| 微米球光催化剂在环境净化及能源转化的研究进展 |
2. 福州大学光催化研究所,能源环境光催化国家重点实验室,福建 福州350002
2. Research Institute of Photocatalysis, State Key Laboratory of Photocatalysis on Energy and Environment, Fuzhou University, Fuzhou 350002, China
自Fujishima和Honda[1]发现TiO2电极能发生水分解析氢反应以来,光催化被视为一项绿色化学技术,促使研究者广泛深入的研究,证实均相或非均相光催化剂能用于太阳能转化,将太阳能最终转化为可再生氢燃料化学能[2-6]。此外,光催化被公认为一种解决环境问题的环境友好型方法[7-11],目前在有机污染物降解、重金属离子还原等方面取得了重大进展。
尽管光催化基础理论方面已有显著进展,而用于实际环境处理中较理想的光催化剂仍缺乏可观突破。要满足实际应用,除了需要实现高效率外,还应进一步降低运行成本。Liu等[12]详细分析过水净化催化剂的经济耗值,结果表明,TiO2光催化剂的成本主要取决于它的用量,一次性使用的TiO2成本太高。如果光催化剂能被高效分离回收并重复利用,能大大降低光催化应用的运行成本。与传统粉末光催化剂相比,微球光催化剂具有以下明显优势:比表面积大、密度低、光捕获效率好、传递能力强以及表面渗透性好,能有效改善光催化性能。更重要的是,微球光催化剂相比粉末光催化剂更易于分离回收并重复使用[13]从而有效降低成本。
设计与制备具有理想形貌的高效稳定光催化剂,成为当下光催化应用研究领域的一大热点,微球光催化剂因其独特性能而受到广泛关注和研究。本文主要介绍了高效微球光催化剂的制备及其应用于有机污染物降解和能源转化方面的最新研究进展。
1 微球光催化剂在环境净化方面的应用随着工业的迅速发展以及农用化学品的大量使用,水体中的有机污染物已成为一个主要问题。通过传统治理方法很难处理大量难降解的水体污染物。近年来,众多研究工作致力于使用光催化剂光催化分解有机污染物,其优势在于:降解反应动力学高,矿化率高,毒性低,降解产物基本无毒。
对于水体中有机污染物的光催化降解,通常是在一种粉末悬浮系统下进行,悬浮系统可以使催化剂颗粒与有机底物良好接触,从而保证颗粒接受光照。然而,悬浮反应系统受光催化剂回收问题影响,大多数纳米级粉体光催化剂易流失,且难以从反应液中分离。为加快粉体分离,需要采取附加措施。例如,加硫酸亚铁混凝,但催化剂被污染不可再用。另外,可将粉末光催化剂固定在固相载体上,如玻璃、纤维或不锈钢。然而,固定化的光催化剂和光源的接触面积大大降低,导致光催化降解效率明显降低。
