| 超重力方向调节电沉积镍箔表面形貌和力学性能 |
功能性金属箔/薄膜已广泛应用于电催化、电池、防腐、电磁屏蔽等领域[1-10].金属箔/薄膜的物理化学性能(如机械性能、催化活性、耐腐蚀性等)取决于其表面结构[11-13].金属电沉积是制备金属箔/薄膜的常用方法.在电沉积过程中, 金属箔/薄膜的表面结构和性能通常通过改变电流密度、溶液组成和电流波形来调节[14-16].
近年来, 在金属箔/薄膜的电沉积过程中, 利用超声、磁场等外场来调节和改善金属箔/薄膜表面结构和理化性能, 受到越来越多的关注[17-20].发现磁场可以使电沉积晶粒细化, 择优取向发生改变[17-18].超声场中也观察到类似现象[19-20]. Cobley发现在超声场下电沉积的镍镀层硬度增加[19].镀层表面结构和性能的变化通常归因于外场对传质的强化作用.
超重力场是一种能够强化"三传一反"化工过程的外场环境, 具有远大于地球常规重力的重力加速度, 因此能够显著促进微观混合、强化传质和相间分离.超重力在电化学反应过程中, 有可能起到增强反应离子对流传质、促进电极/溶液界面微观扰动和相界面更新、加速气泡分离等作用, 从而强化电化学反应过程, 调节产物结构.近十年来, 超重力电化学技术越来越受到人们的关注[21-23].由于气泡与电极表面的快速分离, 超重力场可以对电解水/氯碱电解起到强化作用, 从而实现槽电压的降低和过程节能[24-25].由于超重力强化传质的作用, 金属电沉积速率也可明显增大[26-27].
超重力场电沉积镍基箔/薄膜已经得到了广泛的研究[7, 9, 21, 28-36].结果表明, 超重力场可以对金属箔/薄膜的形貌、晶体结构和化学成分进行大范围的调整, 如微-纳米晶粒、致密-粗糙表面和晶体-非晶结构等, 其力学性能、抗腐蚀性能和催化活性均得到了提高.邵等[35]在超重力场下也一步电沉积了镍/碳纳米管复合阴极, 对析氢反应具有良好的电催化活性.
众所周知, 重力和电场都具有方向性.在电沉积过程中, 由于反应离子的消耗, 阴极附近将产生浓度梯度.基于重力和电场方向, 将产生两种极端情况, 即重力方向分别与电场方向相同(图 1(a))和相反(图 1(b)).因此, 镀层的表面结构和性能也可能会发生变化.在之前的工作中, 仅在超重力与电场方向相同的情况下, 进行了电沉积金属箔的研究[29-31].文中在超重力与电场方向相同或相反的情况下分别电沉积镍箔, 并对其表面形貌和力学性能进行比较, 分析和讨论形貌和力学性能差异性的根源.研究结果将为优化和完善超重力场电沉积金属箔结构和性能, 进而获得高性能金属材料提供理论支撑.另一方面, 随着空间开发的快速发展, 在微重力环境下电沉积功能材料成为长远要求.然而, 在地球上, 微重力场是由自由落体、飞行器和人造卫星获得的[37], 实验成本高、时间短(< 8 s), 实验难度较大.因此, 通过离心机获得的超重力场, 并进行功能材料电沉积研究, 进而预测微重力下电沉积金属结构和性能, 具有重要的意义.
