| 赤泥-钛白废酸综合回收中钪的萃取工艺 |
在自然界中, 钪多以伴生矿物的形式稀散地分布于其他矿物中, 分离和提取的难度较大[1-4].钪及其化合物具有多种优异的性能, 广泛应用于国防、电光源、航天、化工、冶金等领域[5, 6].赤泥属碱性固体废料, 钛白废酸属酸性液体废料, 两种废料均含有一定量的钪和其他有价金属, 将两者酸碱中和后, 再进行有价元素的综合回收利用[7-9], 不仅可以节省生产成本, 也可提高钪和有价元素的品位, 更有利于下一步回收工序的进行.同时, 也解决了碱性固体废料和酸性液体废料的排放问题, 提高了资源的综合利用, 减少了对环境的污染, 具有重要的社会效益、经济效益和环境效益.
目前从含钪原料中提取钪的工艺技术主要有溶剂萃取法[10-17]、离子交换法[18-20]、萃淋树脂色层法[21]、液膜萃取法[22, 23].其中, 溶剂萃取法具有成本低、效果好、处理量大、操作简单等优点, 是从赤泥和钛白废酸中提取钪的主要方法.
文章针对钛白废酸浸出赤泥提取氧化钪生产工艺过程中浸出液容易絮凝,难以直接萃取分离的技术难题,采用先除杂再萃取提钪的工艺方法,具体研究了除杂后浸出液酸度、萃取剂体积分数、相比、萃取时间对钪萃取率的影响. 在较优的萃取工艺条件下,获得了较好的钪萃取率.
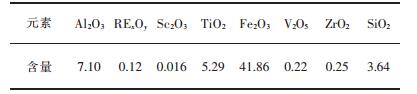
1 实验 1.1 原料取适量的赤泥-钛白废酸浸出液,利用电感耦合等离子体光谱发生仪检测其化学成分, 结果如表 1所示.由表 1可知, 浸出液中硅含量较高, 而硅在酸性条件下易发生水解, 生成硅胶, 从而使浸出液在存放过程中发生胶凝现象, 稳定性差, 难于萃取, 因此需先对浸出液进行除杂.
| 表1 赤泥-钛白废酸浸出液的主要化学成分/(g·L-1) Table 1 The main chemical composition of the red mud-titanium white waste acid/(g·L-1) |
 |
| 点击放大 |
1.2 工艺流程图
对赤泥-钛白废酸浸出液采用先活性炭吸附脱硅, 再萃取的工艺提钪, 具体工艺流程如图 1所示.
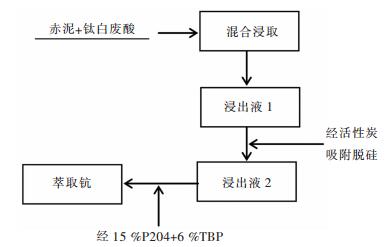 |
| 图 1 赤泥-钛白废酸浸出液萃取钪工艺流程 Fig. 1 Process flow of Sc extraction from leach solution of red mud-titanium white waste acid |
2 结果与讨论 2.1 除杂
由于浸出液中的硅易引起乳化, 且硅在酸性条件下能够发生水解, 形成硅胶, 从而影响后续的萃取试验, 而活性炭的大表面积可以吸附多种化学物质, 从而阻止这些物质的吸收, 是一种有效降低杂质含量的吸附剂.因此先采用木质粉状活性炭吸附法对赤泥浸出液作预处理, 将硅降低至无影响的浓度范围内, 再进行萃取实验.
取1 000 mL赤泥-钛白废酸浸出液, 分别加入0.5、0.8、1、1.5、2g木质粉状活性炭粉, 搅拌均匀, 常温下浸泡6 h, 过滤得除杂后液, 其除硅率如图 2所示.由图可知, 当加入的活性炭质量小于1g时, 硅的去除率快速增大, 而当加入的活性炭质量大于等于1g时, 硅的去除率增加缓慢, 因此, 本文选择在1000 mL赤泥-钛白废酸浸出液中加入1g活性炭为最佳实验条件.