光催化剂回收问题需使用新的方法解决。一方面,应该继续保持光催化剂颗粒与光源能充分接触的悬浮反应体系;另一方面,为使催化剂易于回收,应对其改性处理。研究表明,微球光催化剂能成功适用于该体系[14-15]。如果能控制微球的尺寸,并且通过空气鼓泡或搅拌,使光催化剂能悬浮在体系液中,则能保持催化剂与光源充分接触;同时,在空气鼓泡或机械搅拌停止后,微球因重力作用能迅速自动沉降至反应器底部,处理后的水相可以倒出,微球容易分离。如此,微球光催化剂很容易被重复使用。
已有众多研究致力于将TiO2设计成微球。Li等[16]报道了一种高纯TiO2微球,如图 1所示。这些纳米粒子具备完整的球形结构,表面光滑。TiO2微球的直径范围在30~160 m,平均粒径为80 m;同时,这种TiO2微球有较短的沉降时间,最小沉降速率为150 mm/min,明显高于其他两种TiO2粉末光催化剂(3.3和3.8 mm/min)。催化剂的活性测试在365 nm的紫外光照射下降解水杨酸(SA)和磺基水杨酸(SSA)进行。结果表明,TiO2微球在SSA悬浮液中的反应速率前者比后者更高;此外,所制备的TiO2微球样品在光催化氧化反应中可重复使用 50次以上,且光催化剂无损失、活性无明显降低、颗粒形状不发生改变。TiO2微球在液相有机污染物降解中表现出优异性能,归因于其良好的稳定性和吸附能力。
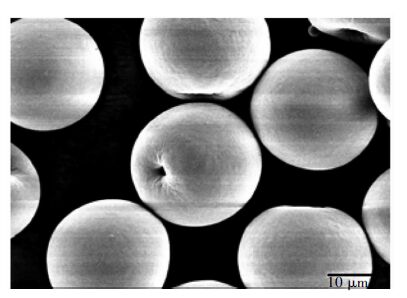 |
| 图 1 TiO2微球样品的SEM图像 Fig. 1 The SEM image of a TiO2 microsphere sample |
图 2显示了另一种尺寸均一的TiO2空心球(约0.2 m)[17],TiO2空心球以苯乙烯-甲基丙烯酸甲酯共聚物微球为模板,结合溶胶-凝胶法制备。如图 2显示,这些均匀的TiO2空心球由稀疏的锐钛矿型纳米颗粒聚合组成。氮气吸附-脱附结果表明,TiO2空心球的比表面积(81.1 m2/g)和孔容(0.23 m3/g)均大于TiO2纳米粒子(BET比表面积:39.1 m2/g;孔容:0.14 m3/g)。这些构造孔隙可能归属于样品颗粒间的空隙率。TiO2空心球因其比表面积更大,多孔结构更丰富,在降解甲基橙(MO)测试中,表现出更高的光催化活性;此外,这些空心球催化剂重复使用3次后,空心球的晶体结构没有发生明显变化,说明稳定性较好。
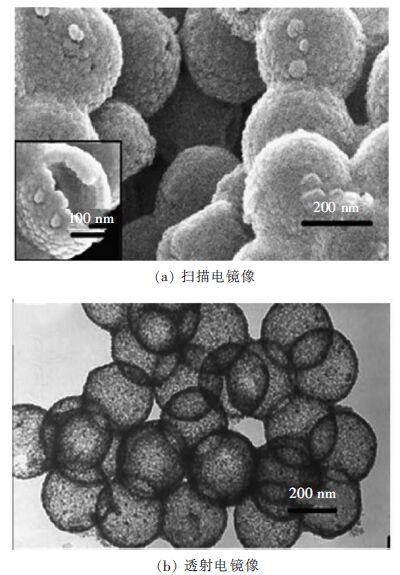 |
| 图 2 TiO2空心球的电镜图像 Fig. 2 Electron microscope images of TiO2 hollow spheres |
Hu等[18]报道了一种奇特的锐钛矿单晶TiO2微球(约2.5 m)。这些TiO2微球由众多的TiO2纳米管在水热条件下自组装而成。敞开的空心结构微球暴露出8个{101}面和1个{ 001 }面,光催化降解亚甲基蓝(MB)活性明显高于P25(商业TiO2)颗粒。
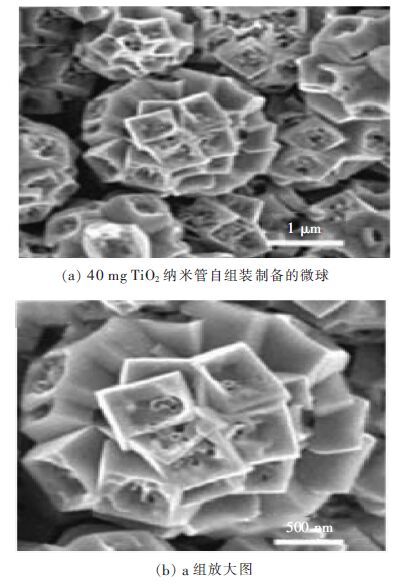 |