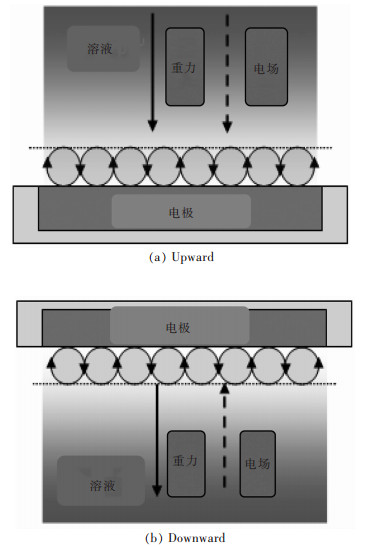 |
| 图 1 重力(G)与电场(E)方向相同(a)与重力(G)与电场(E)方向的方向相反(b)示意 Fig. 1 The illustration for the same (a) and opposite direction (b) between gravity (G) and electric field (E) |
1 实验 1.1 设备
通过离心机获得超重力场(图 2(a)).电解池在超重力场下为水平(即旋转), 在常重力条件下为垂直(即不旋转).重力系数(G)定义为实际重力加速度与地球重力加速度(9.8 m/s2)之比.通过调节转速来改变重力系数, 计算如下:
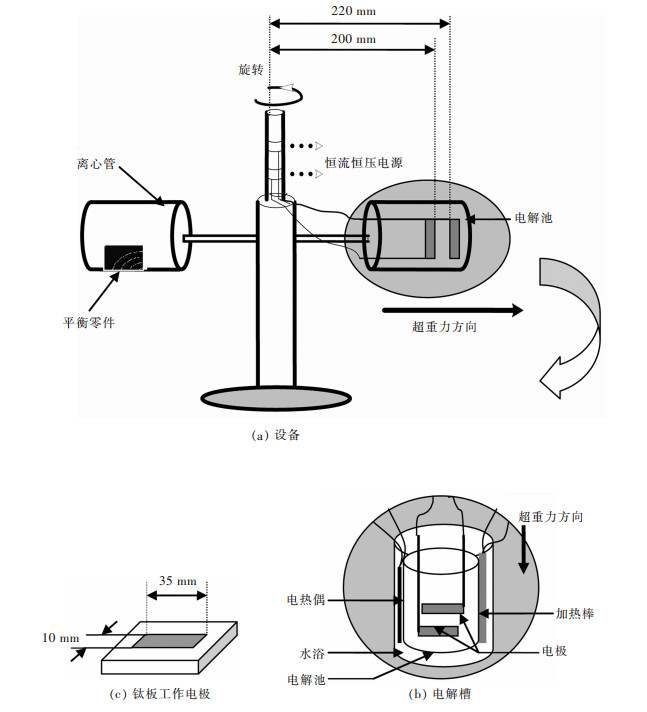 |
| 图 2 超重力场下镍电沉积的结构示意 Fig. 2 The schematic configuration for Ni electrodeposition under super gravity field. (a) Equipment |
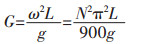
|
(1) |
式(1)中N为转速(r/mim), g为地球重力加速度(9.8 m/s2), L为电极中心到轴的距离, 本实验为0.22 m.常重力条件下G值为1.电解池示意图如图 2(b)所示.镍电沉积溶液采用水浴加热.阴极为纯钛, 阳极为可溶镍, 纯度99 %.电极面积3.5 cm2 (图 2(c)).阳极和阴极之间的距离是2 cm.
1.2 镍箔电沉积镍电沉积溶液为300 g/L NiSO4·6H2O、30 g/L NiCl2、40 g/L H3BO4、0.5 g/L NaC12H25SO4和0.25 g/L糖精钠.用H2SO4调节pH至3.0. Ni电沉积电流密度为0.1 A/cm2和电沉积时间为60 min.电沉积温度为65 ℃.常重力条件下电沉积Ni电极称为A电极.在超重力场下, 当超重力方向与电场方向相同(图 1(a))和相反(图 1(b))时, 电沉积Ni箔分别为电极B和电极C.所有的化学试剂都是分析纯.
1.3 镍箔表征电沉积后, 从Ti基体上剥离镍箔.通过SEM (JEOL, JSM6700F)和AFM (Di MultiMode)表征镍箔表面形貌.采用原子力显微镜检测镍箔表面粗糙度量.采用电子万能试验机(WDW3020)和微硬度计(nMT-3)分别测试了镍箔的拉伸应力和硬度.
2 结果与讨论 2.1 镍箔电沉积当重力和电场方向相反时(图 1(b)), 常重力条件下(G=1)进行电沉积Ni, 发现2 min后可获得Ni薄膜, 此时Ni(OH)2层覆盖整个电极表面, 抑制了镍进一步电沉积.在镍电沉积过程中, 很难避免析氢副反应.氢气泡的运动是由与重力方向相反的浮力所驱动的.因此, 阴极表面被氢气泡覆盖.由于浓差极化以及电极与Ni2+离子被气泡隔离, 镍难以进一步电沉积.随着阴极附近pH值的快速升高, 析氢反应加剧, Ni2+水解为Ni(OH)2.