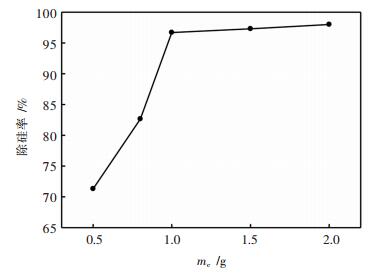 |
| 图 2 活性炭质量对除硅率的影响 Fig. 2 Effect of activated carbon on the removal rate of silicon |
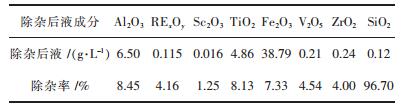
表 2为活性炭除杂后液的化学成分.由表可知, 活性炭除硅率高达96.7%, 而钪的损失率很小, 仅为1.25%, 这样就可以在基本不影响钪含量的条件下除去绝大部分的硅, 有利于后续的萃取实验.
| 表2 除杂后液的化学成分及除杂率 Table 2 The chemical composition and impurities removal efficiency of the solution after removal of impurities |
 |
| 点击放大 |
分别对未除杂的浸出液和除杂后液进行了钪的萃取实验.在萃取未除杂的浸出液时, 由于浸出液中硅含量较高, 萃取过程乳化严重, 且溶液放置几小时即发生胶凝, 阻碍了萃取实验的进行, 因此, 未除杂的浸出液中钪的萃取率很低, 在40%左右.而萃取除杂后液时, 由于溶液中硅含量大幅降低, 萃取时未出现乳化现象, 且溶液可以在常温下放置一星期而不发生胶凝, 因此, 钪的萃取率大大提高, 在90%以上.
2.2 萃取条件实验 2.2.1 浸出液酸度在相比VO/VA=1/25, 萃取剂体积分数15% P204+6% TBP, 萃取时间15 min的条件下, 考察了浸出液的酸度(以H2SO4计)对Sc, Ti, Fe, Al萃取率的影响, 实验结果如图 3所示.由图 3可知, Sc, Ti, Fe萃取率都随着浸出液酸度的增大而增大, Al萃取率随酸度的增大变化不大.当酸度大于1.81 mol∙L-1时, Sc萃取率增速减缓, Ti, Fe萃取率增加显著.这是由于, 当浸出液酸度较低时, 主要是酸性萃取剂P204配合萃取, 而当浸出液酸度较高时, P204不再电离, 主要由中性萃取剂TBP配合萃取.由图 3可知, 在酸度较低时Sc的萃取效果好, 在酸度较高时, Ti, Fe的萃取效果好, 而酸度对Al的萃取无明显影响.此处选择浸出液的最佳酸度为1.81 mol∙L-1.
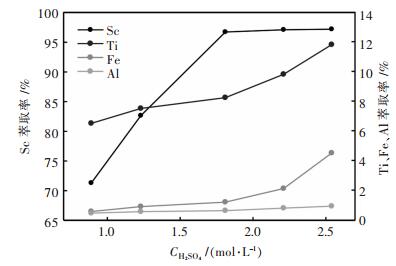 |
| 图 3 浸出液酸度对钪、钛、铁、铝萃取率的影响 Fig. 3 Influence of leach solution acidity on extraction ratio of Sc, Ti, Fe, Al |
2.2.2 相比
在萃取中, 相比太大, 则有机相消耗过高, 不经济; 相比太小, 则萃取率过低, 不利于生产, 成本也高, 因此选择适当的相比, 使萃取率较高, 有机相消耗又省, 是最为理想的.在酸度1.81 mol/L, 萃取剂体积分数15% P204+6% TBP, 萃取时间15 min的条件下, 考察了不同相比对Sc, Ti, Fe, Al萃取率的影响, 结果如图 4所示.由图可知, Sc, Ti, Fe的萃取率随相比的增大而增大, Al的萃取率随相比的增加变化不大.当相比在1/10~1/30之间时, Sc萃取率达到平衡, 但当相比为1/30时, 发生乳化, 难于分离.因此, 选择1/25为最佳条件.