| 图 3 锐钛矿型TiO2微球及TiO2组合体单元的SEM图像 Fig. 3 SEM images of the anatase TiO2 microspheres and the unit TiO2 dous |
为进一步提高TiO2微球的光催化性能,可将微球制备成分级或空心结构,因为这种结构具备更好的光散射性,从而增强光捕获能力。因此,设计由一维构筑单元自组装成分级TiO2光催化剂具有重要意义。图 4显示了尺寸为5 m的分级TiO2微球(HTM)的SEM和TEM图像[19]。HTM样品的高倍透射电镜图像显示,介孔微球由直径约为10 nm、长度为几微米的TiO2纳米管彼此包裹而成。这类HTMs具备较高的比表面积和开放多孔网络,对苯酚的氧化反应及铬还原反应有较好的光催化活性。
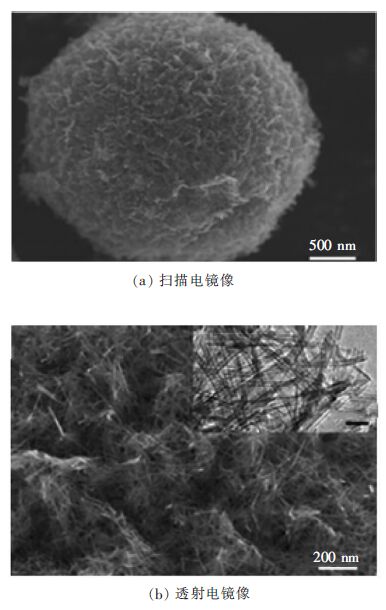 |
| 图 4 分级TiO2微球的电镜图像 Fig. 4 Electron microscope images of the hierarchical TiO2 microspheres |
为进一步提高单纯TiO2微球的综合性能,可对TiO2微球进行掺杂或复合。Kong等[20]采用超声喷雾热解法,在过氧化物前驱体溶液中制备了0.2~1 mm的Nb掺杂的TiO2多孔微球。在可见光照射下,这类Nb掺杂的微球比未掺杂TiO2微球表现出更高的光催化降解乙醛气体活性。为提高TiO2微球的可见光吸收性能,设计一种异质结型g-C3N4/Ag/TiO2微球(2~5 mm)[21]。其中,银纳米颗粒可以通过表面等离子体共振有效增强可见光吸收,而Ag/TiO2和g-C3N4间的紧密界面能阻碍电子空穴的复合。表面等离子体共振和界面间的协同效应导致g-C3 N4/Ag/TiO2微球光催化活性显著提高。为进一步加快TiO2微球在反应溶液中的分离速率,TiO2可附加磁性[22]。例如,Fe3O4@SiO2@TiO2@Pt分级核-壳微球有较大的比表面积(202.42 m2/g)和较高的饱和磁化强度(31.5 emu/g),使这种复合物微球容易在外部磁场的作用下得以分离,分离循环的回收效率高且回收物稳定。
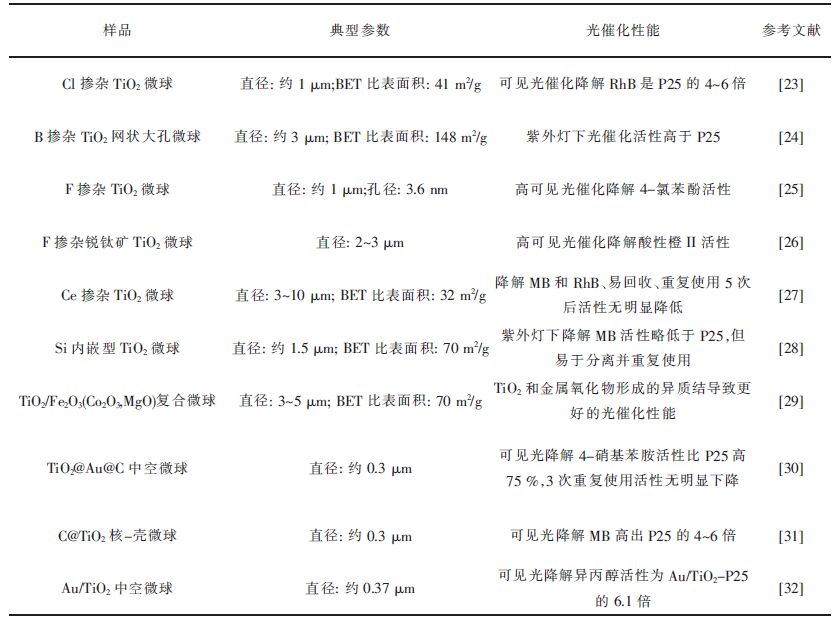
如表 1所示,总结了一系列的掺杂或复合型TiO2微球,以及它们在各类污染物降解,如在水和空气污染物的降解应用中的光催化性能。从表 1可以发现,目前微球光催化剂降解各种污染物优势明显。
| 表1 掺杂或复合型TiO2微球的光催化性能 Table 1 Photocatalytic properties of doped or composite TiO2 microspheres |
 |
| 点击放大 |