在超重力场下, 如图 1(b)(即电极C), 可以成功电沉积镍箔, 但有一个角(图 3)除外, 这是由于惯性的影响, 气泡沿电极表面移动, 在电极边缘快速分离.因此, 有效地降低了气泡覆盖率, 使镍电沉积得以持续进行.然而, 边缘气泡的分离阻碍了Ni2+离子的电化学还原, 因此在该位置没有电沉积获得镍箔.
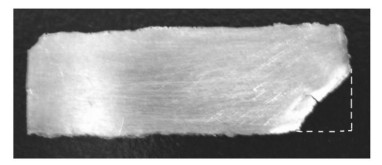 |
| 图 3 在C电极上电镀镍箔 Fig. 3 Ni foil electrodeposited on Electrode C |
用扫描电镜对镍箔表面形貌进行表征, 结果如图 4所示.在A电极上, 镍箔由较大的晶粒组成, 表面粗糙(图 4(a)).超重力场作用下, 在超重力场与电场方向相同的B电极上, Ni箔晶粒细化, 表面变得非常平整致密(图 4(b)).在超重力与电场方向相反的电极C上, 可观察到表面形貌也有类似的改善(图 4(c)).与常规重力相比, 引入超重力场, 将在电极/溶液界面营造微观对流单元(如图 1), 因而可以改善金属离子传质和电流在电极表面的均匀分布, 从而促进金属镍均匀形核, 即晶核数量增大, 因此晶粒细化.
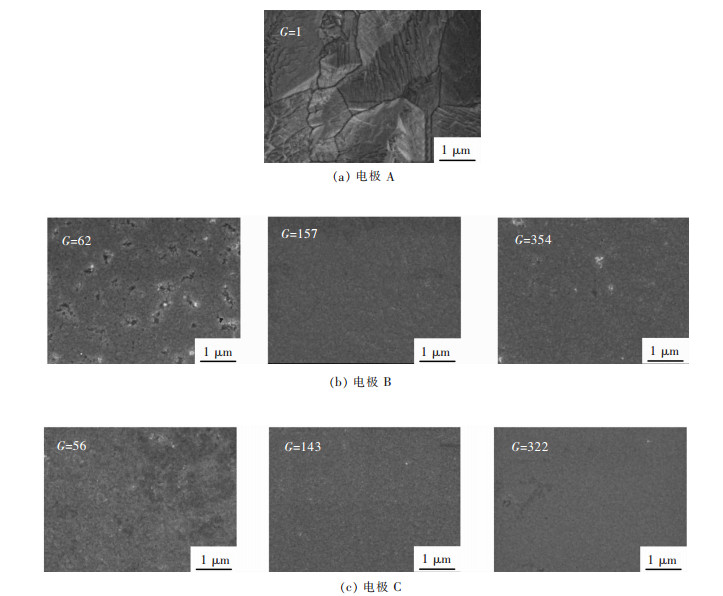 |
| 图 4 电沉积Ni箔的SEM像 Fig. 4 The SEM images of electrodeposited Ni foils |
用AFM进一步检测了镍箔的表面形貌(图 5), 并对其粗糙度进行了评价(图 6).可以发现A电极上沉积的镍箔不均匀, 出现了较大的波动(图 5(a)).这意味着Ni晶粒的不均匀生长.而在B和C电极上, 波动得到有效抑制, 表面趋于均匀(图 5(b)和图 5(c)).另外, C电极的表面比B电极更平整致密.