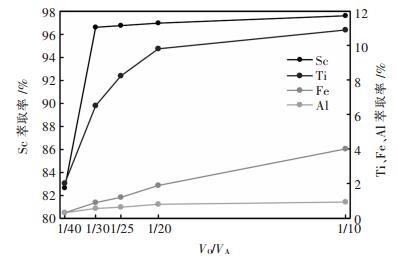 |
| 图 4 相比对萃取率的影响 Fig. 4 Influence of phase ratio on extraction ratio |
2.2.3 萃取时间
在相比VO/VA=1/25, 酸度1.81 mol/L, 萃取剂体积分数15% P204+6% TBP的条件下, 考察了萃取时间对Sc, Ti, Fe, Al萃取率的影响, 结果如图 5所示.由图可知, 随着萃取时间的增加, Sc, Ti, Fe萃取率增大, Al萃取率变化不大.当萃取时间大于15 min时, Sc萃取率增幅减缓, 而Ti, Fe萃取率继续增大, 因此, 选择萃取时间15 min为较优条件.
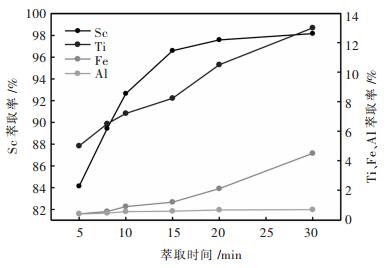 |
| 图 5 萃取时间对萃取率的影响 Fig. 5 Influence of extraction time on extraction ratio |
2.2.4 萃取剂浓度
萃取剂是采用P204、TBP与煤油按一定比例混和而成的有机相, 合理的萃取剂配比可使萃取过程中分配比尽可能大, 分相时间短, 同时也可使操作过程方便, 防止乳化和第三相的形成.在相比VO/VA=1/25, 酸度1.81 mol/L, 6% TBP, 萃取时间15 min的条件下, 考察了P204体积分数的改变对Sc, Ti, Fe, Al萃取率的影响, 结果如图 6(a)所示.由图图 6(a)可知, Sc, Ti, Fe的萃取率随着P204体积分数的增大而增大, Al的萃取率随P204体积分数的增加变化不大.当P204的体积分数大于15%时, Sc的萃取率基本达到平衡, 而Ti, Fe的萃取率继续增大.在保证Sc的萃取率的前提下更多地分离Ti, Fe, Al, 这里, P204体积分数以15%较优.
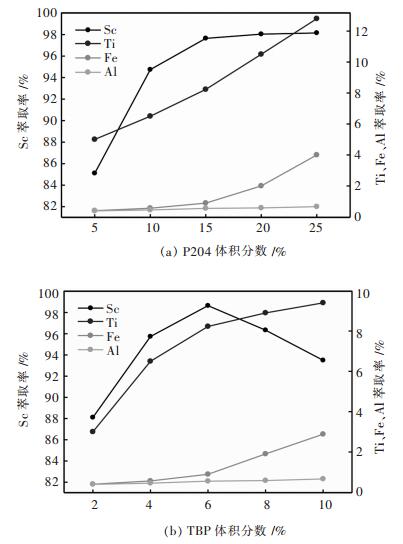 |
| 图 6 P204和TBP体积分数对钪萃取率的影响 Fig. 6 Influence of volume fraction of P204 and TBP on extraction ratio of scandium |
在相比VO/VA=1/25, 酸度1.81 mol/L, 15% P204, 萃取时间15 min的条件下, 考察了TBP体积分数的改变对Sc, Ti, Fe, Al萃取率的影响, 结果如图 6(b)所示.由图图 6(b)可知, Ti, Fe的萃取率随TBP体积分数的增大而增大, Al的萃取率随TBP体积分数的增加变化不大, 当TBP的体积分数小于6%时, Sc的萃取率随TBP体积分数的增大而增大, 当TBP的体积分数大于6%时, Sc的萃取率随TBP体积分数的增大而减小.这可能是由于浸出液中离子的浓度差异大以及迁移速度不同所致, Ti, Fe的浓度干扰了萃取剂TBP对Sc的萃取, Ti, Fe与Sc发生竞争萃取.因此, 选择TBP体积分数以6%为较优.
