| 多孔氧化镁/硅藻土吸附材料的制备及其吸附性能 |
b. 江西理工大学, 应用科学学院, 江西 赣州 341000
b. College of Applied Science, Jiangxi University of Science and Technology, Ganzhou 341000, China
在我们的生活中,使用染料的制品无处不在,据统计结果,商业用途的染料种类已超过100 000种,世界上染料的年产量约为80~90万t,而我国染料年产量约为15万t,位居世界染料产量前列[1-3].染料的广泛使用为我们提供了便利,也对我们的生态环境造成了极大的污染和破坏.有一部分染料废水排放到环境中,很多染料有毒且难以降解,降低了光透过率,影响水生植物的生长,危害水体生物,影响了人们的饮用水安全,甚至有些染料还具有致畸、致癌的潜在威胁[4-5].因此,去除水体中的染料成为科研工作者所关注的热点之一.对于废水中有机物污染物的处理方法有萃取法、吸附法、浓缩法、膜分离等方法[6-9].其中由于吸附法具有操作简单、处理费用低等优点,而广泛应用于水中有机物污染物的处理[10].目前吸附材料的开发主要集中在金属氧化物、多孔矿物质、碳材料等[11, 12],但它们的饱和吸附量较低,而且有些还需要调节pH值,这大大降低了处理效率,增加了处理费用.在水资源极度匮乏及不断受到污染的现状下,开发研究一种高效低廉的新型吸附材料显得极为重要.
MgO具有价格低廉、吸附速率快、饱和吸附量大等优点,是一种非常有潜力的吸附材料[13-16]. YU等[17]制备具有微米/纳米结构的MgO,其对砷具有良好的吸附能力,对三价砷和五价砷的饱和吸附量分别高达644 mg/g和379 mg/g,而且吸附时不用调节pH值,这大大提高了处理效率,降低了处理成本. CAO等[18]制备具有高效吸附能力的分等级花状MgO,对Cd2+、Pb2+离子的饱和吸附量分别高达1 500 mg/g和1 980 mg/g.综上所述,可以发现MgO对阴、阳离子都具有优异的吸附性能,而目前大部分吸附材料只对阳或阴离子中的一种离子具有良好的吸附效果,因此MgO基材料在废水处理应用中具有良好的应用前景.
具有应用前景的高效吸附剂既要满足价格低廉、对污染物响应速度快、饱和吸附量大等优点,同时也必须具备处理后容易与水分离[19-21].文中以多孔硅藻土作为载体,通过预处理后,在其孔道及表面负载一定量的MgO,制作一种氧化镁/硅藻土复合多孔吸附材料.由于硅藻土密度较大,同时尺寸达数微米,因此通过沉降的方式就可以水体分离.文中所制备的氧化镁/硅藻土复合多孔吸附材料对有机染料具有优异的吸附性能,具有较好的应用前景.
1 实验方案用蒸馏水将硅藻土淘洗2~3次,过滤后再加入1 mol/L的HCl溶液,置于磁力搅拌器上2 h,静置24 h后过滤,用蒸馏水清洗干净,干燥后备用.分别称取0.5 g、1 g、1.5 g、2 g乙酸镁,加入20 mL蒸馏水,溶解,再添加2.5 g改性硅藻土,将烧杯置于恒常温空间的磁力搅拌机上,搅拌24 h.再将烧杯置于50 ℃烘干箱中烘干,之后将其研磨,然后置于管式炉中400 ℃保温2 h,研磨后得到不同质量比的氧化镁/硅藻土多孔吸附材料.
在空气气氛下,所制备样品采用PERKIN ELMER TG/DTA 6300热重-差热(DTA-TG)分析仪进行分析,升温速率为5 ℃/min;物相分析在Rigaku D-max 2500型X射线衍射仪进行,采用Cu靶,电压为40 kV,电流为30 mA,入射波长λ=0.154 18 nm,扫描速度为2°/min,扫描范围为20°≤2 θ≤80°,石墨单色器,NaI探测器;采用HITACHI S4800场发射扫面电子显微镜(SEM)观察不同热处理工艺制备的试样形貌特征,工作电压1 kV.