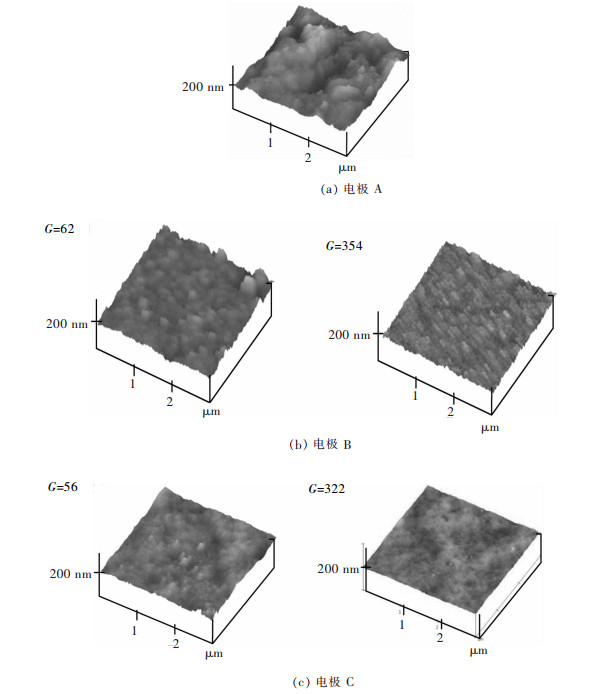 |
| 图 5 电沉积Ni箔的AFM图像 Fig. 5 The AFM images of electrodeposited Ni foils |
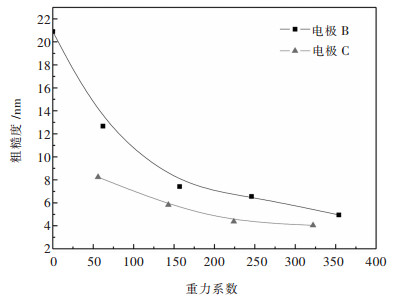
图 6所示为AFM图像中沿直线的高度分布.对于在常重力条件下电沉积的镍箔, 线呈现出明显的波动, 最大高度差可达117 nm(图 6(a)).而相似重力系数(G)下, B电极和C电极的高度差分别为25 nm和20 nm.表明超重力可以均匀化电沉积镍箔表面.其中, C电极表面较光滑.
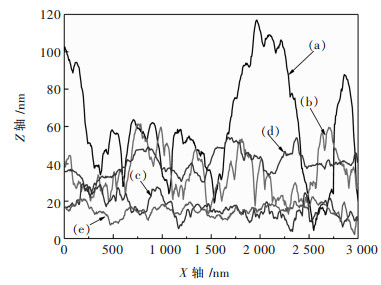 |
| A电极(a); 电极B上G=65(b)和G=354(c); C电极上G=56(d)和G=322(e) (a) On Electrode A; (b) G=65 and (c) G=354 on Electrode B; (d) G=56 and (e) G=322 on Electrode C 图 6 晶粒高度分布剖面 Fig. 6 The profile of height distribution of grains |
根据式(2)[28]计算出图 6中基于高度的均方根粗糙度(RMS).
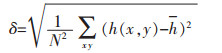
|
(2) |
其中, N2为图像的像素数, h(x, y)为每个点的高度, 为平均高度.镍箔的表面粗糙度扫描面积3×3 μm2, 如图 7所示.在常重力条件下, 粗糙度可达20.9 nm.粗糙度随G值的增大而减小. C电极的粗糙度小于B电极. G值为322时, C电极的粗糙度仅为4.04 nm.
 |
| 图 7 电沉积镍箔表面粗糙度 Fig. 7 The surface roughness of electrodeposited Ni foils |
众所周知, 电结晶过程中晶粒的大小是由成核和生长决定的.当晶核的形成速率大于生长速率时, 晶粒细化.相反, 颗粒粗大.通常成核时间TN(即诱导周期)约为1 ms[29].然而, 消除反应离子浓度差的微混合时间为Tm, 大约是5 ~ 50 ms[29]. Tm比TN大很多.也就是说, 成核是在非均匀溶液中进行的.因此, Ni晶粒的成核和生长也是不均匀的, 导致晶粒较大, 表面粗糙(图 4(a)和图 5(a)).在超重力场作用下, 电极表面强化微混合加速了Ni2+的传质.据估计, Tm下降到约0.04~0.4 ms[28].这个值小于TN.晶粒的成核和生长可以在均匀溶液中进行.因此, 在超重力场下电沉积的镍箔颗粒细小均匀, 表面光滑致密.
另一方面, 在垂直超重力场下(图 1), 形成了许多对流单元[30], 也抑制了树枝状突起的产生.因此, 镍箔的表面比常重力条件下更平坦.在电极B上, 超重力方向与离子传质方向相同, 而在电极C上, 两者方向相反.因此, 相比于电极B, 金属离子向电极C表面的传质速率较慢, 导致更大的浓差极化和反应过电位, 有利于形核, 不利于长大.因此, 电极C上获得的镍箔晶粒更加细小.
