Advances in chemistry and bioactivity of the genus Erythroxylum
Abstract
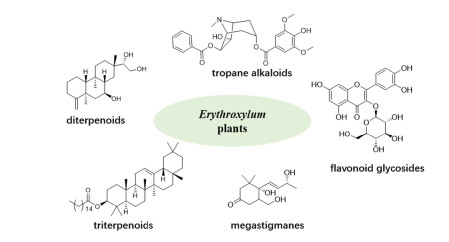
Erythroxylum P. Browne is the largest and most representative genus of Erythroxylaceae family. It contains approximately 230 species that are mainly distributed in tropical and subtropical regions. Some species in this genus, such as E. monogynum and E. coca, have been used as folk medicines in India or South America for a long history. It is well known that Erythroxylum plants are rich in tropane alkaloids, and the representative member cocaine shows remarkable activity in human central nervous system. However, many other types of active compounds have also been found in Erythroxylum along with the broadening and deepening of phytochemical research. To date, a total of 383 compounds from Erythroxylum have been reported, among which only 186 tropane alkaloids have been reviewed in 2010. In this review, we summarized all remained 197 compounds characterized from 53 Erythroxylum species from 1960 to 2021, which include diterpenes, triterpenes, alkaloids, flavonoids, and other derivates, providing a comprehensive overview of phytoconstituents profile of Erythroxylum plants. In addition, the biological activities of representative phytochemicals and crude extracts were also highlighted.Graphical Abstract
Keywords
Erythroxylum Natural products Phytoconstituent Bioactivity1 Introduction
In the long evolutionary process of nature, plants have acquired the ability to synthesize various compounds to better adapt to stimulations in the environment. The accumulation of practical experience has made human realize that these substances are also of significant importance for the treatment of human diseases and the improvement of the quality of life. With the support of technology in compound extraction, separation and structural identification, the active substances in traditional herbs are gradually being discovered by humans. Therefore, modern medicine based on a single or several compounds has been developed. With the deepening of research on plant natural products, new biologically active compounds are constantly being discovered and further applied in medicine, health care and agriculture. Sorting out and summarizing the plant distribution, structure, and activity characteristics of these newly discovered phytocompounds will confer us effective information in rational use of plant resources.
Erythroxylum P. Browne, the representative genus of Erythroxylaceae family, is especially well known for its phytoconstituents of tropane alkaloids (TAs), such as cocaine [1, 2]. Species of this genus are mainly distributed in tropical and subtropical regions including South America, South Africa, Southeast Asia and Australian flora [2]. As the largest genus of the Erythroxylaceae family, approximately 230 species are included in Erythroxylum [2], among which E. coca and E. novogranatense are the famous plant sources of cocaine. Before achieving the purification of cocaine from plants in 1859 [3], the leaves of E. coca or E. novogranatense had been chewed by the Indigenous South American as stimulant and hunger-suppressant for over a thousand years. The remarkable biological activity of cocaine in human central nervous system attracted widespread attention to compounds in plants of this genus. Accordingly, numerous of cocaine analogs (TAs), as well as other bioactive compounds have been found in Erythroxylum [4, 5].
To date, no comprehensive summary on chemical compositions found in Erythroxylum species and their bioactivities has been reported, though Oliveira et al. [6] presented an excellent review focusing on structures of TAs isolated from this genus in 2010 and Dr. John D'Auria' s group discussed application potentials of Erythroxylum species worldwide in mental health, nutrition, agriculture, and commercialization based on studies on representative compounds discovered in this genus [7]. Attracted by the diverse biological activity of compounds found in Erythroxylum, which included anaesthetic [8], antioxidative [9, 10], anti-inflammatory [9], cytotoxic [11], anticancer [12], and insecticidal activities [13], as well as neutralization of snake venom [14], we therefore aimed to provide a comprehensive review of all compounds reported in Erythroxylum species from 1960 to 2021 and an update of alkaloids isolated after 2010 here, which is supposed to be essential for further effective development and utilization of plant resources in the genus in the future. Additionally, we also presented an overview of the biological activities of representative phytochemicals and crude extracts at the end of the review, providing medicinal and commercial application prospects of Erythroxylum species.
2 Chemical composition
Based on the published results dedicated to study chemical composition of Erythroxylum species, 383 compounds, including diterpenes, triterpenes, flavonoids, alkaloids, and other derivates, have been found in 67 Erythroxylum species. Among these, 186 TAs compounds identified in Erythroxylum plants before 2010 have been systematically reviewed by Oliveira et al. [6]. Therefore, here we summarized all remained 197 compounds characterized from 53 Erythroxylum species from 1960 to 2021, which include diterpenes, triterpenes, alkaloids, flavonoids, and other derivates.
According to the literature, Erythroxylum plants are rich in alkaloids. Especially E. coca, E. coca var. coca, and E. novogranatense var. novogranatense, the content of total alkaloids varies from 0.5% to 2.4% in leaves (dry mass, Table 1) [15]. Particularly, high cocaine content (0.13%-0.76% dry mass) was found in E. coca and E. novogranatense leaves [16]. In 2006, Stefan Bieri et al. [17] analyzed the cocaine distribution in 51 plant species and cocaine was detected only in 23 Erythroxylum species with the content less than 0.001% (dry leaves). High production of total phenols, total tannins and total flavonoids of up to 17.97%, 8.4%, and 3.87% (dry leaves), respectively, was reported in E. suberosum, E. tortuosum, and E. deciduum [18] (Table 1). Additionally, the total diterpenes content determined in stems of E. australe and E. pictum ranged from 0.09% to 1.8% (dry mass, Table 1).
Tabel 1 The content of principal components in several Erythroxylum species
2.1 Diterpenes
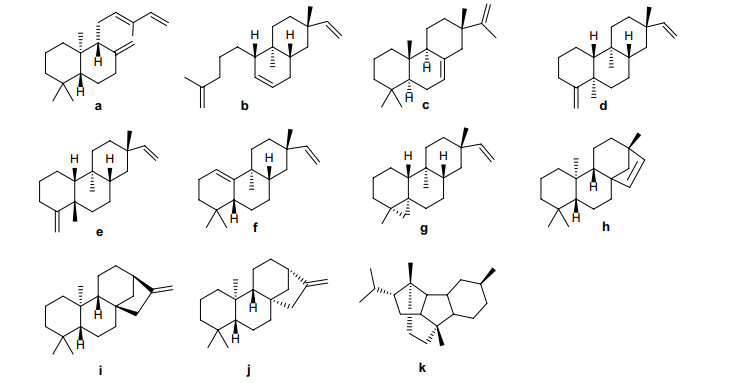
Plants of Erythroxylum are rich in diterpenoids, which have been extensively studied since the last century. In particular, Connolly [21-24] and Kapadi [25-27], who focused on investigating diterpenoids of E. monogynum in 1960s, provided the earliest knowledge of diterpenoids in Erythroxylum species. Using nuclear magnetic resonance (NMR) spectroscopy and chemical reactions, they and their coworkers elucidated the structures of 17 diterpenoids in E. monogynum. To date, about 11 types of diterpene skeletons (a–k) have been identified from plants in this genus (Fig. 1). Based on the number of rings in the diterpene skeletons, diterpenes found in Erythroxylum species could be divided into bicyclic diterpenes, tricyclic diterpenes, and tetracyclic diterpenes.
Skeletons of diterpenes found in Erythroxylum plants
2.1.1 Bicyclic diterpenes
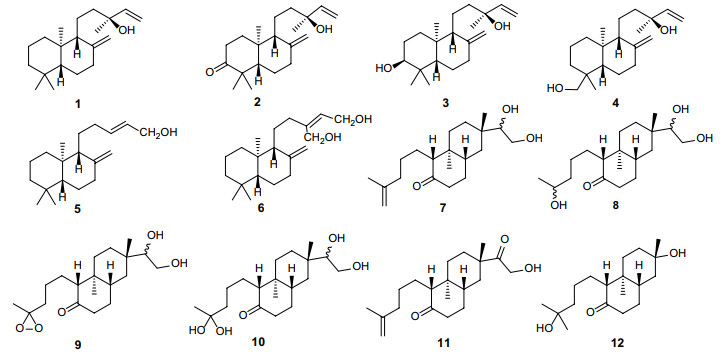
Labdane is a typical bicyclic diterpene, which forms the structural skeleton for many diterpene compounds found in plants [28-30]. In Erythroxylum, six ent-labdane derivatives (1–6) have been isolated and characterized from nine species of this genus in the past decades [20, 31, 32] (Table 2). Additionally, Ansell [20] et al. first found six 4, 5-seco-rosane derivative diterpenoids (7–12) from E. pictum in 1993 (Table 2). Since these derivatives were shown to be characteristic of E. pictum, they named this novel bicyclic diterpene skeleton, 4, 5-seco-rosane, as pictane. Later, they found one of these derivatives of pictane, ent-15ξ, 16-dihydroxypictan-4(18)-en-5-one (7), was also present in other six species of Erythroxylum [31]. The distribution and structures of these bicyclic diterpenes are listed in Table 2 and Fig. 2, respectively.