2 结果与讨论为了得到多孔MgO的合理煅烧工艺,在样品煅烧之前,取部分粉末样进行热重-差热分析(TG-DTA),结果如图 1所示.从TG曲线可以看出,从251 ℃到1 000 ℃样品的质量随着温度的升高而降低,在这温度区间,试样的质量出现了2次快速降低,对应的温度区间分别25~120 ℃,300~385 ℃.从DTA曲线可以看出,从25 ℃到1 000 ℃出现了2次明显的吸热峰.在温度为74 ℃出现了一个明显的吸热峰,这是由于乙酸镁失去结晶水[4]需要吸收热量的缘故;在温度337 ℃出现了一个吸热峰,这是由于温度在300~385 ℃区间吸收热量将乙酸镁转化成MgO.负载后为了获得MgO/硅藻土复合材料,热处理温度应该在385 ℃以上,本试验煅烧温度定于400 ℃.
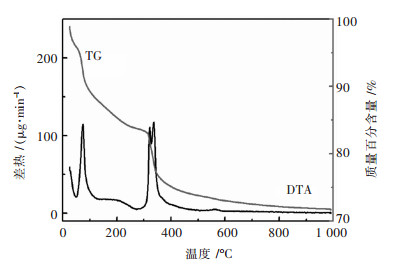 |
| 图 1 乙酸镁/硅藻土TG-DTA曲线 Fig. 1 TG-DTA curves for Mg(AC)2/Diatomite |
对改性后硅藻土、乙酸镁与硅藻土不同质量比的4组试样煅烧后进行XRD分析,结果如图 2所示.图 2中曲线a为纯的改性硅藻土,对照PDF卡521379.在XRD曲线中并没有发现Al2O3、Fe2O3、CaO等杂质的衍射峰,这是由于硅藻土原矿中的Al2O3、Fe2O3、CaO等杂质在酸洗过程中被溶解变成可溶性物质,其反应如反应方程式(1)~式(3)所示.
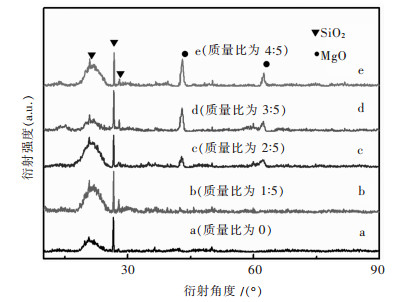 |
| 图 2 乙酸镁与硅藻土不同质量比样品XRD分析结果 Fig. 2 XRD patterns of the samples synthesized with different Mg(AC)2 concentrations |
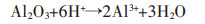
|
(1) |
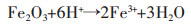
|
(2) |
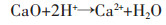
|
(3) |
当乙酸镁与硅藻土质量比为1:5,由于乙酸镁含量较低,因此热处理后MgO的含量也较低,因此只在2θ为43°出现较为明显的衍射峰(如图 2(b)所示);当乙酸镁与硅藻土质量比为2:5、3:5、4:5时,在2 θ为43°及62.4°出现非常明显的衍射峰,且衍射强度随着乙酸镁的含量增加而增加(如图 2中曲线c、曲线d、曲线e所示),通过检索为MgO的衍射峰.从XRD分析结果表明负载后,在400 ℃保温2 h,能得到氧化镁/硅藻土复合吸附材料.
硅藻土的孔道负载MgO情况及孔道是否堵塞是影响其吸附性能的关键因素之一,采用SEM对不同含量乙酸镁制备的试样进行微观结构观察,结果如图 3所示.改性硅藻土为圆盘状,硅藻土盘很干净,孔道均匀且明显,无堵塞,其中圆盘中间孔径约为200 nm左右(如图 3(a)所示),对圆盘边缘放大,发现圆盘边缘也具有多孔结构,其孔径大小约为50 nm(如图 3(b)所示).当乙酸镁与硅藻土质量比分别为1:5,硅藻土少量孔道负载了少量的MgO(如图 3(c)所示);当乙酸镁与硅藻土质量比分别为2:5,硅藻土大多数孔道中负载了MgO(如图 3(d)所示);当乙酸镁与硅藻土质量比分别为3:5,硅藻土所有孔道几乎都负载了MgO,而且有少量孔道口被MgO堵住(如图 3(e)所示);当乙酸镁与硅藻土质量比分别为4:5,硅藻土所有孔道中都负载了MgO,大量孔道口被MgO堵住(如图 3(f)所示).为了使所制备的氧化镁/硅藻土多孔吸附材料具有良好的吸附性能,既要尽可能使所有孔道负载MgO,同时又不能使硅藻土的孔道堵住.