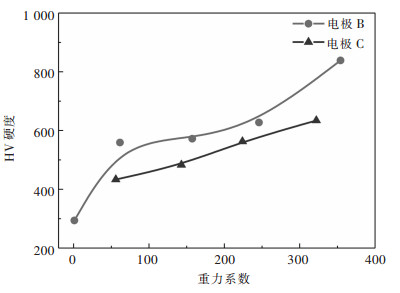
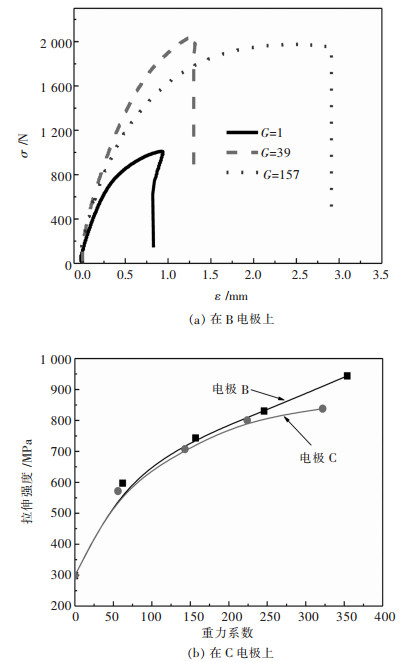
2.2 镍箔力学性能测试了镍箔的硬度和拉伸强度.由图 8可知, 在常重力条件下电沉积的镍箔HV硬度仅为294.在B电极和C电极上, 硬度均随着G值的增加而增加.例如, 在B电极上, 当G值为354时, HV硬度可达839.同样, 随着G值的增大, 拉伸强度也增大(图 9).在B电极上G值为354时, 抗拉强度增加到944 MPa.
 |
| 图 8 镍箔硬度 Fig. 8 The hardness of Ni foils |
 |
| 图 9 镍箔的应力-应变曲线(a)和拉伸强度(b) Fig. 9 The stress-strain curve (a) and the tensile strength (b) of Ni foils |
金属箔的硬度和拉伸强度与晶粒尺寸和内应力有关.金属的抗拉强度和硬度通常可用Hall-Petch方程[36]表示:
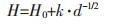
|
(3) |
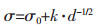
|
(4) |
H和σ分别是金属的硬度和抗拉强度, d是平均颗粒直径, σ0、H0和k是物质本身的常数.通过降低镍箔的晶粒尺寸, 可以提高镍箔的抗拉强度和硬度.根据图 4和图 5的结果, 晶粒随重力系数的增大而减小.因此, 拉伸强度和硬度的提高应归因于晶粒的细化.
此外, 从图 8和图 9中还可以发现, B电极电沉积镍箔的硬度和抗拉强度均大于C电极上电沉积镍箔的硬度和抗拉强度.浮力的方向与超重力相反.气泡在C电极上的停留时间比B电极上的长, 氢更容易被C电极上的镍箔吸收, 导致镍箔内应力增大, 硬度和抗拉强度降低.
3 结论1) 垂直超重力场作用下电沉积镍箔表面致密, 晶粒细小, 硬度和抗拉强度均明显高于常重力条件下的镍箔;
2) 镍箔表面粗糙度和晶粒尺寸随重力系数的增大而减小.在C电极上电沉积镍箔粗糙度和晶粒尺寸略低于B电极上的镍箔;
3) 在超重力场与电场方向相同时, 电沉积镍箔具有较好的力学性能, 这可能与镍箔内部低的内应力有关.
| [1] |
刘柏雄, 钟素文. 电沉积法制备泡沫镍的研究[J].
有色金属科学与工程, 2011, 2(3): 28–31.
|
| [2] |
张荣伟, 孙军伟, 李升燕, 等. 锰元素对铜镍合金电化学性能的影响[J].
有色金属科学与工程, 2018, 9(4): 60–65.
|
| [3] |
陈敏, 肖玄, 汤爱涛. 钛精矿制备Fe-TiCN金属陶瓷的研究[J].
有色金属科学与工程, 2015, 6(5): 70–72.
|
| [4] |
宋高阳, 宋波, 杨玉厚, 等. 利用超重力分离5052铝合金熔体中的非金属夹杂[J].
有色金属科学与工程, 2015, 6(1): 29–34.
|
| [5] |
高启瑞, 宋波, 杨占兵, 等. 含钛高炉渣碳化及超重力分离碳化钛的研究[J].
有色金属科学与工程, 2017, 8(2): 1–7.
|
| [6] |
PLOWMAN B J, JONES L A, BHARGAVA S K. Building with bubbles: the formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition[J].
