白表达的影响 ×400 Fig.2 Effect of Rho kinase inhibitor Y-27632 pretreatment on caspase-3 expression in mouse kidney after LPS challenge ×400 |
2.3 ROCK抑制剂Y-27632降低内毒素血症小鼠肾脏炎性细胞因子的表达水平
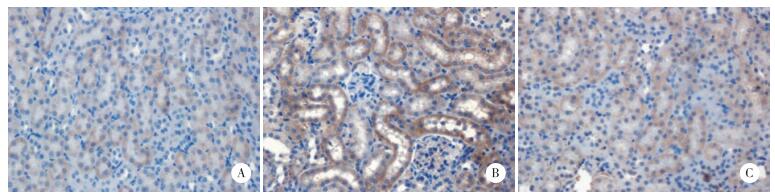
注射LPS后8 h,LPS组小鼠肾脏TNF-α和IL-1β mRNA的表达较对照组显著升高(P < 0.001);与LPS组相比,Y-27632预处理则能显著下调内毒素诱导的TNF-α和IL-1β mRNA表达(P < 0.001)。见图 3。
|
| A, TNF-α; B, IL-1β. * P < 0.001 vs control group; # P < 0.001 vs LPS group. 图 3 ROCK抑制剂Y-27632预处理对内毒素诱导的肾脏炎性细胞因子表达的影响 Fig.3 Effect of Rho kinase inhibitor Y-27632 pretreatment on proinflammatory cytokine expression in mouse kidney after LPS challenge |
2.4 ROCK抑制剂Y-27632下调内毒素血症小鼠肾脏TLR4蛋白表达及NF-κBp65磷酸化水平
如图 4所示,与对照组相比,LPS组内毒素血症小鼠的肾脏组织中TLR4蛋白的表达以及NF-κBp65磷酸化均显著增加(P < 0.001);与LPS组相比,Y-27632预处理则明显降低内毒素血症小鼠肾脏TLR4蛋白的表达以及NF-κBp65磷酸化水平(P < 0.01)。
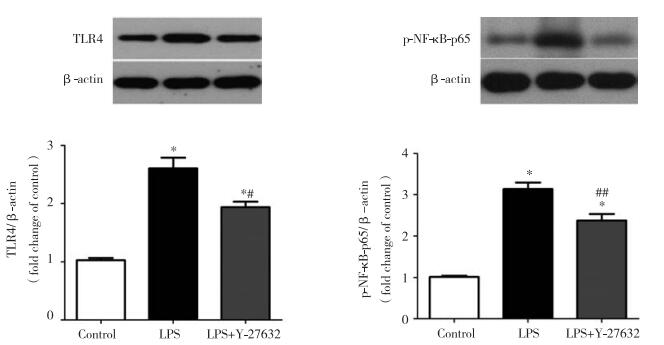
|
| * P < 0.001 vs control group; # P < 0.01 and ## P < 0.001 vs LPS group. 图 4 ROCK抑制剂Y-27632预处理对内毒素血症小鼠肾脏TLR4表达以及NF-κB磷酸化的影响 Fig.4 Effect of Rho kinase inhibitor Y-27632 pretreatment on TLR4 protein expression and NF-κBp65 phosphorylation in kidney tissue of endotoxemia mice |
3 讨论
本研究成功构建了内毒素肾损伤的动物模型,LPS显著上调了小鼠肾脏中炎性细胞因子TNF-α和IL-1β的表达。这些细胞因子已被证明在LPS诱导的AKI的发病机制中发挥着重要的作用[11-13],抑制这些炎性细胞因子的表达可以防止LPS诱导的AKI [14]。
越来越多的研究[10, 15-16]表明,ROCK参与了机体的炎症与免疫反应,包括炎症细胞的迁移、趋化因子和炎性细胞因子的产生。有文献[16]报道,ROCK抑制剂可能在脓毒症模型中发挥有益的作用。HASAN等[17]的研究也表明,ROCK能够调控腹腔感染小鼠T淋巴细胞功能障碍,ROCK抑制剂预处理可以降低脓毒症小鼠的全身炎症反应,抑制脓毒症诱发T细胞凋亡。研究[10]表明,ROCK抑制剂能够减轻LPS诱导的全身炎症反应,降低内毒素血症小鼠的死亡率。本研究中,进一步评估了ROCK抑制剂Y-27632对LPS诱导的小鼠AKI的保护作用,结果表明,Y-27632通过抑制炎性细胞因子TNF-α和IL-1β的生成缓解了LPS诱发的AKI。另外,Y-27632还显著下调了内毒素血症小鼠肾脏caspase-3的表达,提示Y-27632还可能通过抑制LPS诱导的细胞凋亡发挥肾脏保护作用。
Toll样受体(Toll like receptor,TLR)做为一种重要的模式识别受体,参与生物体的先天性免疫反应和炎症反应[18-20]。病原体感染机体时,各种不同的疾病相关分子模式被TLR识别,帮助先天免疫细胞识别微生物病原体,并引发适当的免疫反应[19-20]。LPS可通过与细胞膜上的受体TLR4结合,使TLR4胞内段招募特异接头蛋白MyD88,在信号介导分子肿瘤坏死因子相关因子6的作用下激活IκB激酶复合体,后者磷酸化IκB蛋白,导致IκB蛋白被泛素化和溶酶体降解,于是NF-κB被释放出来。活化的NF-κB进一步被磷酸化激活并转移入核,进而诱导下游一系列特异基因的表达[21-22]。以往的研究[23-25]表明,TLR4以及NF-κB的活化在LPS诱导的AKI中发挥重要作用。而且,NF-κB亚基p65的磷酸化水平常被用来间接反映NF-κB的活化[26]。本研究进一步探讨了ROCK抑制剂调控内毒素血症小鼠肾脏炎症反应的可能机制,结果显示,Y-27632能够显著抑制LPS诱导的TLR4表达以及NF-κB的活化。
综上所述,本研究结果表明,ROCK抑制剂Y-27632可以明显减轻内毒素诱导的肾损伤,且Y-27632可能通过抑制TLR4和NF-κB信号通路降低内毒素血症小鼠肾脏的炎症反应。
| [1] |
SUH SH, KIM CS, CHOI JS, et al. Acute kidney injury in patients with sepsis and septic shock:risk factors and clinical outcomes[J]. Yonsei Med J, 2013, 54(4): 965-972. DOI:10.3349/ymj.2013.54.4.965 |