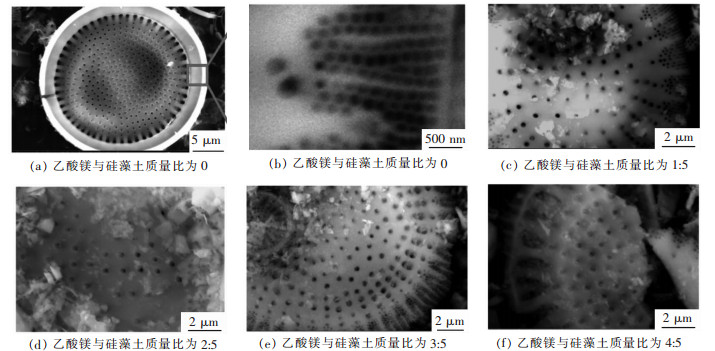 |
| 图 3 乙酸镁与硅藻土不同质量比样品的SEM照片 Fig. 3 Typical FESEM images of samples synthesized with different Mg(AC)2 concentrations |
由于固液界面的吸附过程涉及多相体系,同时涉及溶质、溶剂以及吸附剂三者之间错综复杂的作用,因此,等温吸附模型较多,常用描述等温吸附模型有Langmuir、Freundlich、Redlich-Person、Temkin模型等.文中采用Langmuir等温吸附模型来研究在恒温条件下,溶液中吸附质平衡浓度与吸附剂表面吸附量的关系.
Langmuir等温吸附模型假设吸附为单分子层吸附、吸附剂表面均一、各处吸附能相同,其表达式为:
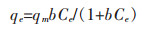
|
(4) |
式(4)中,qe为平衡时吸附量,Ce为平衡时溶液中吸附质浓度,qm为最大吸附量,b为平衡常数.
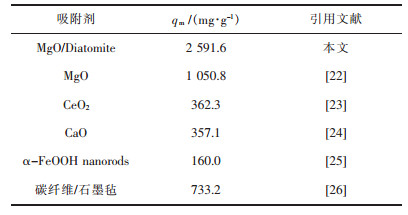
采用不同浓度的刚果红溶液,吸附24 h后测得溶液中吸附质的平衡浓度Ce,计算对应的平衡吸附量qe,用Langmuir等温吸附模型对所得的数据点进行拟合,拟合结果如图 4所示.根据Langmuir等温吸附模型估计各试样对刚果红的最大吸附量分别为427.5 mg/g,1 598.8 mg/g,2 591.6 mg/g,2 122.7 mg/g,相关系数R2都大于0.96,因此采用Langmuir等温吸附模型对所得的数据点进行拟合具有很高的可信度.当乙酸镁与硅藻土质量比分别为1:5及2:5时,由于负载氧化镁较少,因此其吸附性能较低.当乙酸镁与硅藻土质量比分别为3:5时,负载的氧化镁较多,而且孔道几乎没有堵上,因此所制备的氧化镁/硅藻土多孔吸附材料对刚果红具有优异的吸附性能,最大饱和吸附量达2 591.6 mg/g,表 1列举了常用于刚果红吸附剂及其吸附量,从表 1中数据可以看出,该文所制备的氧化镁/硅藻土多孔吸附材料在刚果红吸附方面具有明显的优势,在有机污染物废水处理中具有良好的应用前景.当乙酸镁与硅藻土质量比分别为4:5时,虽然硅藻土所有孔道几乎都负载了MgO,但由于部分孔道口被MgO堵住,从而降低其比表面积,进而降低其吸附性能.