Chemical Communication, 2015, 51: 4331–4346. DOI: 10.1039/C4CC06638C. |
| [7] |
WANG M Y, WANG Z, GUO Z C. Electrodeposited free-crack niw films under super gravity filed: structure and excellent corrosion property[J].
Materials Chemistry and Physics, 2014, 148: 245–252. DOI: 10.1016/j.matchemphys.2014.07.041. |
| [8] |
NIU X H, LAN M B, ZHAO H L, et al. Highly sensitive and selective nonenzymatic detection of glucose using three-dimensional porous nickel nanostructures[J].
Analytical Chemistry, 2013, 85: 3561–3569. DOI: 10.1021/ac3030976. |
| [9] |
LIU T, GUO Z C, WANG Z, et al. Structure and mechanical properties of iron foil electrodeposited in super gravity field[J].
Surface and Coatings Technology, 2010, 204: 3135–3140. DOI: 10.1016/j.surfcoat.2010.02.060. |
| [10] |
NIA N S, CREUS J, FEAUGAS X, et al. Influence of metallurgical parameters on the electrochemical behavior of electrodeposited ni and ni-w nanocrystalline alloys[J].
Applied Surface Science, 2016, 370: 149–159. DOI: 10.1016/j.apsusc.2016.02.101. |
| [11] |
GAO S, LIN Y, JIAO X C, et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel[J].
Nature, 2016, 529: 68–71. DOI: 10.1038/nature16455. |
| [12] |
YU X T, WANG M Y, WANG Z, et al. The structure evolution mechanism of electrodeposited porous Ni films on Ni4Cl concentration[J].
Applied Surface Science, 2016, 360: 502–509. DOI: 10.1016/j.apsusc.2015.10.174. |
| [13] |
QIAN X, HANG T, LI M, et al. Decoration of micro-nanoscale noble metal particles on 3d porous nickel using electrodeposition technique as electrocatalyst for hydrogen evolution reaction in alkaline electrolyte[J].
ACS applied materials & Interface, 2015, 7: 15716–15725. |
| [14] |
KUO Y, LIAO W, YAU S. Effects of Anions on the electrodeposition of cobalt on pt(111) electrode[J].
Langmuir, 2014, 30: 13890–13897. DOI: 10.1021/la503513s. |
| [15] |
MALLIK M, MITRA A, SENGUPTA S, et al. Effect of current density on the nucleation and growth of crystal facets during pulse electrodeposition of Sn-Cu lead-free solder[J].
Crystal Growth & Design, 2014, 14: 6542–6549. |
| [16] |
LIU Z, ABEDIN S Z E, BORISENKO N, et al. Influence of an additive on zinc electrodeposition in the ionic liquid 1-ethyl-3-methylimidazolium trifluoromethylsulfonate[J].
Chemelectrochem, 2015, 2: 1159–1163. DOI: 10.1002/celc.201500108. |
| [17] |
MATSUSHIMA H, BUND A, PLIETH W, et al. Copper electrodeposition in a magnetic field[J].
Electrochimica Acta, 2007, 53: 161–166. DOI: 10.1016/j.electacta.2007.01.043. |
| [18] |
OLVERA S, ESTRADA E M A. Effect of the low magnetic field on the electrodeposition of CoxNi100-x alloys[J].
Materials Characterization, 2015, 105: 136–143. DOI: 10.1016/j.matchar.2015.05.002. |
| [19] |
TUDELA I. Ultrasound-assisted electrodeposition of nickel: effect of ultrasonic power on the characteristics of thin coatings[J].
Surface and Coatings Technology, 2015, 264: 49–59. DOI: 10.1016/j.surfcoat.2015.01.020. |
| [20] |
BOOPATHI S, KUMAR S S. Impact of ultrasonic waves in direct electrodeposition of nanostructured aupt -alloy catalyst on carbon substrate: structural characterization and its superior electrocatalytic activity for methanol oxidation reaction[J].
Journal of Physical Chemistry c, 2014, 118: 29866–29873. DOI: 10.1021/jp509248e. |
| [21] |
WANG M Y, WANG Z, GONG X Z, et al. The progress toward electrochemistry intensified by using supergravity field[J].
Chemelectrochem, 2015(2): 1879–1887. |
| [22] |
DU J P, SHAO G J, QIN X J. High specific surface area MnO2 electrodeposited under supergravity field for supercapacitors and its electrochemical properties[J].