Bicyclic diterpenes isolated from Erythroxylum plants
Chemical structures of bicyclic diterpenes (1–12) found in Erythroxylum plants
2.1.2 Tricyclic diterpenes
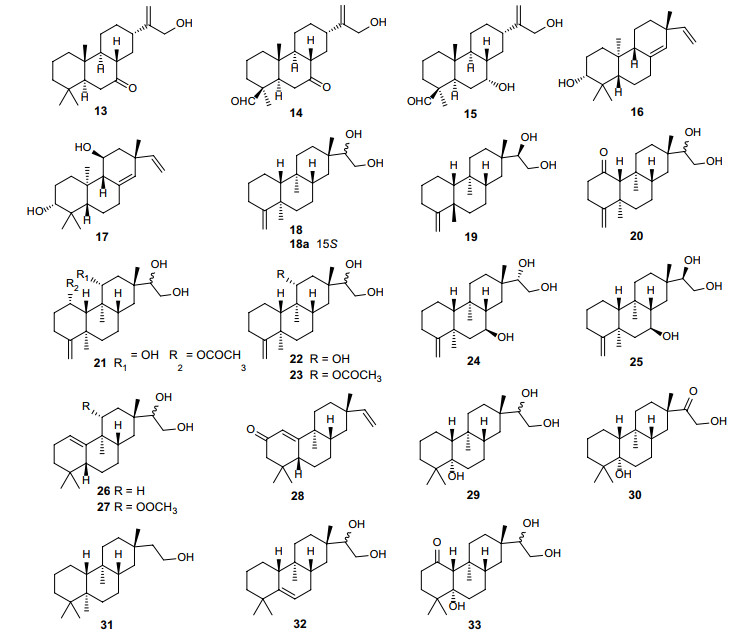
21 tricyclic diterpene compounds with four skeleton types (abietane, pimarane, dolarbrane, and rosane) have been isolated from Erythroxylum genus (Fig. 3; Table 3). Among these compounds, there are three abietane (13–15) [33] and two pimarane diterpenoids (16–17) [31] obtained from E. suberosum and E. cuneatum, respectively. Dolarbrane-type diterpene was first found in the leaves of Thujopsis dolabrata of Cupressaceae in 1964 [34]. Almost at the same time, Connolly [21], who focused on the phytochemistry of E. monogynum, characterized erythroxydiol Y (18) from this Erythroxylum plant. In 1993, seven new dolarbrane-type derivatives (19–25) were reported by Ansell et al. [20, 31]. In addition, they identified seven rosane-type (26–32) diterpenoids from several Erythroxylum species. Another rosane-type compound (33) was found in E. barbatum by dos Santos [35].
Chemical structures of tricyclic diterpenes (13–33) found in Erythroxylum plants
Tricyclic diterpenes isolated from Erythroxylum plants
2.1.3 Tetracyclic diterpenes
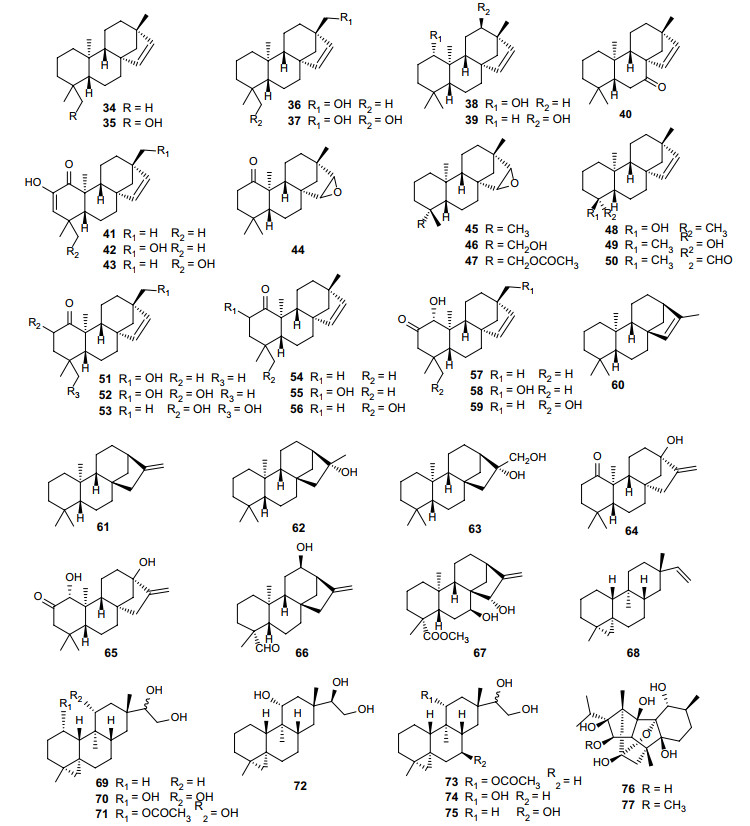
Erythroxylum is a prolific source of beyerene diterpenes [5, 24, 31]. More than 20 beyerene derivatives (34–59) have been identified from nine Erythroxylum species [5, 24, 25, 31, 37-40], while diterpenoids isolated from E. australe consisted preponderantly of beyerene derivatives [5, 24] (Fig. 4; Table 4). Importantly, in a recent research, auto-oxidation of the aldehyde group of ent-beyer-15-en-19-al (50) isolated from E. monogynum to a carboxylic acid group was observed, and this auto-oxidation could take place both with and without the concurrent epoxidation of the 15, 16-double bond, indicating that some beyerene type diterpenoids identified previously may be artefacts arising from the auto-oxidation reaction [40]. Tetracyclic diterpene ent-kaurene is a critical intermediate in gibberellin hormones biosynthesis pathway in plants, and kaurene diterpenes are widely distributed in nature. Seven kaurene diterpenes (61–67) have been isolated and identified from Erythroxylum plants [5, 20, 31, 33, 41] (Fig. 4; Table 4), among which erythroxylisin A (64) and erythroxylisin B (65) obtained from roots of E. barbatum are unusual kaurene diterpenes with a cis-orientation of the C-20 methyl and the CH2-15 methylene groups [41]. Devadarane is a tetracyclic diterpene skeleton completely different from the two mentioned above (Fig. 4; Table 4). Devadarane-type diterpene compounds (68–75) were first discovered in E. monogynum [21, 42]. However, the structure of triol Q (72) was not determined until McCrindle. R [43] undertook an X-ray analysis two years later. Although devadarane-type diterpenes have been identified in five species of this genus, only eight devadarane derivatives (68–75) have been reported so far (Fig. 4; Table 4). Ryanodane diterpenes (76–77) [13] were originally isolated from ripe fruits of E. passerinum, and later ryanodanol (76) was also identified in E. nummularia leaves (Fig. 4; Table 4). This type of diterpenoids has a complicated skeleton. According to reports in the literature since 1960, only two compounds (76–77) of this type have been discovered in the genus Erythroxylum.
Chemical structures of tetracyclic diterpenes (34–77) found in Erythroxylum plants
Tetracyclic diterpenes isolated from Erythroxylum plants
2.2 Triterpenoids
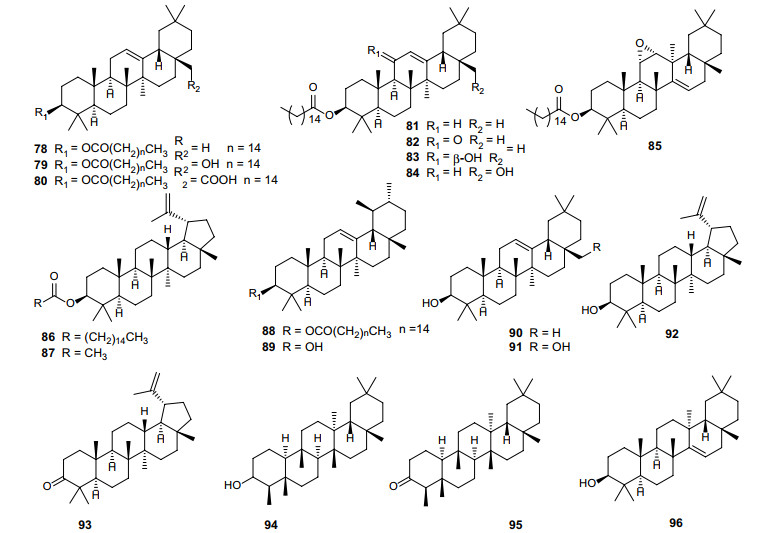
To date, a total of 19 triterpenoids have been identified in Erythroxylum plants (Fig. 5; Table 5), ten of which are fatty acid esters of triterpenes (78–86, 88) from E. nummularia [44], E. leal-costae [45], E. rimosum [46] or E. passerinum [47]. Lupenyl acetate (87) [45], α-amyrin (89) [46], β-amyrin (90) [44, 46, 47] and erythrodiol (91) [47] are other four triterpenoids found in E. leal-costae, E. nummularia, E. rimosum or E. passerinum (Fig. 5; Table 5). Besides, recent studies reported five triterpenes (92–96) from E. ovalifolium [14], E. daphnites [48] or E. macrocalyx [49]. Interestingly, all the triterpenoids identified in this genus are pentacyclic triterpenes.