采用不同的方法制备的非TiO2型微球,也表现出优异的光催化性能。例如,由水热法制备的分级结构PbWO4微球(图 5,直径:2~3 μm),对酸性橙II染料的光催化降解活性较高[33]。PbWO4微球的分级结构具有较高的比表面积,加之对染料适宜的吸附能力,丰富的表面羟基,从而提高了活性。此外,PbWO4微球在在5次循环后活性无明显下降(新制样品降解率为96%;重复使用5次后:91%),表明此类光催化剂在环境净化中具备潜在的应用价值。
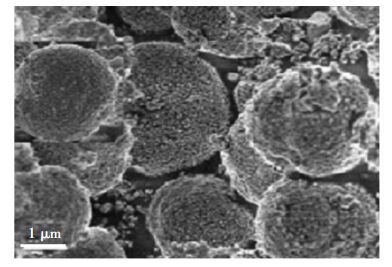 |
| 图 5 分级PbWO4微球的SEM图像 Fig. 5 SEM image of the hierarchical PbWO4 microspheres |
空心分级结构的Pt-ZnO纳米复合微球可以通过温和的溶剂热法制备[34]。图 6(a)和图 6(b)显示分级空心Pt-ZnO微球由结晶良好的Pt-ZnO纳米晶自组装而成。图 7显示,与纯ZnO纳米粉体和相应的Pt沉积的ZnO纳米粉体相比,对酸性橙Ⅱ染料的降解,Pt-ZnO微球有极高的光催化活性和稳定性。Pt-ZnO微球的显著光催化性能归因于其独特的纳米结构,以及Pt嵌入ZnO纳米晶导致的低电子-空穴复合率。对于有机污染物降解及其它环境净化方面的应用,这种中空Pt-ZnO纳米复合光催化微球是一种理想的材料。
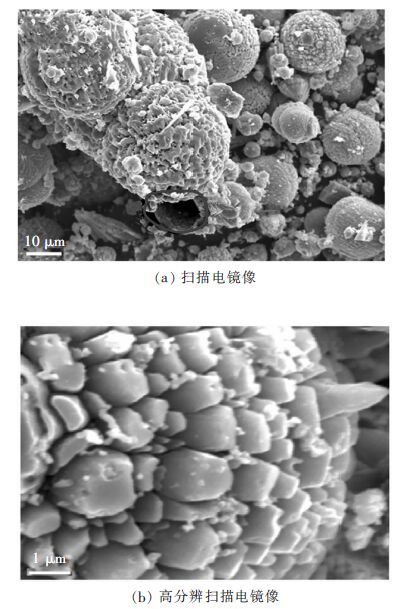 |
| 图 6 Pt (2%)-ZnO的电镜图像 Fig. 6 Electron microscope images of a microsphere in Pt (2%)-ZnO |
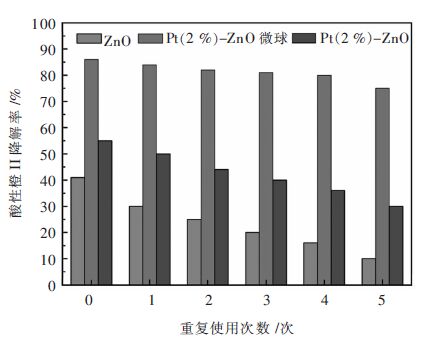 |
| 图 7 纯ZnO纳米粉体,Pt(2%)-ZnO复合微球及Pt(2%)/ZnO纳米粉体的光催化循环试验 Fig. 7 Photocatalytic recycling test of pure ZnO nano-powder,Pt (2%)-ZnO composite microspheres and Pt(2%)/ZnO nano-powder |
BiOX(X=Cl,Br,I)半导体具有独特的各向异性层状结构,有助于光生电子和空穴的快速分离和迁移。此外,这类半导体可以通过改变卤素成分及含量精确调控光吸收性能。因此,BiOX半导体能将有机污染物高效光催化降解为CO2和H2O[35-37]。但超细粉末颗粒的BiOX光催化剂往往存在比表面积小,难分离的缺点。Xia等[38]用离子液体制备的中空BiOI的组装体,比相应的BiOI纳米片具有更高的光催化活性。Yu等[39]发现BiOBr微球具有较强的捕光能力,并且更容易吸附水溶液中重金属和染料。另一种颗粒均一的BiOCl微球(直径:6.5 m,比表面积:9.3~27 m2/g)通过溶剂热合成,由纳米片组装而成[40],这种BiOCl微球在可见光和紫外光照射下循环使用4次后,光催化活性(高于商业P25)无明显下降。