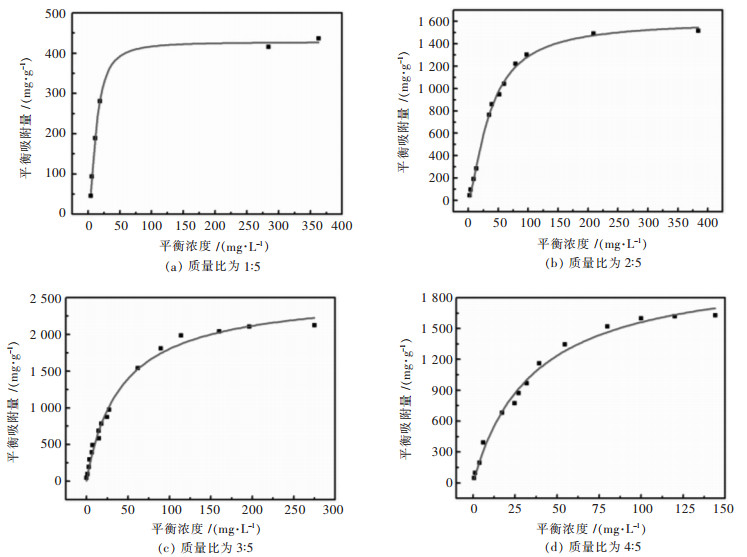 |
| 图 4 采用Langmuir等温吸附模型拟合试样吸附刚果红 Fig. 4 Fitting absorption isotherms for Congo Red with Langmuir |
| 表 1 不同吸附剂对刚果红溶液的吸附量对照表 Table 1 Comparison of adsorption capacities twoard congo red on different adsorbents |
 |
| 点击放大 |
3 结论
1) 硅藻土负载乙酸镁后为了得到氧化镁/硅藻土多孔吸附材料,其煅烧温度至少需要385 ℃;通过对煅烧后物相进行分析,煅烧温度为400 ℃,保温时间2 h,得到纯净的氧化镁/硅藻土多孔吸附材料;
2) 当乙酸镁与硅藻土质量比分别为3:5,硅藻土所有孔道几乎都负载了的MgO,只有少量孔道口被MgO堵住;当乙酸镁与硅藻土质量比进一步增加时,硅藻土所有孔道中都负载了的MgO,大量孔道口被MgO堵住;
3) 当乙酸镁与硅藻土质量比分别为3:5时,所制备的氧化镁/硅藻土多孔吸附材料对有机染料具有优异的吸附性能,采用Langmuir等温吸附模型估算其对刚果红的饱和吸附量为2 591.6 mg/g.
| [1] |
TIAN Y, LI H, RUAN Z, et al. Synthesis of NiCo2O4 nanostructures with different morphologies for the removal of methyl orange[J].
Applied Surface Science, 2017, 393: 434–440. DOI: 10.1016/j.apsusc.2016.10.053. |
| [2] |
白羽, 吴榛, 刘仁月, 等. 花状Pt/Bi2WO6微米晶合成、表征及其高可见光催化性能[J].
有色金属材料与工程, 2016, 7(2): 60–66.
|
| [3] |
QU X, ALVAREZ P J, LI Q. Applications of nanotechnology in water and wastewater treatment[J].
Water Research, 2013, 47(12): 3931–3946. DOI: 10.1016/j.watres.2012.09.058. |
| [4] |
CHAWLA S, UPPAL H, YADAV M, et al. Zinc peroxide nanomaterial as an adsorbent for removal of Congo red dye from waste water[J].
Ecotoxicology and Environmental Safety, 2017, 135: 68–74. DOI: 10.1016/j.ecoenv.2016.09.017. |
| [5] |
LI X, XIAO W, HE G, et al. Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: High adsorption capability to azo dyes[J].
Colloids & Surfaces A Physicochemical & Engineering Aspects, 2012, 408: 79–86. |
| [6] |
ZIETSCHMANN F, STUTZER C, JEKEL M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater-aligning breakthrough curves and capacities[J].
Water Research, 2016, 92: 180–187. DOI: 10.1016/j.watres.2016.01.056. |
| [7] |
CHEN H, WANG X X, LI J X, et al. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions[J].
Journal of Materials Chemistry A, 2015, 11(3): 6073–6081. |
| [8] |
CHEN D H, CAO L, HANLEY T L, et al. Facile synthesis of monodisperse mesoporous zirconium titanium oxide microspheres with varying compositions and high surface areas for heavy metal ion sequestration[J].
Advanced Functional Materials, 2012, 22(9): 1966–1971. DOI: 10.1002/adfm.201102878. |
| [9] |
LIANG H W, CAO X, ZHANG W J, et al. Robust and highly efficient free-standing carbonaceous nanofiber membranes for water purification[J].
Advanced Functional Materials, 2011, 21(20): 3851–3858. DOI: 10.1002/adfm.v21.20. |
| [10] |
LIU B X, WANG J S, WU J S, et al. Controlled fabrication of hierarchical WO3 hydrates with excellent adsorption performance[J].
Journal of Materials Chemistry A, 2014(2): 1947–1954. |
| [11] |
WANG B, WU H B, YU L, et al. Template-free formation of uniform urchin-like α-FeOOH hollow spheres with superior capability for water treatment[J].