Chemical structures of triterpenoids (78–96) found in Erythroxylum plants
Triterpenoids isolated from Erythroxylum plants
2.3 Alkaloids
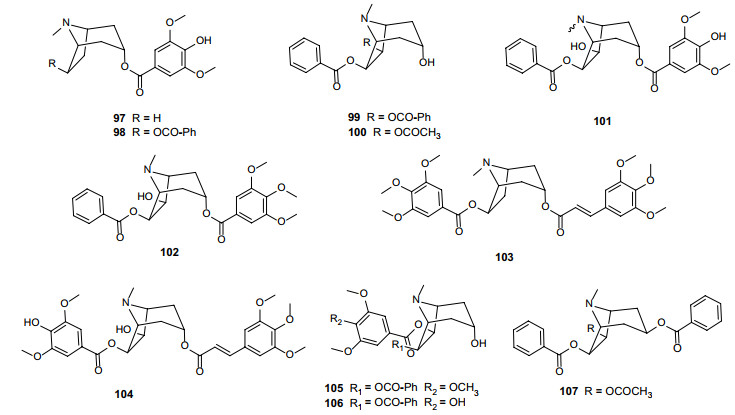
TAs are alkaloids with a tropane skeleton (8-azabicyclo[3.2.1]octane). As characteristic alkaloids widely distributed in Erythroxylum species, TAs exhibit a range of pharmacological activities like vasorelaxation [50], antiproliferative [49], anesthesia [51], antimicrobial and anticancer [12]. In 2010, a fascinating review by Oliveira et al. [6] comprehensively summarized structures of 186 TAs found in 35 species of Erythroxylum. As an update, we here found 11 more new TAs reported in studies since then (Fig. 6; Table 6). Among these newly identified TAs, two members were isolated from E. pungens (97) [52] and E. caatingae (98) [53], respectively; 6β, 7β-dibenzoyloxytropan-3α-ol (99) was obtained from E. subsessile [54]; 7β-acetoxy-6β-benzoyloxy-3α-hydroxytropane (100) was isolated from the twigs of E. macrocalyx [49]; six members named as erythrobezerrines A-F (101–106) were isolated from the stem bark of E. bezerrae [55]; and 7β-acetoxy-3β, 6β-dibenzoyloxytropane (107) was isolated from the leaves of E. rimosum [46]. Previous studies have also reported the isolation of non-TA alkaloids by GC–MS analysis [56-58]. However, since most of them were potential precursors or side products of TA biosynthetic pathway [56], we will not include them here. Readers interested in the details of these compounds are referred to the review by Brachet Anne and coworkers [56].
Chemical structures of TAs (97–107) found in Erythroxylum plants after 2010
Alkaloids isolated from Erythroxylum plants
2.4 Flavonoids
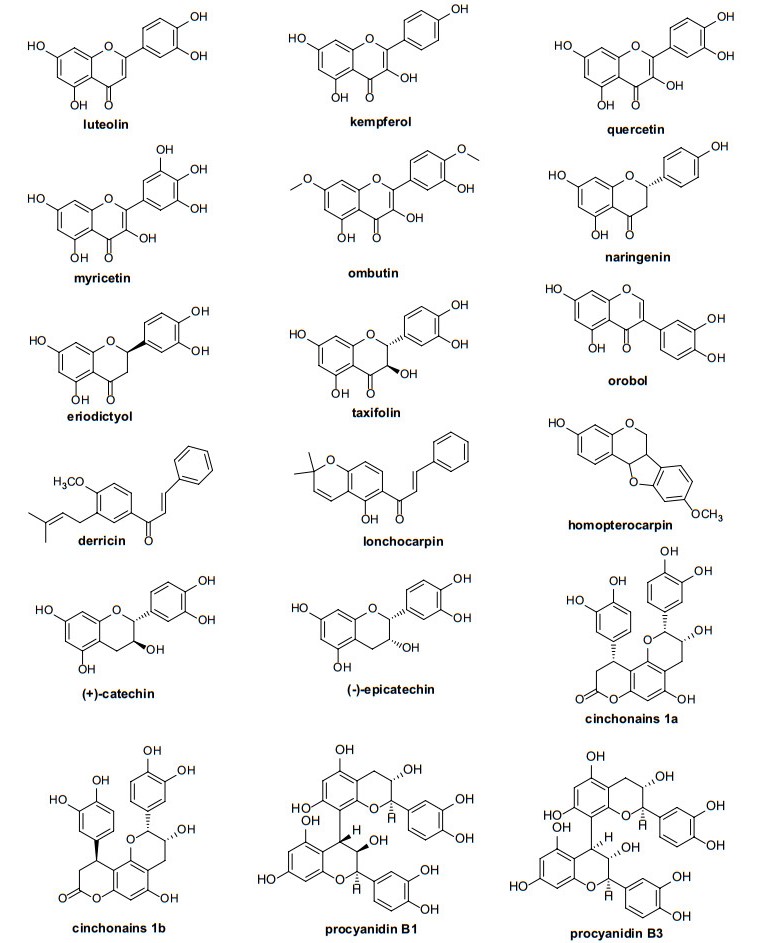
Flavonoids are a large and complex group of constituents found in almost all plants. Flavonoid variation in thirteen species of Erythroxylum has been studied systematically by Plowman et al. in 1988 [59]. They found kaempferol, ombuin (7, 4ʹ-dimethylquercetin), and quercetin were predominant flavonoid aglycones in Erythroxylum plants analyzed. Besides, Johnson and coworkers [60-65], based on their work on flavonoids profiles of six species or variants and flavonoids that had been reported in Erythroxylum, proposed that some unique flavonoids could be used as chemotaxonomic markers for taxon. Overall, flavonoid aglycones in Erythroxylum mainly consist of quercetin, ombuin, fisetin, kaempferol, epicatechin, eriodictyol and taxifolin. In addition to these, isoflavone, isoflavanone and other flavone derivatives were also found in Erythroxylum. Chemical structures of flavonoid aglycones that have been found in Erythroxylum plants were summarized and presented in Fig. 7. Moreover, the major glycosides of these flavonoids include mono-glucosyl-rhamnosyls and dirhamnosyl-glucosides, as well as mono-galactosyl and mono-arabinosyl. In total, 73 flavonoids from 37 species of Erythroxylum have been studied (Table 7), though some structures lack NMR data support in the literature.
Chemical structures of flavonoid aglycones found in Erythroxylum plants
Flavonoids isolated from Erythroxylum plants
2.5 Other constituents
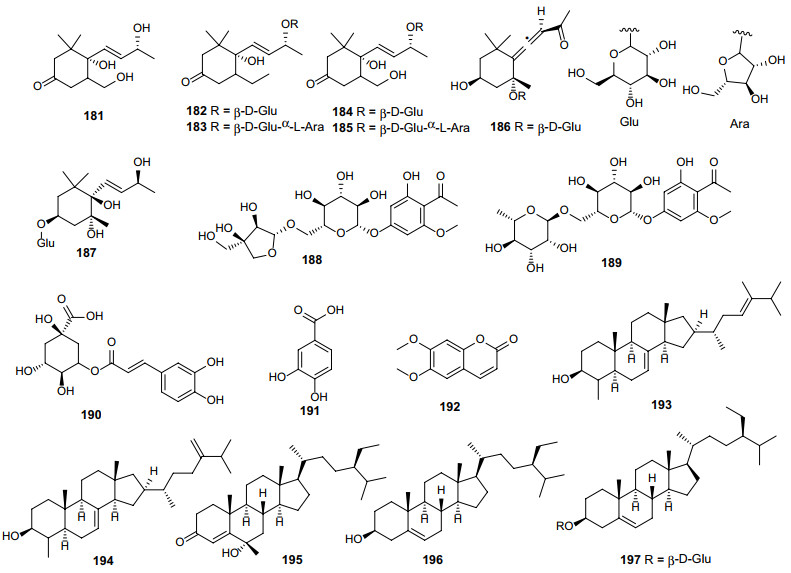
Norisoprenoid compounds (megastigmanes, 181–187) have been characterized in E. cuneatum [74] and E. cambodianum [72] by Kanchanapoom et al. (Fig. 8; Table 8). Phenolic derivatives and their glycosides were also obtained (Fig. 8; Table 8), which include two acetophenone diglycosides (188–189) isolated from E. cambodianum [72], neochlorogenic acid (190) and protocatechuic acid (191) extracted from E. lucidum [68], and scoparon (192) yielded from E. barbatum [76]. Additionally, five steroids (193–197) have been identified in this genus according to the previous studies [35, 44, 46-48, 76, 77] (Fig. 8; Table 8). Importantly, compounds 193 and 194 showed significant anti-oxidant and anti-glycation activities in vitro [77].