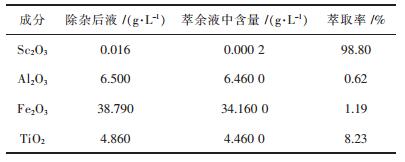
由于P204对钪的选择性较差, 使杂质元素的共萃现象比较明显, 而向P204有机相中加入一定量的TBP, 形成P204与TBP协同萃取效应可有效减少共萃现象, 表 3为在相比VO/VA=1/25, 酸度1.81 mol/L, 萃取剂体积分数15% P204+6% TBP, 萃取时间15 min的最优条件下, Sc, Ti, Fe, Al的萃取率结果, 由表 3可知, Sc的萃取率达98.80%, 而杂质Al、Fe的萃取率仅为0.62%、1.19%, 几乎不被萃取, Ti的萃取率为8.23%, 但Ti在萃取液中浓度较低, 因此对后续反萃Sc的影响较小, 可忽略不计.试验结果表明, 采用P204+TBP协同萃取钪具有良好的提钪效果.
| 表3 萃取结果 Table 3 Results of extraction |
 |
| 点击放大 |
3 结论
1) 采用活性炭对浸出液进行脱硅, 除硅率高达96.70%, 有效控制了浸出液的胶凝现象.同时, 除硅工艺中, 钪的损失率很小, 仅为1.25%, 这样就可以在基本不影响钪含量的条件下除去绝大部分的硅, 有利于后续萃取试验的进行.
2) 对赤泥-钛白废酸浸出液萃取钪的研究结果表明:在相比VO/VA=1/25, 酸度1.81 mol/L, 萃取剂体积分数15% P204+6% TBP, 萃取时间15 min的最佳试验条件下, 钪的萃取率可达98.80%.
3) 扩大试验条件下, 经除硅工艺, 硅去除率达到95%以上, 经钪萃取工艺, 钪的萃取率仍然高达96%以上, 试验可重复性好.
| [1] | 李亮星, 宋祥莉, 黄茜琳. 含钪废料的回收处理方法[J]. 江西有色金属, 2008, 22(2): 23–25. |
| [2] |
WANG W W, PRANOLO Y, CHENG C Y. Metallurgical processes for scandium recovery from various resources: A review[J].
Hydrometallurgy, 2011, 108(1-2): 100–108. DOI: 10.1016/j.hydromet.2011.03.001. |
| [3] |
ZHANG N, LI H X, LIU X M. Recovery of scandium from bauxite residue-red mud: a review[J].
Rare Metals, 2016, 35(12): 887–900. DOI: 10.1007/s12598-016-0805-5. |
| [4] | 杨海琼, 董海刚, 赵家春, 等. 钪的回收技术研究进展[J]. 有色金属(冶炼部分), 2014(3): 29–33. |
| [5] |
JAYASHREE B, SUNIL G, HARISH J P, et al. Synthesis, characterization, neutron activation, and application of scandium oxide microsphere in radioactive particle tracking experiments[J].
Industrial & Engineering Chemistry Research, 2016, 55(1): 3–12. |
| [6] |
LIAC H, CAOA F, GUOA S, et al. Microstructures and properties evolution of spray-deposited Al-Zn-Mg-Cu-Zr alloys with scandium addition[J].
Journal of Alloys and Compounds, 2017, 691: 482–488. DOI: 10.1016/j.jallcom.2016.08.255. |
| [7] |
BIEKE O, KOEN B. Recovery of scandium(Ⅲ) from aqueous solutions by solvent extraction with the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide[J].
Industrial & Engineering Chemistry Research, 2015, 54(6): 1887–1898. |
| [8] | 司秀芬, 邓佐国, 徐廷华. 赤泥提抗综述[J]. 江西有色金属, 2003, 17(2): 28–31. |
| [9] |
WANG W, PRANOLO Y, CHENG C Y. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA[J].