Advanced Materials, 2012, 24: 5713–5721. DOI: 10.1002/adma.v24.42. |
| [12] |
YANG J Q, JIAO L F, ZHAO Q Q, et al. Facile preparation and electrochemical properties of hierarchical chrysanthemum-like WO3·0.33H2O[J].
Journal Material Chemistry, 2012, 22(9): 3699–3701. DOI: 10.1039/c2jm15837j. |
| [13] |
CAI Y, LI C, WU D, et al. Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution[J].
Chemical Engineering Journal, 2017, 312: 158–166. DOI: 10.1016/j.cej.2016.11.134. |
| [14] |
BIAN S W, BALTRUSAITIS J, GALHOTRA P, et al. A template-free, thermal decomposition method to synthesize mesoporous MgO with a nanocrystalline framework and its application in carbon dioxide adsorption[J].
Journal of Materials Chemistry, 2010, 20(39): 8705–8710. DOI: 10.1039/c0jm01261k. |
| [15] |
MOHAMED R M, SHAWKY A, MKHALID I A. Facile synthesis of MgO and Ni-MgO nanostructures with enhanced adsorption of methyl blue dye[J].
Journal of Physics & Chemistry of Solids, 2017, 101: 50–57. |
| [16] |
CHOWDHURY A H, CHOWDHURY I H, NASKAR M K. A facile synthesis of grainy rod-like porous MgO[J].
Materials Letters, 2015, 158(1): 190–193. |
| [17] |
YU X Y, LUO T, JIA Y, et al. Porous Hierarchically Micro-/Nanostructured MgO: Morphology Control and Their Excellent Performance in As(Ⅲ) and As(Ⅴ) Removal[J].
The Journal of Physical Chemistry C, 2011, 115(45): 22242–22250. DOI: 10.1021/jp207572y. |
| [18] |
CAO C Y, QU J, WEI F, et al. Superb adsorption capacity and mechanism of flowerlike magnesium oxide nanostructures for lead and cadmium ions[J].
Accounts of Chemical Research Applied Materials & Interfaces, 2012, 4(8): 4283–4287. |
| [19] |
JIA Y, YU X, LUO T, et al. Necklace-like mesoporous MgO/TiO2 heterojunction structures with excellent capability for water treatment[J].
Dalton Transactions, 2014, 43(6): 2348–2351. DOI: 10.1039/C3DT52199K. |
| [20] |
CHOWDHURY I H, CHOWDHURY A H, Bose P, et al. Effect of anion type on the synthesis of mesoporous nanostructured MgO, and its excellent adsorption capacity for the removal of toxic heavy metal ions from water[J].
Royal Society of Chemistry Advance, 2016, 6(8): 6038–6047. |
| [21] |
WALDRON K, WU Z, WU W D, et al. Formation of uniform large SBA-15 microspheres via spray drying[J].
Journal of Materials Chemistry A, 2014, 2(45): 19500–19508. DOI: 10.1039/C4TA05002A. |
| [22] |
DHAL J P, SETHI M, MISHRA B G, et al. MgO nanomaterials with different morphologies and their sorption capacity for removal of toxic dyes[J].
Materials Letters, 2015, 141: 267–271. DOI: 10.1016/j.matlet.2014.10.055. |
| [23] |
WU J, WANG J, DU Y, et al. Adsorption mechanism and kinetics of azo dye chemicals on oxide nanotubes: a case study using porous CeO2 nanotubes[J].
Journal of Nanoparticle Research, 2016, 18(7): 191. DOI: 10.1007/s11051-016-3497-8. |
| [24] |
HAN M X, LI F C, YUN J F. Highly efficient removal of congo red from wastewater by nano-CaO[J].
Separation Science & Technology, 2013, 48(17): 2681–2687. |
| [25] |
MAITI D, MUKHOPADHYAY S, DEVI P S. Evaluation of mechanism on selective, rapid, and superior adsorption of congo red by reusable mesoporous α-Fe2O3 nanorods[J].
ACS Sustainable Chemistry & Engineering, 2017, 5(12): 11255–11267. |
| [26] |
SHEN Y, LI L, XIAO K, et al. Constructing three-dimensional hierarchical architectures by integrating carbon nanofibers into graphite felts for water purification[J].
ACS Sustainable Chemistry & Engineering, 2016, 4(4): 2351–2358. |
 2018, Vol. 9
2018, Vol. 9