Chemical structures of other constituents (181–197) found in Erythroxylum plants
Other constituents isolated from Erythroxylum plants
3 Biological activities of natural products in Erythroxylum
3.1 Bioactivities of diterpenes, triterpenes and sterols
Pharmacological investigation of diterpenes isolated from Erythroxylum species are still scarce despite the large resource found. Diterpene 14-O-methyl-ryanodanol (77) showed insecticidal activity against Aedes aegypti larvae [13], as well as a dose-dependent cytotoxic effect to astrocytes (GL-15 cell line) [78]. Cytotoxicity activities against five tumor cell lines of devadarane derivatives (69, 73–75) were also investigated, but no activity was observed [35]. Exploring and evaluating bioactivities of the numerous diterpenoids found in Erythroxylum species will be essential for further effective utilization of these natural product resources in this genus. For triterpenes, compounds 93–95 were major constituents of the hexane extract of E. daphnites leaves which showed a cytotoxic effect against SCC-9 oral squamous cell carcinoma cell line [48]. Additionally, sterols (193, 194) isolated from E. monogynum possess good anti-oxidant and anti-glycation activities [77]. Additionally, although a large number of flavonoids have been found in Erythroxylum species, these compounds are not specifically distributed in this genus. Readers interested in the details of bioactive flavonoids are referred to the review by Shashank Kumar and coworkers [79].
3.2 Bioactivities of TAs
In Erythroxylaceae family, TAs specially occur in species of Erythroxylum. Until now, a total of 197 TAs compounds have been characterized in Erythroxylum plants. There are plenty of researches on pharmacology activities of TAs in Erythroxylum, especially cocaine. Ophthalmologist Carl Koller first demonstrated the ability of cocaine to induce local anesthesia in eyes [8]. Later, it was extended to dentistry, urology, laryngology and other fields as a local anaesthetic [80]. In addition, a review by Drake [51] highlighted that cocaine could act as a psychomotor stimulant and also showed toxicity in coabuse and overdoses. Cocaine acts on the mesolimbic dopamine system whose origins begins in the ventral tegmental area and projects to the nucleus accumbens, the amygdala, the hippocampus, and the prefrontal cortex, resulting in a higher concentration of dopamine release into the nucleus accumbens and prefrontal cortex [81]. Previous study also showed that acute cocaine at a dose used by cocaine abusers for recreational purposes induced large increases in intracellular calcium in the cortex of the rat brain and the mechanism were related to the local anesthetic actions of cocaine and not its sympathomimetic effects [82]. The cardiovascular mitochondrial dysfunction induced by cocaine is involved in the mechanisms of oxidative stress [83]. Also, Ca2+/calmodulin-dependent protein kinase II and inhibitory G-protein coupled receptor signaling are involved in the mechanism of the effect of cocaine- and amphetamine-regulated transcript in cocaine reward [84]. There are a number of excellent reviews on the bioactivity, toxicity, and biological mechanisms of cocaine [84-88], and therefore we will not repeat more details here. Additionally, for cocaine-producing Erythroxylum plants, cocaine could function as a natural insecticide to protect the leaves [89].
Pervilleine A (reviewed in ref. [6]) from E. pervillei demonstrated weak nonspecific anticholinergic and vascular antiadrenergic activities [90]. Catuabine B and 3α, 6β-dibenzoyloxytropane from E. vaccinifolium [53] (reviewed in ref. [6]) showed antimicrobial activity on gram-positive bacteria and fungi [12]. It has also been demonstrated that the E. cuneatum leaf alkaloid extract possessed both antioxidative and anti-inflammatory properties [9]. Among the reported biological activities of TAs, cytotoxicity is also noticeable. Araújo Neto et al. [91] summarized the cytotoxic activity of 21 species of Erythroxylum against 45 different cell lines and found the species with presence of disubstituted TAs had the highest cytotoxic potentials. Recently, a newly identified tropane alkaloid (6β-benzoyloxy-3α[(4-hydroxy-3, 5-dimethoxybenzoyloxy] tropane) (98) was demonstrated to possess high antiproliferative activity on liver hepatocellular carcinoma cells (HepG2) with IC50 value of 3.66 μg mL−1. Meanwhile, it showed no cytotoxicity on human lymphoblast cell line [49]. In addition, erythrobezerrine C (103) showed moderate cytotoxicity activity on HCT-116 and NCI-H460, with IC50 values of 3.38 and 5.43 μM, respectively [55]. TAs with antimicrobial [12] and diuretic [92, 93] activities have also been reported. In 1984, Novak [94] reported the bioactivities of TAs from E. coca and E. novogranatense contained stimulant activity, inhibiting effect on dopamine uptake, and cholinolytic action.
3.3 Bioactivities of crude extracts
In addition to research on single compound, many studies have been carried out on the biological activities of crude extracts of Erythroxylum plants (Table 9). E. monogynum is rich in alkaloids and diterpenes. In 2019, Dhanunjaya et al. [95] summarized that crude extracts of this species had multiple bioactivities, such as antioxidant, antihyperlipidemic, antidiabetic, antiplasmodial and hepatoprotective. Particularly, leaf and bark extracts of E. delagoense, E. emarginatum, or E. pictum, showed great antibacterial activities [96]. Ethanolic extract obtained from the roots of E. pungens could induce dose-dependent hypotension and tachycardia in conscious rats, as well as vasorelaxation in mesenteric artery ring preparations in vitro [50]. Ethanolic extract of E. caatingae has a relaxant effect on ovine cervical contractions [97]. Besides, low-polarity fractions of this species showed significantly high cytotoxicity activity against the NCI-H292, HEp-2 and K562 cell lines [12]. Furthermore, acetone/water (70/30, v/v) extract of E. macrocarpum is a significant inhibitor of acetylcholinesterase [98]. Hydroalcoholic extracts of E. areolatum or E. confusum showed antiherpetic activity [99]. For the antitumor activity, when mice were treated with different doses of methanol extract of E. caatingae, a significant reduction in their tumor weight was observed [53]. Moreover, extracts of E. minutifolium or E. confusum showed hepatoprotective effects [100]. Crude extracts, fractions, or isolated products of E. ovalifolium or E. subsessile were demonstrated to inhibit toxic effects of the snake (Lachesis muta) venom, providing a new strategy for antivenom treatment [14].
Biological activities of crude extracts of Erythroxylum plants
4 Conclusions and prospecting
Based on the current progress in phytochemistry of the Erythroxylum [6], there is no doubt that TAs are the largest class of compounds found in this genus (197 of 383 compounds). In the past years, their remarkable pharmacological activities have made this class of compounds receive more attention than others [49, 52, 104]. However, many other types of active compounds have been found in Erythroxylum along with the broadening and deepening of phytochemical research. A summary of the structure and distribution of these compounds is essential for in-depth understanding and utilization of plant resources of this genus. Based on the literature, a total of 383 compounds from Erythroxylum have been reported, among which only 186 tropane alkaloids have been reviewed in 2010. In this review, we summarized all remained 197 compounds characterized from 53 Erythroxylum species from 1960 to 2021, including 11 skeleton-types of diterpenes (1–77) isolated from 18 Erythroxylum species, 19 triterpenoids obtained from 8 Erythroxylum species, 11 TAs found in 6 species after 2010, 73 flavonoids from 37 Erythroxylum species, and 17 other constituents (norisoprenoids, phenolic derivatives and their glycosides, and steroids). Among these compounds, most diterpenes were isolated from the timber or roots of the plants, triterpenes were identified from aerial organs, flavonoids were distributed in leaves or branches, while others had no obvious tissue- or organ-specific distributions. Significant biological activities, including anaesthetic [8], antioxidative [9, 10], anti-inflammatory [9], cytotoxic [11], anticancer [12], and insecticidal activities [13], as well as neutralization of snake venom [14], have been demonstrated for isolated products or crude extracts from some species of Erythroxylum. However, potential activity of most compounds is still unknown. In-depth biological activity studies on compounds obtained will be the basis for exploring potential medicinal resources in this genus. Additionally, some of the diterpenes were suggested to serve as the defensive components to protect the Erythroxylum plants from herbivores, pathogens, or other environmental challenges. Therefore, they could be used as potential bioinsecticides in agriculture in the future.