同样,基于自组装和Ostwald熟化生长机制,制备了BiOI空心微球(直径:1~3 m,比表面积:15.81 m2/g)[41],BiOI空心微球光催化降解罗丹明B(RhB)的速率常数分别为BiOI纳米片、TiO2和g-C3N4的4倍、10.3倍和19.8倍。此外,BiOI空心微球的光电流强度是BiOI纳米片的1.5倍,表明增强了光生电子和空穴的分离效率。Pan等[42]也制得了类似的BiOI微球(直径:1.6~1.7 μm),实验结果表明,在可见光照射下光催化降解双酚A,暴露{110}面的BiOI微球活性比暴露{001}面的BiOI微球更高。
如表 2所示,列出了其他常见的微球光催化剂,如碳量子点(CQDs)/Bi2WO6[43],BiFeO3[44],Ag3PO4/Bi2MoO6[45]和CeO2[46],进一步证实微球光催化剂在水处理应用中的优越性,包括催化活性高、光吸收效率好、连续运行保持高稳定性、易于从悬浆体系分离等。
| 表2 非TiO2微球及其光催化性能 Table 2 Photocatalytic properties of non-TiO2 microspheres |
 |
| 点击放大 |
微球光催化剂不仅在水体净化方面优势明显,在空气污染物降解方面也具备优势。例如,Ai等[75]发现气溶胶流动法制备的纳米晶InVO4中空微球(颗粒尺寸:0.5~1.5
之前分析表明,相对纳米粉体颗粒,微球光催化剂在水净化中效果显著,是因其孔径可调、比表面积大、光吸收好、载流子有效分离、易沉降、渗透性好、耐久性及传递能力高。由于TiO2独特的性质,TiO2微球在能源转化方面也受到广泛关注,利用其光催化分解水生成清洁可再生氢燃料[78]或将二氧化碳还原成太阳能燃料[79-83]的形式,使太阳能转换成化学能。氢燃料是低碳排放经济发展中的理想能源。在过去的几十年里,许多研究工作一直致力于制备TiO2及其他半导体微球以达到高效产氢效率。Yan等[84]总结了TiO2微球光催化剂的合成及其在太阳能高效分解水制氢燃料应用中的一些最新进展。
通过简易水热法,制得由锐钛矿型纳米十面体组装成的分级TiO2微球(直径:3~6
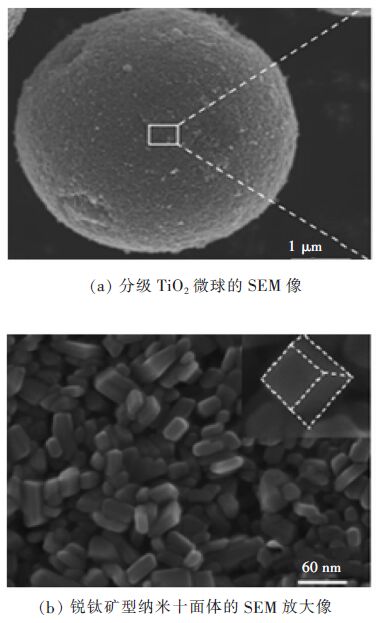 |
| 图 8 样品的扫描电镜图像 Fig. 8 SEM image of sample |
Cheng等[86]通过阴离子交换法,基于微观柯肯达尔效应,成功制备了Bi2WO6空心微球(直径:2~5 m;BET比比表面积:23.8 m2/g)。得到的Bi2WO6空心微球,在可见光照射,没有任何助催化剂的条件下,具有高吸附CO2能力和CO2转化甲醇的效率。这种分级中空结构使Bi2WO6具有更多的表面活性位点和更好的渗透性,有利于空间电荷转移,因此导致更佳的光催化性能。
最近,有报道[87]通过简单的水热法制备了一种由均匀纳米棒组成的锐钛矿型TiO2多孔微球(直径:1 m;BET比表面积:79 m2/g)。所制备的微球在最佳实验条件下,光电流和产氢速率(约3.85 μmol/h)分别是P25(约1.58 μmol/h)的4.8倍和2.4倍。所合成TiO2微球具有较高的比表面积,这使得微球能更有效地捕获光能,因而具有更高的反应活性。