Separation and Purification Technology, 2013, 108: 96–102. DOI: 10.1016/j.seppur.2013.02.001. |
| [10] | 钟学明. 伯胺萃取法提取氧化钪的工艺研究[J]. 稀有金属, 2002, 26(6): 527–529. |
| [11] | 徐廷华, 邓佐国, 李伟, 等. 从钨渣浸出液中提取钪的研究[J]. 江西有色金属, 1997, 11(4): 32–36. |
| [12] |
ZHAO Z G, KUBOTA F, KAMIYA N, et al. Selective extraction of scandium from transition metals by synergistic extraction with 2-thenoyltrifluoroacetone and tri-n-octylphosphine oxide[J].
Solvent Extraction Research and Development, 2016, 23(2): 137–143. DOI: 10.15261/serdj.23.137. |
| [13] |
DENISOVA S A, GOLOVKINA A V, LESNOV A E. Extraction of scandium by diantipyrylalkanes from naphthalene-2-sulfonate solutions in the extraction systems of different types[J].
Journal of Analytical Chemistry, 2015, 70(2): 107–112. DOI: 10.1134/S1061934815020033. |
| [14] |
XU S Q, LI S Q. Review of the extractive metallurgy of scandium in China (1978~1991)[J].
Hydrometallurgy, 1996, 42(3): 337–343. DOI: 10.1016/0304-386X(95)00086-V. |
| [15] |
BIEKE O, CHENNA R B, TOM V G, et al. Recovery of scandium from sulfation-roasted leachates of bauxite residue by solvent extraction with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide[J].
Separation and Purification Technology, 2017, 176: 208–219. DOI: 10.1016/j.seppur.2016.12.009. |
| [16] |
TURANOV A N, KARANDASHEV V K, BAULIN V E, et al. Extraction of rare earths and scandium by 2-phosphorylphenoxyacetic acid amides in the presence of ionic liquids[J].
Russian Journal of Inorganic Chemistry, 2016, 61(3): 377–383. DOI: 10.1134/S0036023616030232. |
| [17] |
DEPUYDT D, DEHAEN W, BINNEMANS K. Solvent Extraction of Scandium(Ⅲ) by an Aqueous Biphasic System with a Nonfluorinated Functionalized Ionic Liquid[J].
Industrial & Engineering Chemistry Research, 2015, 54(36): 8988–8996. |
| [18] |
HSU C G, XU Q, PAN J M. Determination of trace scandium by ion-exchanger phase spectrophotometry with p-nitrochlorophosphonazo[J].
Microchimica Acta, 1997, 126(1-2): 83–86. DOI: 10.1007/BF01242666. |
| [19] |
MALGORZATA B, KRZYSZTOF M, JERZY K. Determination of aluminum, barium, molybdenum, scandium, berylium, titanium, vanadium, fluoride and boron in highly salinated waters[J].
Water Science & Technology, 2015, 33(6): 349–356. |
| [20] |
MAHINDRAKAR A N, CHANDRA S, SHINDE L P. Chemical characterization of Al-Li alloys for scandium by hyphenated technique using ion exchange chromatography[J].
Asian Journal of Chemistry, 2009, 21(3): 1775–1780. |
| [21] |
SHANG Q K, LI D Q, QI J X. Separation of scandium, yttrium and lanthanum in high-performance centrifugal partition chromatography with S-octyl phenyloxy acetic acid[J].
Journal of Solid State Chemistry, 2003, 171(1): 358–361. |
| [22] |
WANG C Z, ZHOU G Z, ZHENG Z L. Extraction of scandium from red mud using ELM with P204 as carrier[J].
Advanced Materials Research, 2012(602-604): 1116–1119. |
| [23] |
YANG X J, GU Z M, WANG D X. Extraction and separation of scandium from rare earths by electrostatic pseudo liquid membrane[J].
Journal of Membrane Science, 1995, 106(1): 131–145. |
 2017, Vol. 8
2017, Vol. 8