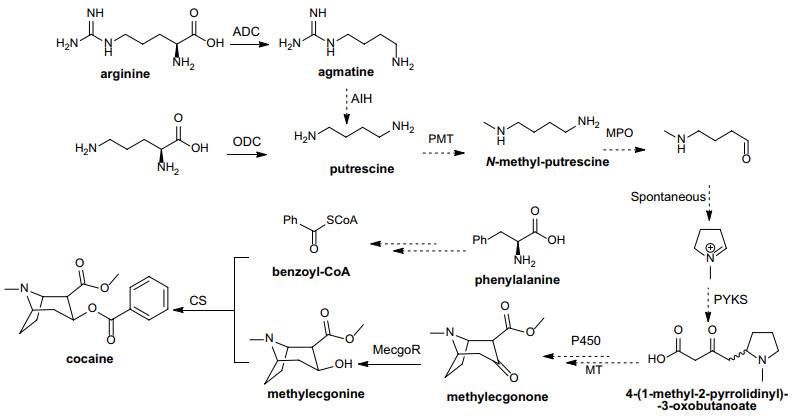
Elucidation of natural product biosynthetic pathways has been proved to be highly useful for natural products discovery, structure identification and subsequent heterologous synthesis. In Erythroxylum plants, TAs and diterpenes are representative phytoconstituents. Biochemists and molecular biologists have long sought to identify the biosynthetic pathways of TAs, especially cocaine, through isotope labeled precursor feeding studies and gene cloning and characterization [105-110]. As a result, incomplete biosynthetic route of cocaine starting from arginine and ornithine and passing through putrescine, methylecgonone, and methylecgonine has been established [7, 110] (Fig. 9), though further studies are still essential to elucidate the missing steps. Studies focusing on the biosynthesis pathway of diterpenes in Erythroxylum plants have not been reported till now. However, the kaurene-type (Fig. 1i) diterpene synthase that is responsible for the formation of ent-kaurene, the universal biosynthetic intermediate of gibberellin, has been identified in many other plants [111-113]. Besides, ent-beyerene synthase, which is the key diterpene cyclase required for generating ent-beyerene type diterpenes (Fig. 1h), has been characterized in monocotyledonous rice (Oryza sativa L.) [114]. Still, much more researches needed to be done for better understanding the biosynthetic mechanisms and diversity of diterpenes identified in Erythroxylum.
The proposed cocaine biosynthesis pathway in E. coca. The following enzymes are depicted in the figure above: ADC (arginine decarboxylase), ODC (ornithine decarboxylase), AIH (armatine iminohydrolase), PMT (putrescine methyltransferase), MPO (N-methylputrescine oxidase), PYKS (pyrrolidine ketide synthase), P450 (cytochrome 450), MT (methyltransferase), MecgoR (methylecgonone reductase), CS (cocaine synthase)
Notes
Acknowledgements
This research was supported by the National Key R&D Program of China (2018YFA0900600), the National Science Foundation of China (U1902212 and 32000239), the Foundations of Yunnan Province (2019FJ007 and 2019ZF011–2), the Strategic Priority Research Program of the CAS (XDB27020205), and the Key Research Program of Frontier Sciences of the CAS (QYZDB-SSW-SMC051).
Author contributions
YL, TT and Y-J W collected the related references and prepared chemical compounds structures; YL and J-P H worte the manuscript; J-P H and S-X H reviewed and edited the manuscript. All authors read and approved the final manuscript.
Declarations
Competing interests
The authors declare no conflict of interest.
References
-
1.Evans WC. The comparative phytochemistry of the genus Erythroxylon[J]. J Ethnopharmacol 3, 265-77 (1981) CrossRef PubMed Google Scholar
-
2.Plowman T, Hensold N. Names, types, and distribution of neotropical species of Erythroxylum (Erythroxylaceae)[J]. Brittonia 56, 1-53 (2004) CrossRef PubMed Google Scholar
-
3.Niemann A. Ueber eine neue organische base in den cocablättern[J]. Arch Pharm (Weinheim) 153, 129-55 (1860) CrossRef PubMed Google Scholar
-
4.Hegnauer R. Chemotaxonomy of Erythroxylaceae (including some ethnobotanical notes on old world species)[J]. J Ethnopharmacol 3, 279-92 (1981) CrossRef PubMed Google Scholar
-
5.Ansell SM, Pegel KH, Taylor DAH. Diterpenes from the timber of Erythroxylum australe[J]. Phytochemistry 32, 937-43 (1993) CrossRef PubMed Google Scholar
-
6.Oliveira SL, da Silva MS, Tavares JF, Sena-Filho JG, Lucena HFS, Romero MAV, Barbosa-Filho JM. Tropane alkaloids from Erythroxylum genus: distribution and compilation of 13C-NMR spectral data[J]. Chem Biodivers 7, 302-26 (2010) CrossRef PubMed Google Scholar
-
7.Restrepo DA, Saenz E, Jara-Munoz OA, Calixto-Botia IF, Rodriguez-Suarez S, Zuleta P, Chavez BG, Sanchez JA, D'Auria JC. Erythroxylum in focus: an interdisciplinary review of an overlooked genus[J]. Molecules 24, 3788 (2019) CrossRef PubMed Google Scholar
-
8.Redman M. Cocaine: what is the crack? A brief history of the use of cocaine as an anesthetic[J]. Anesth Pain Med 1, 95-7 (2011) CrossRef PubMed Google Scholar
-
9.Li LS, Chiroma SM, Hashim T, Adam SK, Mohd Moklas MA, Yusuf Z, Rahman SA. Antioxidant and anti-inflammatory properties of Erythroxylum cuneatum alkaloid leaf extract[J]. Heliyon 6, e04141 (2020) CrossRef PubMed Google Scholar
-
10.De I, Barros C, Leite B, Leite C, Fagg C, Gomes S, Resck I, Fonseca Y, Magalhães P, Silveira D. Chemical composition and antioxidant activity of extracts from Erythroxylum suberosum A.St. Hil. Leaves[J]. J Appl Pharm Sci 7, 88-94 (2017) PubMed Google Scholar
-
11.Satoh M, Satoh Y, Fujimoto Y. Cytotoxic constituents from Erythroxylum catuaba isolation and cytotoxic activities of cinchonain[J]. Nat Med 54, 97-100 (2000) PubMed Google Scholar
-
12.Aguiar JS, Araujo RO, Rodrigues Mdo D, Sena KX, Batista AM, Guerra MM, Oliveira SL, Tavares JF, Silva MS, Nascimento SC, da Silva TG. Antimicrobial, antiproliferative and proapoptotic activities of extract, fractions and isolated compounds from the stem of Erythroxylum caatingae Plowman[J]. Int J Mol Sci 13, 4124-40 (2012) CrossRef PubMed Google Scholar
-
13.Barreiros ML, David JP, David JM, Lopes LMX, de Sa MS, Costa JFO, Almeida MZ, de Oueiroz LP, Sant'Ana AEG. Ryanodane diterpenes from two Erythroxylum species[J]. Phytochemistry 68, 1735-9 (2007) CrossRef PubMed Google Scholar
-
14.Coriolano de Oliveira E, Alves Soares Cruz R, de Mello Amorim N, Guerra Santos M, Carlos Simas Pereira Junior L, Flores Sanchez EO, Pinho Fernandes C, Garrett R, Machado Rocha L, Lopes Fuly A. Protective effect of the plant extracts of Erythroxylum sp. against toxic effects induced by the venom of Lachesis muta snake[J]. Molecules 21, 1350 (2016) CrossRef PubMed Google Scholar
-
15.Aynilian GH, Duke JA, Gentner WA, Farnsworth NR. Cocaine content of Erythroxylum species[J]. J Pharm Sci 63, 1938-9 (1974) CrossRef PubMed Google Scholar
-
16.Holmstedt B, Jaatmaa E, Leander K, Plowman T. Determination of cocaine in some South American species of Erythroxylum using mass fragmentography[J]. Phytochemistry 16, 1753-5 (1977) CrossRef PubMed Google Scholar
-
17.Bieri S, Brachet A, Veuthey JL, Christen P. Cocaine distribution in wild Erythroxylum species[J]. J Ethnopharmacol 103, 439-47 (2006) CrossRef PubMed Google Scholar
-
18.Rodrigues GA, Souza WC, Godinho MGC, Ferreira HD, Vila Verde GM. Determinação de parâmetros farmacognósticos para as folhas de Erythroxylum suberosum A. St. -hilaire (Erythroxylaceae) coletadas no município de Goiânia, GO[J]. Rev Bras de Plantas Med 17, 1169-76 (2015) CrossRef PubMed Google Scholar
-
19.Johnson EL, Foy CD. Biomass accumulation and alkaloid content in leaves of Erythroxylum coca and Erythroxylum novogranatense var. novogranatense grown in soil with varying pH[J]. J Plant Physiol 149, 444-50 (1996) CrossRef PubMed Google Scholar
-