通过浸渍-还原法在TiO2微球(5~10 μm)上负载1.2 %(质量分数)Pt,提高了TiO2微球的分解水效率[88]。Pt/TiO2微球比未改性的TiO2微球的产氢速率高出约125倍。此外,Pt/TiO2微球表现出较高的稳定性,3次循环使用后光催化活性无明显下降。
其他微球,如CuInS2[89],Cu掺杂的ZnIn2S4[90]和ZnGa2O4[91],也被用于制氢和二氧化碳还原。例如,Zheng等[89]开发了一种简易原位形成模板-溶剂热法制备了CuInS2分级微球(平均直径:3.5~4.0 m),产物在循环使用中具有很高的可见光催化产氢活性,在可见光照射下,氢气的平均产率达59.4 mol/(h·g),这可能是因为CuS模板的优点:如高比表面积、单分散性、渗透性好,保证了充分的可见光响应和在液相光催化反应中丰裕的反应位点。Liu等[91]报道了一种由超薄ZnGa2O4纳米片架构成的均匀分级ZnGa2O4微球。这种既继承了块体材料高结晶度又继承了纳米片高比表面积优点的三维分级结构在CO2还原成CH4光催化反应中表现出显著活性。
如表 3所示,列出了其他一些微球光催化剂在能源转化中的应用。上述结果表明,分级中空微球光催化剂有助于提高光催化产氢和二氧化碳转化效率。
| 表3 微球光催化剂在光催化分解水及还原二氧化碳成太阳能燃料方面的应用 Table 3 Applications of microsphere photocatalysts in water splitting or CO2 reduction to solar fuels |
 |
| 点击放大 |
3 结论与展望
为使环境光催化进一步发展,应用于实际水净化和能源转换中,还需要着力提高光催化效率,降低运行成本。微球光催化剂与其粉末对应物相比,在回收应用方面展现独特的优势。此外,微球具有分级结构,可以展现出较大的比表面积、高效的光捕获能力、丰富的表面活性位点以及更好的渗透性。这些独特的结构有利于空间电荷转移,从而显著提升光催化性能。因此,设计及制备所需结构或形貌的高品质光催化剂具有重要意义。未来的工作将致力于开发简单、绿色的合成方法以制备微球光催化剂。此外,基于材料角度探索不同微球光催化剂的生长机制,系统研究产物活性与晶体的晶相、结晶度、表面结构、形貌等之间的关系,有利于设计高效微球光催化剂;基于密度泛函理论计算和第一性原理研究可以有效确定具有特定形貌的微球光催化剂,并得到合理的处理和制备条件,这将有助于对微球光催化剂的可控高效制备。
| [1] |
FUJISHIMA A, HONDA K. Electrochemical photolysis of water at a semiconductor electrode[J].