Materials Letters, 2012, 84: 13–15. DOI: 10.1016/j.matlet.2012.06.059. |
| [23] |
TONG H, KONG L B, WANG C M. Electroless deposition of Ag onto p-Si(100) surface under the condition of the centrifugal fields[J].
Thin Solid Films, 2006, 496: 360–363. DOI: 10.1016/j.tsf.2005.09.079. |
| [24] |
WANG M Y, WANG Z. The intensification technologies to water electrolysis for hydrogen production-A review[J].
Renewable and Sustainable Energy Review, 2014, 29: 573–588. DOI: 10.1016/j.rser.2013.08.090. |
| [25] |
LAO L, RAMSHAW C, YEUNG H. Process intensification: water electrolysis in a centrifugal acceleration field[J].
Journal of Applied Electrochemistry, 2011, 41: 645–656. DOI: 10.1007/s10800-011-0275-2. |
| [26] |
WANG M Y, WANG Z, GUO Z C. Deposit structure and kinetic behavior of metal electrodeposition under enhanced gravity-induced convection[J].
Journal of Electroanalytical Chemistry, 2015, 744: 25–31. DOI: 10.1016/j.jelechem.2015.03.003. |
| [27] |
WANG M Y, WANG Z, GUO Z C. Preparation of electrolytic copper powders with high current efficiency enhanced by super gravity field and its mechanism[J].
Transactions of Nonferrous Metals Society fo China, 2010, 20: 1154–1160. DOI: 10.1016/S1003-6326(09)60271-5. |
| [28] |
MORISUE M, FUKUNAKA Y. Effect of gravitational strength on nucleation phenomena of electrodeposited copper onto at tin substrate[J].
Journal of Electroanalytical Chemistry, 2003, 559: 155–163. DOI: 10.1016/j.jelechem.2003.08.021. |
| [29] |
LIU T, GUO Z C. Structure and corrosion resistance of nickel foils deposited in a vertical gravity field[J].
Applied Surface Science, 2010, 256: 6634–6640. DOI: 10.1016/j.apsusc.2010.04.062. |
| [30] |
WANG M Y, WANG Z. Facile one-step electrodeposition preparation of porous NiMo film as electrocatalyst for hydrogen evolution reaction[J].
International Journal of Hydrogen Energy, 2015, 40: 2173–2181. DOI: 10.1016/j.ijhydene.2014.12.022. |
| [31] |
LIU T, GUO Z C, WANG Z, WANG M Y. Effects of gravity on the electrodeposition and characterization of nickel foils[J].
International Journal of Minerals Metallurgy and Materials, 2011, 18: 59–65. DOI: 10.1007/s12613-011-0400-6. |
| [32] |
CHEN Z H, MA Z P. Novel one-step synthesis of wool-ball-like Ni-carbon nanotubes composite cathodes with favorable electrocatalytic activity for hydrogen evolution reaction in alkaline solution[J].
Journal of Power Sources, 2016, 324: 86–96. DOI: 10.1016/j.jpowsour.2016.04.101. |
| [33] |
CHEN J F.
The application and technology of super gravity[M]. Beijing: Chemical Industry Press, 2002.
|
| [34] |
TONG H, KONG L B, WANG C M. Electroless deposition of Ag onto P-Si(100) surface under the condition of the centrifugal fields[J].
Thin Solid Films, 2006, 496: 360–363. DOI: 10.1016/j.tsf.2005.09.079. |
| [35] |
SATO M, YAMADA A, AOGAKI R. Electrochemical reaction in a high gravity field vertical to an electrode surface-analysis of diffusion process with a gravity electrode[J].
Japanese Journal of Applied Physics, 2003, 42: 4520–4528. DOI: 10.1143/JJAP.42.4520. |
| [36] |
MOTI E, SHARIAT M H, BAHROLOLOOM M E. Electrodeposition of nanocrystalline nickel by using rotating cylindrical electrodes[J].
Materials chemistry and physics, 2008, 111: 469–474. DOI: 10.1016/j.matchemphys.2008.04.051. |
| [37] |
KIUCHI D, MATSUSHIMA H. Ohmic resistance measurement of bubble froth layer in water electrolysis under microgravity[J].
Journal of Electrochem Soc, 2006, 153: E138–E143. DOI: 10.1149/1.2207008. |
 2019, Vol. 10
2019, Vol. 10