20.Ansell SM, Pegel KH, Taylor DAH. Diterpenes from the timber of Erythroxylum pictum[J]. Phytochemistry 32, 945-52 (1993) CrossRef PubMed Google Scholar
-
21.Connolly JD, McCrindle R, Murray RDH, Overton KH. Constituents of Erythroxylon monogynum Roxb. II. Erythroxydiols X and Y-2 novel skeletal types of diterpenoids[J]. Tetrahedron Lett 5, 1859-66 (1964) CrossRef PubMed Google Scholar
-
22.Connolly JD, McCrindle R, Murray RDH, Renfrew AJ, Overton KH, Melera A. Constituents of Erythroxylon monogynum Roxb. II. Erythroxydiols X, Y and Z-2 novel skeletal types of diterpenoids[J]. J Chem Soc C, 268-273 (1966) CrossRef PubMed Google Scholar
-
23.Connolly JD, Gunn DM, McCrindle R, Murray RDH, Overton KH. Constituents of Erythroxylon monogynum Roxb. III. Erythroxytriols P and Q[J]. Tetrahedron Lett, 2109-2113 (1966) PubMed Google Scholar
-
24.J.D. Connolly, A.E. Harding. Constituents of Erythroxylon species. VII. Diterpenoids from Erythroxylon australe[J]. J Chem Soc-Perkin Trans 1, 1996-2000 (1972) PubMed Google Scholar
-
25.Kapadi AH, Dev S. The diterpenoids of Erythroxylon monogynum. I Monogynol[J]. Tetrahedron Lett 5, 1171-80 (1964) CrossRef PubMed Google Scholar
-
26.Kapadi AH, Dev S. The diterpenoids of Erythroxylon monogynum. III. Futher constituents, the absolute stereochemistry of monogynol, and hydroxymonogynol[J]. Tetrahedron Lett 5, 2751-7 (1964) CrossRef PubMed Google Scholar
-
27.Kapadi AH, Sobti RR, Dev S. Diterpenoids of Erythroxylon monogynum. V. Atisirene isoatisirene and devadarene[J]. Tetrahedron Lett 6, 2729-35 (1965) CrossRef PubMed Google Scholar
-
28.Nakatani N, Kikuzaki H, Yamaji H, Yoshio K, Kitora C, Okada K, Padolina WG. Labdane diterpenes from rhizomes of Hedychium coronarium[J]. Phytochemistry 37, 1383-8 (1994) CrossRef PubMed Google Scholar
-
29.Tasdemir D, Sticher O, Çalis I, Linden A. Further labdane diterpenoids isolated from Leonurus persicus[J]. J Nat Prod 60, 874-9 (1997) CrossRef PubMed Google Scholar
-
30.Chimnoi N, Pisutjaroenpong S, Ngiwsara L, Dechtrirut D, Chokchaichamnankit D, Khunnawutmanotham N, Mahidol C, Techasakul S. Labdane diterpenes from the rhizomes of Hedychium coronarium[J]. Nat Prod Res 22, 1249-56 (2008) CrossRef PubMed Google Scholar
-
31.Ansell SM, Pegel KH, Taylor DAH. Diterpenes from the timber of 20 Erythroxylum species[J]. Phytochemistry 32, 953-9 (1993) CrossRef PubMed Google Scholar
-
32.Ribeiro EMDO, David JP, David JM, Guedes MLS, Lopes LMX, Krskova Z, Dusek J. Ent-labdane and beyerane diterpenes from Erythroxylum betulaceum Mart[J]. Biochem Syst Ecol 50, 90-2 (2013) CrossRef PubMed Google Scholar
-
33.do Nascimento CJ, PovoaViolante IM, Garcez WS, Pott A, Garcez FR. Biologically active abietane and ent-kaurane diterpenoids and other constituents from Erythroxylum suberosum[J]. Phytochem Lett 5, 401-6 (2012) CrossRef PubMed Google Scholar
-
34.Kitahara Y, Yoshikoshi A. The structure of dolabradiene[J]. Tetrahedron Lett 5, 1755-61 (1964) CrossRef PubMed Google Scholar
-
35.Santos CCD, Lima MAS, Braz Filho R, Silveira ER. Diterpenes from Erythroxylum barbatum[J]. J Braz Chem Soc 17, 1304-8 (2006) PubMed Google Scholar
-
36.Soman R, Dev S, Misra R, Pandey RC. The diterpenoids of Erythroxylon monogynum. IV. Allodevadarool, devadarool and hydroxydevadarool[J]. Tetrahedron Lett 5, 3767-73 (1964) CrossRef PubMed Google Scholar
-
37.McCrindle R, Martin A, Murray RDH. Constituents of Erythroxylon monogynum Roxb. I. (+)-hibaene [(+)-stachene], erythroxylol A (monogynol), erythroxylol B and erythroxydiol A[J]. J Chem Soc C, 2349-2354 (1968) CrossRef PubMed Google Scholar
-
38.Martin A, Murray RDH. Constituents of Erythroxylon monogynum Roxb. IV. 2 norditerpenoid tertiary alcohols and 3 diterpenoid epoxides[J]. J Chem Soc C, 2529-2533 (1968) CrossRef PubMed Google Scholar
-
39.Kapadi AH, Dev S. The diterpenoids of Erythroxylon monogynum. III. Futher constituents, the absolute stereochemistry of monogynol and hydroxymonogynol[J]. Tetrahedron Lett, 2751-2757 (1964) PubMed Google Scholar
-
40.Tennakoon T, Gunaherath G, De Silva KTD, Padumadasa C, Wijesundara DSA, Abeysekera AM. Auto-oxidation of ent-beyer-15-en-19-al isolated from the essential oil of the heartwood of Erythroxylum monogynum Roxb.: formation of 15, 16-epoxy-ent-beyeran-19-oic acid and other products[J]. BMC Chem 14, 18 (2020) CrossRef PubMed Google Scholar
-
41.dos Santos CC, Lima MAS, Braz Filho R, de Simone CA, Silveira ER. NMR assignments and X-ray diffraction spectra for two unusual kaurene diterpenes from Erythroxylum barbatum[J]. Magn Reson Chem 43, 1012-5 (2005) CrossRef PubMed Google Scholar
-
42.Soman R, Dev S. The diterpenoids of Erythroxylon monogynum. II. Devadarool, a new type in tetracyclic diterpenoids[J]. Tetrahedron Lett 5, 1181-5 (1964) PubMed Google Scholar
-
43.Ferguson G, Fulke JWB, McCrindle R. The crystal structural and stereochemistry of triol Q from Erythroxylon monogynum Roxb[J]. Chemical Commun (London), 691-692 (1966) PubMed Google Scholar
-
44.Barreiros ML, David JM, de Queiroz LP, David JP. Flavonoids and triterpenes from leaves of Erythroxylum nummularia[J]. Biochem Syst Ecol 33, 537-40 (2005) CrossRef PubMed Google Scholar
-
45.Chavez JP, DosSantos ID, Cruz FG, David JM. Flavonoids and triterpene ester derivatives from Erythroxylum leal costae[J]. Phytochemistry 41, 941-3 (1996) CrossRef PubMed Google Scholar
-
46.Ribeiro EMDO, Lima LS, David JM, do Vale AE, Lopes LMX, David JP. A new tropane alkaloid and other constituents of Erythroxylum rimosum (Erythroxylaceae)[J]. Phytochem Lett 6, 232-5 (2013) CrossRef PubMed Google Scholar
-
47.Barreiros ML, David JM, Pereira PAD, Guedes MLS, David JP. Fatty acid esters of triterpenes from Erythroxylum passerinum[J]. J Braz Chem Soc 13, 669-73 (2002) CrossRef PubMed Google Scholar
-
48.Elias ST, Macedo CCS, Simeoni LA, Silveira D, Magalhaes PO, Lofrano-Porto A, Coletta RD, Neves FAR, Guerra ENS. Cytotoxic effect of Erythroxylum daphnites extract is associated with G1 cell cycle arrest and apoptosis in oral squamous cell carcinoma[J]. Cell Cycle 15, 948-56 (2016) CrossRef PubMed Google Scholar
-
49.da Silva Junior LJC, Alves LA, Silva VR, Santos LS, Bezerra DP, Soares MBP, Doriguetto AC, Barbosa LCA, do Nascimento JC, Macedo GEL, Queiroz RF, de Paula VF. A new tropane alkaloid and other metabolites from Erythroxylum macrocalyx (Erythroxylaceae) and their antiproliferative activities[J]. Phytochem Lett 41, 168-74 (2021) CrossRef PubMed Google Scholar
-
50.Oliveira AC, Sena-Filho JG, Mendes-Junior LG, Anjos RM, Ribeiro TP, Barbosa-Filho JM, Braga VA, Medeiros IA. Erythroxylum pungens elicits vasorelaxation by reducing intracellular calcium concentration in vascular smooth muscle cells of rats[J]. Rev Bras Farmacogn-Braz J Pharmacogn 22, 436-42 (2012) CrossRef PubMed Google Scholar
-
51.Drake LR, Scott PJH. Dark classics in chemical neuroscience: cocaine[J]. ACS Chem Neurosci 9, 2358-72 (2018) CrossRef PubMed Google Scholar