Nature, 1972, 238: 37–38. DOI: 10.1038/238037a0. |
| [2] |
UDDIN M T, BABOT O, THOMAS L, et al. New insights into the photocatalytic properties of RuO2/TiO2 mesoporous heterostructures for hydrogen production and organic pollutant photodecomposition[J].
Journal of Physical Chemistry C, 2015, 119(13): 7006–7015. DOI: 10.1021/jp512769u. |
| [3] |
ZHOU X M, LIU G, YU J G, et al. Surface plasmon resonance-mediated photocatalysis by noble metal-based composites under visible light[J].
Journal of Materials Chemistry, 2012, 22(40): 21337–21354. DOI: 10.1039/c2jm31902k. |
| [4] |
ZHANG J Y, WANG Y H, ZHANG J, et al. Enhanced photocatatytic hydrogen production activities of Au-loaded ZnS flowers[J].
ACSApplied Materials andInterfaces, 2013, 5(3): 1031–1037. DOI: 10.1021/am302726y. |
| [5] |
ZHOU X, LI X, GAO Q, et al. Metal-free carbon nanotube-SiC nanowire heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation[J].
Catalysis Science and Technology, 2015, 5(5): 2798–2806. DOI: 10.1039/C4CY01757A. |
| [6] |
YUAN Y P, RUAN L W, BARBER J, et al. Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion[J].
Energyand Environmental Science, 2014, 7(12): 3934–3951. DOI: 10.1039/C4EE02914C. |
| [7] | 薛霜霜, 何洪波, 余长林. 稀土上转换用于提高半导体化合物光催化效率的研究进展[J]. 有色金属科学与工程, 2015, 6(4): 97–103. |
| [8] | 何洪波, 薛霜霜, 余长林. 钨基半导体光催化剂研究进展[J]. 有色金属科学与工程, 2015, 6(5): 32–39. |
| [9] | 陈建钗, 薛霜霜, 余长林. 稀土在非TiO2光催化剂的改性研究[J]. 有色金属科学与工程, 2015, 6(1): 99–105. |
| [10] | 魏龙福, 余长林, 陈建钗, 等. 水热法合成Ag2CO3/ZnO异质结复合光催化剂及其光催化性能[J]. 有色金属科学与工程, 2014, 5(1): 47–53. |
| [11] | 魏龙福, 余长林. 石墨烯/半导体复合光催化剂的研究进展[J]. 有色金属科学与工程, 2013, 4(3): 34–39. |
| [12] |
LIU H, YU C L, WANG C. Economics and its implication of photocatalyst recycling for water purification, handbook of photocatalysts: preparation, structure and application[J].
US: Nova Science Publishers, 2009, Chapter 19: 527–534. |
| [13] |
GRTZEL M. Dye-sensitized solarcells[J].
JournalofPhotochemistryandPhotobiology C:PhotochemistryReviews, 2003, 4(2): 145–153. |
| [14] |
YU C L, YU J C, CHAN M. Sonochemical fabrication of fluorinated mesoporous titanium dioxide microspheres[J].
Journal ofSolidStateChemistry, 2009, 182(5): 1061–1069. |
| [15] |
YU J C, YU J G, ZHANG L Z, et al. Enhancing effects of water content and ultrasonic irradiation on the photocatalytic activity of nano-sized TiO2 powders[J].