| [2] |
LAFRANCE JP, MILLER DR. Acute kidney injury associates with increased long-term mortality[J]. J Am Soc Nephrol, 2010, 21(2): 345-352. DOI:10.1681/ASN.2009060636 |
| [3] |
ZARJOU A, AGARWAL A. Sepsis and acute kidney injury[J]. J Am Soc Nephrol, 2011, 22(6): 999-1006. DOI:10.1681/ASN.2010050484 |
| [4] |
MATEJOVIC M, CHVOJKA J, RADEJ J, et al. Sepsis and acute kidney injury are bidirectional[J]. Contrib Nephrol, 2011, 174: 78-88. DOI:10.1159/000329239 |
| [5] |
GUPTA A, RHODES GJ, BERG DT, et al. Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2[J]. Am J Physiol Renal Physiol, 2007, 293(1): F245-F254. DOI:10.1152/ajprenal.00477.2006 |
| [6] |
DOI K, LEELAHAVANICHKUL A, YUEN PS, et al. Animal models of sepsis and sepsis-induced kidney injury[J]. J Clin Invest, 2009, 119(10): 2868-2878. DOI:10.1172/JCI39421 |
| [7] |
RAMESH G, REEVES WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity[J]. J Clin Invest, 2002, 110(6): 835-842. DOI:10.1172/JCI15606 |
| [8] |
RIENTO K, RIDLEY AJ. Rocks:multifunctional kinases in cell behaviour[J]. Nat Rev Mol Cell Biol, 2003, 4(6): 446-456. DOI:10.1038/nrm1128 |
| [9] |
BOKOCH GM. Regulation of innate immunity by Rho GTPases[J]. Trends Cell Biol, 2005, 15(3): 163-171. DOI:10.1016/j.tcb.2005.01.002 |
| [10] |
DING RY, ZHAO DM, ZHANG ZD, et al. Pretreatment of Rho kinase inhibitor inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in mice[J]. J Surg Res, 2011, 171(2): e209-e214. DOI:10.1016/j.jss.2011.08.009 |
| [11] |
CUNNINGHAM PN, DYANOV HM, PARK P, et al. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney[J]. J Immunol, 2002, 168(11): 5817-5823. DOI:10.4049/jimmunol.168.11.5817 |
| [12] |
PATEL NS, CHATTERJEE PK, DI PAOLA R, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion[J]. J Pharmacol Exp Ther, 2005, 312(3): 1170-1178. DOI:10.1124/jpet.104.078659 |
| [13] |
FAGGIONI R, FANTUZZI G, FULLER J, et al. IL-1 beta mediates leptin induction during inflammation[J]. Am J Physiol, 1998, 274(1 Pt 2): R204-R208. |
| [14] |
XU C, CHANG A, HACK BK, et al. TNF-mediated damage to glomerular endothelium is an important determinant of acute kidney injury in sepsis[J]. Kidney Int, 2014, 85(1): 72-81. DOI:10.1038/ki.2013.286 |
| [15] |