-
52.Sena-Filho JG, da Silva MS, Tavares JF, Oliveira SL, Romero MAV, Xavier HS, Barbosa Filho JM, Braz Filho R. Cytotoxic evaluation of pungencine: a new tropane alkaloid from the roots of Erythroxylum pungens O. E. Schulz[J]. Helv Chim Acta 93, 1742-4 (2010) CrossRef PubMed Google Scholar
-
53.de Oliveira SL, Tavares JF, Castello Branco MVS, Lucena HFS, Barbosa-Filho JM, Agra MDF, do Nascimento SC, Aguiar JDS, da Silva TG, de Simone CA, de Araujo-Junior JX, da Silva MS. Tropane alkaloids from Erythroxylum caatingae Plowman[J]. Chem Biodivers 8, 155-65 (2011) CrossRef PubMed Google Scholar
-
54.Soares Cruz RA, Almeida H, Fernandes CP, Joseph-Nathan P, Rocha L, Leitao GG. A new tropane alkaloid from the leaves of Erythroxylum subsessile isolated by pH-zone-refining counter-current chromatography[J]. J Sep Sci 39, 1273-7 (2016) CrossRef PubMed Google Scholar
-
55.Brito LSDO, Pinto FDCL, de Filho MOM, Rocha DD, Mendoza MFM, Ayala AP, Bezerra BP, Loiola MIB, Canuto KM, Silveira ER, Pessoa ODL. Tropane alkaloids from the stem bark of Erythroxylum bezerrae[J]. Phytochemistry 178, 112458 (2020) CrossRef PubMed Google Scholar
-
56.Brachet A, Munoz O, Gupta M, Veuthey JL, Christen P. Alkaloids of Erythroxylum lucidum stem-bark[J]. Phytochemistry 46, 1439-42 (1997) CrossRef PubMed Google Scholar
-
57.Christen P, Roberts MF, Phillipson JD, Evans WC. Alkaloids of the genus Erythroxylum.11. Alkaloids of Erythroxylum zambesiacum stem-bark[J]. Phytochemistry 34, 1147-51 (1993) PubMed Google Scholar
-
58.Novak M, Salemink CA. The essential oil of Erythroxylum coca[J]. Planta Med 53, 113-113 (1987) CrossRef PubMed Google Scholar
-
59.Bohm BA, Loo T, Nicholls KW, Plowman T. Flavonoid variation in Erythroxylum[J]. Phytochemistry 27, 833-7 (1988) CrossRef PubMed Google Scholar
-
60.Johnson EL, Schmidt WF, Norman HA. Leaf flavonoids as chemotaxonomic markers for two Erythroxylum taxa[J]. Z Naturforsch (C) 52, 577-85 (1997) PubMed Google Scholar
-
61.Johnson EL, Schmidt WL, Norman HA. Leaf flavonoids as chemotaxonomic markers for two Erythroxylum taxa[J]. Am J Bot 84, 157-157 (1997) PubMed Google Scholar
-
62.Johnson EL, Schmidt WF, Norman HA. Flavonoids as markers for Erythroxylum taxa: E. coca var. ipadu and E. novogranatense var truxillense[J]. Biochem Syst Ecol 26, 743-59 (1998) PubMed Google Scholar
-
63.Johnson EL, Schmidt WF. Flavonoids as chemotaxonomic markers for Erythroxylum ulei[J]. Z Naturforsch (C) 54, 881-8 (1999) PubMed Google Scholar
-
64.Johnson EL, Schmidt WF, Cooper D. Flavonoids as chemotaxonomic markers for cultivated Amazonian coca[J]. Plant Physiol Biochem 40, 89-95 (2002) CrossRef PubMed Google Scholar
-
65.Johnson EL, Schmidt WF. Flavonoids as chemotaxonomic markers for Erythroxylum australe[J]. Z Naturforsch (C) 59, 769-76 (2004) PubMed Google Scholar
-
66.Cordova WHP, Matos MG, Tabart J, Sipel A, Kevers C, Dommes J. In vitro characterization of antioxidant properties of cuban endemic varieties of Erythroxylum alaternifolium. A. Rich isolation of two flavonol glycosides[J]. J Chil Chem Soc 57, 1340-3 (2012) PubMed Google Scholar
-
67.Iñigo RPA, Pomilio AB. Flavonoids from Erythroxylon argentinum[J]. Phytochemistry 24, 347-9 (1985) CrossRef PubMed Google Scholar
-
68.Guldbrandsen N, De Mieri M, Gupta M, Seiser T, Wiebe C, Dickhaut J, Reingruber R, Sorgenfrei O, Hamburger M. Screening of Panamanian plant extracts for pesticidal properties and HPLC-based identification of active compounds[J]. Sci Pharm 83, 353-67 (2015) CrossRef PubMed Google Scholar
-
69.Bonefeld M, Friedrich H, Kolodziej H. (+)-catechin 3-rhamnoside from Erythroxylum novogranatense[J]. Phytochemistry 25, 1205-7 (1986) CrossRef PubMed Google Scholar
-
70.de Albuquerque CH, Tavares JF, de Oliveira SL, Silva TS, Goncalves GF, de Oliveira Costa VC, Agra MDF, Freire Pessoa HDL, da Silva MS. Flavonoid glycosides from Erythroxylum pulchrum A. St. -hil. (Erythroxylaceae)[J]. Quim Nova 37, 663-128 (2014) PubMed Google Scholar
-
71.Inigo RPA, Deiglesias DIA, Catalan CAN. Kaempferol 3-α-D-glucopyranoside-7-α-L-rhamnopyranoside from Erythroxylon cuneifolium[J]. Phytochemistry 27, 1230-1 (1988) CrossRef PubMed Google Scholar
-
72.Kanchanapoom T, Noiarsa P, Tiengtham P, Otsuka H, Ruchirawat S. Acetophenone diglycosides from Erythroxylum cambodianum[J]. Chem Pharm Bull 53, 579-81 (2005) CrossRef PubMed Google Scholar
-
73.Bohm BA, Phillips DW, Ganders FR. Flavonoids of Erythroxylum rufum and Erythroxylum ulei[J]. J Nat Prod 44, 676-9 (1981) CrossRef PubMed Google Scholar
-
74.Kanchanapoom T, Sirikatitham A, Otsuka H, Ruchirawat S. Cuneatoside, a new megastigmane diglycoside from Erythroxylum cuneatum Blume[J]. J Asian Nat Prod Res 8, 747-51 (2006) CrossRef PubMed Google Scholar
-
75.Gurib-Fakim A, Marie D, Narod F. The pharmacological properties of the isolated bioactive compounds from endemic medicinal plants of Mauritius. Paper Presented at the 3rd World Congress on Medicinal and Aromatic Plants (WOCMAP-III), Chiang Mai, Thailand, Feb 03–07, 2003. PubMed Google Scholar
-
76.dos Santos CC, Lima MAS, Silveira ER. Micromolecular secondary metabolites of Erythroxylum barbatum[J]. Biochem Syst Ecol 31, 661-4 (2003) CrossRef PubMed Google Scholar
-
77.Bindu G, Suripeddi RK. Development and validation of HPTLC method for identification and quantification of sterols from leaves of Erythroxylum monogynum Roxb. and in vitro evaluation of anti-oxidant and anti-glycation activities[J]. S Afr J Bot 137, 24-34 (2021) PubMed Google Scholar
-
78.Menezes-Filho NDJ, Souza CDS, Costa TCS, Da Silva VDA, Ribeiro CSDO, Barreiros ML, Costa JFO, David JM, David JPL, Costa SL. Cytotoxicity of the diterpene 14-O-methyl-ryanodanol from Erythroxylum passerinum in an astrocytic cells model[J]. Nat Prod Commun 9, 1245-8 (2014) PubMed Google Scholar
-
79.Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview[J]. Sci World J 2013, 162750 (2013) PubMed Google Scholar
-
80.Bauer I. Travel medicine, coca and cocaine: demystifying and rehabilitating Erythroxylum—a comprehensive review[J]. Trop Dis Travel Med Vaccines 5, 20 (2019) CrossRef PubMed Google Scholar
-
81.Nassar P, Ouanounou A. Cocaine and methamphetamine: pharmacology and dental implications[J]. Can J Dental HygiCJDH 54, 75-82 (2020) PubMed Google Scholar
-
82.Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects[J]. J Neurosci 26, 11522-31 (2006) CrossRef PubMed Google Scholar
-
83.Graziani M, Sarti P, Arese M, Magnifico MC, Badiani A, Saso L. Cardiovascular mitochondrial dysfunction induced by cocaine: biomarkers and possible beneficial effects of modulators of oxidative stress[J]. Oxidative Med Cell Longev 2017, 3034245 (2017) PubMed Google Scholar
-
84.Yu C, Zhou X, Fu Q, Peng Q, Oh KW, Hu Z. A new insight into the role of CART in cocaine reward: involvement of CaMKII and inhibitory G-protein coupled receptor signaling[J]. Front Cell Neurosci 11, 244 (2017) PubMed Google Scholar
-
85.Levine BS, Kerrigan S. Principles of forensic toxicology. 2020. https://doi.org/10.1093/jat/bkab020 PubMed Google Scholar
-
86.Yu DW, Gatley SJ, Wolf AP, MacGregor RR, Dewey SL, Fowler JS, Schlyer DJ. Synthesis of carbon-11 labeled iodinated cocaine derivatives and their distribution in baboon brain measured using positron emission tomography[J]. J Med Chem 35, 2178-83 (1992) CrossRef PubMed Google Scholar
-
87.Dinis-Oliveira RJ. Metabolomics of cocaine: implications in toxicity[J]. Toxicol Mech Methods 25, 494-500 (2015) PubMed Google Scholar
-
88.Glauser J, Queen JR. An overview of non-cardiac cocaine toxicity[J]. J Emerg Med 32, 181-6 (2007) CrossRef PubMed Google Scholar
-
89.Nathanson JA, Hunnicutt EJ, Kantham L, Scavone C. Cocaine as a naturally occurring insecticide[J]. Proc Natl Acad Sci USA 90, 9645-8 (1993) CrossRef PubMed Google Scholar
-
90.Chin YW, Kinghorn AD, Patil PN. Evaluation of the cholinergic and adrenergic effects of two tropane alkaloids from Erythroxylum pervillei[J]. Phytother Res 21, 1002-5 (2007) CrossRef PubMed Google Scholar
-
91.Araujo Neto JF, de Ribeiro OREM, do Vale AE, David JM, David JP. Cytotoxic activity of tropane alkaloides of species of Erythroxylum[J]. Mini-Rev Med Chem 21, 2458-80 (2021) CrossRef PubMed Google Scholar
-
92.Alsaid MS, Evans WC, Grout RJ. Alkaloids of the genus Erythroxylum. 6. Alkaloids of Erythroxylum macrocarpum and Erythroxylum sideroxyloides[J]. Phytochemistry 25, 851-3 (1986) CrossRef PubMed Google Scholar
-
93.Mahomoodally MF, Fakim A-G, Subratty AH. Effects of Erythroxylum macrocarpum (Erythroxylaceae), an endemic medicinal plant of Mauritius, on the transport of monosaccharide, amino acid and fluid across rat everted intestinal sacs in vitro[J]. J Cell Mol Biol 4, 93-8 (2005) PubMed Google Scholar
-
94.Novak M, Salemink CA, Khan I. Biological activity of the alkaloids of Erythroxylum coca and Erythroxylum novogranatense[J]. J Ethnopharmacol 10, 261-74 (1984) CrossRef PubMed Google Scholar
-
95.Kumar CD, Anitha S, Varalakshmi P, Rao DM. Evaluation of potential phytochemicals and phyto pharmacological activities of Erythroxylum monogynum Roxb[J]. Biosci Biotechnol Res Asia 16, 441-9 (2019) CrossRef PubMed Google Scholar
-
96.Wet HD. Antibacterial activity of the five South African Erythroxylaceae species[J]. Afr J Biotechnol 10, 11511-4 (2011) PubMed Google Scholar
-
97.Santos KC, Monte APO, Lima JT, Ribeiro LAA, Palheta Junior RC. Role of NO-cGMP pathway in ovine cervical relaxation induced by Erythroxylum caatingae Plowman[J]. Anim Reprod Sci 164, 23-30 (2016) CrossRef PubMed Google Scholar
-
98.Ramhit P, Ragoo L, Bahorun T, Neergheen-Bhujun VS. Multi-targeted effects of untapped resources from the Mauritian endemic flora[J]. S Afr J Bot 115, 208-16 (2018) CrossRef PubMed Google Scholar
-
99.Gonzalez-Guevara JL, Gonzalez-Lavaut JA, Pino-Rodriguez S, Garcia-Torres M, Carballo-Gonzalez MT, Echemendia-Arana OA, Molina-Torres J, Prieto-Gonzalez S. Phytochemical screening and in vitro antiherpetic activity of four Erythroxylum species[J]. Acta Farm Bonaerense 23, 506-9 (2004) PubMed Google Scholar
-
100.Rodeiro I, Donato MT, Martinez I, Hernandez I, Garrido G, Gonzalez-Lavaut JA, Menendez R, Laguna A, Castell JV, Gomez-Lechon MJ. Potential hepatoprotective effects of new Cuban natural products in rat hepatocytes culture[J]. Toxicol In Vitro 22, 1242-9 (2008) CrossRef PubMed Google Scholar
-
101.Syed SH, Namdeo AG. Hepatoprotective effect of leaves of Erythroxylum monogynum Roxb. on paracetamol induced toxicity[J]. Asian Pac Trop Biomed 3, 877-81 (2013) CrossRef PubMed Google Scholar
-
102.Kalayou S, Haileselassie M, Gebre-egziabher G, Tiku'e T, Sahle S, Taddele H, Ghezu M. In-vitro antimicrobial activity screening of some ethnoveterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray Region, Ethiopia[J]. Asian Pac Trop Biomed 2, 516-22 (2012) CrossRef PubMed Google Scholar
-
103.Santos ASS, Silva MVR, Oliveira SL, Tavares JF, Araújo LA, Lima JT. Avaliação da atividade relaxante do extrato etanólico bruto obtido de Erythroxylum caatingae, Erythroxylum subrotundum e Erythroxylum revolutum (Erythroxylaceae) em traquéia isolada de cobaia[J]. Evol Sci 1, 33-119 (2013) PubMed Google Scholar
-
104.Kohnen-Johannsen KL, Kayser O. Tropane alkaloids: chemistry, pharmacology, biosynthesis and production[J]. Molecules 24, 796 (2019) CrossRef PubMed Google Scholar
-
105.Leete E. Biosynthesis of cocaine and cuscohygrine from [1-14C]-labeled acetate and [4-3H]-labeled phenylalanine in Erythroxylon coca[J]. Phytochemistry 22, 699-704 (1983) CrossRef PubMed Google Scholar
-
106.Bjorklund JA, Leete E. Biosynthesis of the benzoyl moiety of cocaine from cinnamic acid via (R)-(+)-3-hydroxy-3-phenylpropanoic acid[J]. Phytochemistry 31, 3883-7 (1992) CrossRef PubMed Google Scholar
-
107.Newquist ML, Abraham TW, Leete E. Biosynthetic incorporation of ethyl (RS) [2, 3–13C2, 3–14C]-4-(1-methyl-2-pyrrolidinyl)-3-oxobutanoate into cuscohygrine in Erythroxylum coca[J]. Phytochemistry 33, 1437-40 (1993) CrossRef PubMed Google Scholar
-
108.Docimo T, Reichelt M, Schneider B, Kai M, Kunert G, Gershenzon J, D'Auria JC. The first step in the biosynthesis of cocaine in Erythroxylum coca: the characterization of arginine and ornithine decarboxylases[J]. Plant MolBiol 78, 599-615 (2012) PubMed Google Scholar
-
109.Schmidt GW, Jirschitzka J, Porta T, Reichelt M, Luck K, Torre JC, Dolke F, Varesio E, Hopfgartner G, Gershenzon J, D'Auria JC. The last step in cocaine biosynthesis is catalyzed by a BAHD acyltransferase[J]. Plant Physiol 167, 89-101 (2015) CrossRef PubMed Google Scholar
-
110.Huang JP, Wang YJ, Tian T, Wang L, Yan YJ, Huang SX. Tropane alkaloid biosynthesis: a centennial review[J]. Nat Prod Rep 38, 1634-58 (2021) CrossRef PubMed Google Scholar
-
111.Graebe JE. Gibberellin biosynthesis and control[J]. Annu Rev Plant Physiol Plant Molec Biol 38, 419-65 (1987) CrossRef PubMed Google Scholar
-
112.Su P, Tong Y, Cheng Q, Hu Y, Zhang M, Yang J, Teng Z, Gao W, Huang L. Functional characterization of ent-copalyl diphosphate synthase, kaurene synthase and kaurene oxidase in the Salvia miltiorrhiza gibberellin biosynthetic pathway[J]. Sci Rep 6, 23057 (2016) CrossRef PubMed Google Scholar
-
113.Zhou K, Xu M, Tiernan M, Xie Q, Toyomasu T, Sugawara C, Oku M, Usui M, Mitsuhashi W, Chono M, Chandler PM, Peters RJ. Functional characterization of wheat ent-kaurene(-like) synthases indicates continuing evolution of labdane-related diterpenoid metabolism in the cereals[J]. Phytochemistry 84, 47-55 (2012) CrossRef PubMed Google Scholar
-
114.Tezuka D, Ito A, Mitsuhashi W, Toyomasu T, Imai R. The rice ent-kaurene synthase like 2 encodes a functional ent-beyerene synthase[J]. Biochem Biophys Res Commun 460, 766-71 (2015) CrossRef PubMed Google Scholar
Copyright information
© The Author(s) 2022
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.