PALANI K, RAHMAN M, HASAN Z, et al. Rho-kinase regulates adhesive and mechanical mechanisms of pulmonary recruitment of neutrophils inabdominal sepsis[J]. Eur J Pharmacol, 2012, 682(1/3): 181-187. DOI:10.1016/j.ejphar.2012.02.022 |
| [16] |
HASAN Z, PALANI K, RAHMAN M, et al. Rho-kinase signaling regulates pulmonary infiltration of neutrophils in abdominal sepsis via attenuation of CXC chemokine formation and Mac-1 expression on neutrophils[J]. Shock, 2012, 37(3): 282-288. DOI:10.1097/SHK.0b013e3182426be4 |
| [17] |
HASAN Z, PALANI K, ZHANG S, et al. Rho kinase regulates induction of T-cell immune dysfunction in abdominal sepsis[J]. Infect Immun, 2013, 81(7): 2499-2506. DOI:10.1128/IAI.00126-13 |
| [18] |
CHAO W. Toll-like receptor signaling:a critical modulator of cell survival and ischemic injury in the heart[J]. Am J Physiol Heart Circ Physiol, 2009, 296(1): H1-H12. DOI:10.1152/ajpheart.00995.2008 |
| [19] |
KHAKPOUR S, WILHELMSEN K, HELLMAN J. Vascular endothelial cell Toll-like receptor pathways in sepsis[J]. Innate Immun, 2015, 21(8): 827-846. DOI:10.1177/1753425915606525 |
| [20] |
SAVVA A, ROGER T. Targeting toll-like receptors:promising therapeutic strategies for the management of sepsis-associated pathology and infectious diseases[J]. Front Immunol, 2013, 4: 387. DOI:10.3389/fimmu.2013.00387 |
| [21] |
CARMODY RJ, CHEN YH. Nuclear factor-kappaB:activation and regulation during toll-like receptor signaling[J]. Cell Mol Immunol, 2007, 4(1): 31-41. |
| [22] |
KAWAI T, AKIRA S. Signaling to NF-kappaB by Toll-like receptors[J]. Trends Mol Med, 2007, 13(11): 460-469. DOI:10.1016/j.molmed.2007.09.002 |
| [23] |
CUNNINGHAM PN, WANG Y, GUO R, et al. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure[J]. J Immunol, 2004, 172(4): 2629-2635. DOI:10.4049/jimmunol.172.4.2629 |
| [24] |
ZHANG B, RAMESH G, UEMATSU S, et al. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity[J]. J Am Soc Nephrol, 2008, 19(5): 923-932. DOI:10.1681/ASN.2007090982 |
| [25] |
SANZ AB, SANCHEZ-NINO MD, RAMOS AM, et al. NF-κB in renal inflammation[J]. J Am Soc Nephrol, 2010, 21(8): 1254-1262. DOI:10.1681/ASN.2010020218 |
| [26] |
CHUNZHI G, ZUNFENG L, CHENGWEI Q, et al. Hyperin protects against LPS-induced acute kidney injury by inhibiting TLR4 and NLRP3 signaling pathways[J]. Oncotarget, 2016, 7(50): 82602-82608. DOI:10.18632/oncotarget.13010 |
 2018, Vol. 47
2018, Vol. 47