Journal of Photochemistry and PhotobiologyA: Chemistry, 2002, 148(1): 263–271. |
| [16] |
LI X Z, LIU H, CHENG L F, et al. Photocatalytic oxidation using a new catalytic TiO2 microspherefor water and wastewater treatment[J].
Environmental Science and Technology, 2003, 37(17): 3989–3994. DOI: 10.1021/es0262941. |
| [17] |
SONG C Y, YU W J, ZHAO B, et al. Efficient fabrication and photocatalytic properties of TiO2 hollow spheres[J].
Catalysis Communications, 2009, 10(5): 650–654. DOI: 10.1016/j.catcom.2008.11.010. |
| [18] |
HU X Y, ZHANG T C, JIN Z, et al. Single-crystalline anatase TiO2 dous assembled micro-sphere and their photocatalytic activity[J].
CrystalGrowthand Design, 2009, 9(5): 2324–2328. |
| [19] |
ZHENG Z K, HUANG B B, QIN X Y, et al. Strategic synthesis of hierarchical TiO2 microspheres with enhanced photocatalytic activity[J].
Chemistry-A European Journal, 2010, 16(37): 11266–11270. DOI: 10.1002/chem.v16:37. |
| [20] |
KONG L N, WANG C H, ZHENG H, et al. Defect-induced yellow color in Nb-doped TiO2 and its impact on visible-light photocatalysis[J].
Journal of Physical Chemistry C, 2015, 119(29): 16623–16632. DOI: 10.1021/acs.jpcc.5b03448. |
| [21] |
CHEN Y F, HUANG W X, HE D L, et al. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-Light irradiation[J].
ACSApplied Materials andInterfaces, 2014, 6(16): 14405–14414. DOI: 10.1021/am503674e. |
| [22] |
LI X Y, LIU D P, SONG S Y, et al. Fe3O4@SiO2@TiO2@Pt hierarchical core-shell microspheres: controlled synthesis, enhanced degradation system, and rapid magnetic separation to recycle[J].
CrystalGrowthand Design, 2014, 14(11): 5506–5511. |
| [23] |
XU H, ZHENG Z, ZHANG L Z, et al. Hierarchical chlorine-doped rutile TiO2 spherical clusters of nanorods: large-scale synthesis and high photocatalytic activity[J].
Journal ofSolidStateChemistry, 2008, 181(9): 2516–2522. |
| [24] |
FEI H L, LIU Y P, LI Y P, et al. Selective synthesis of borated meso-macroporous and mesoporous spherical TiO2 with high photocatalytic activity[J].
Microporous and Mesoporous Materials, 2007, 102(1): 318–324. |
| [25] |
YU J C. Synthesis of hierarchical nanoporous F-doped TiO2 spheres with visible light photocatalytic activity[J].
Chemical Communications, 2006(10): 1115–1117. DOI: 10.1039/b515513d. |
| [26] |
YU C L, ZHOU W Q, YANG K, et al. Hydrothermal synthesis of hemisphere-like F-doped anatase TiO2 with visible light photocatalytic activity[J].
Journal of Materials Science, 2010, 45(21): 5756–5761. DOI: 10.1007/s10853-010-4646-6. |
| [27] |
XIE J M, JIANG D L, CHEN M, et al. Preparation and characterization of monodisperse Ce-doped TiO2 microspheres with visible light photocatalytic activity[J].
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 372(1): 107–114. |
| [28] |
LI G, LIU F, ZHANG Z. Enhanced photocatalytic activity of silica-embedded TiO2 hollow microspheres prepared by one-pot approach[J].
Journal ofAlloysand Compounds, 2010, 493(1): 1–7. |
| [29] |
LU X J, HUANG F Q, WU J J, et al. Intelligent hydrated-sulfate template assisted preparation of nanoporous TiO2 spheres and their visible-light application[J].
ACSApplied Materials andInterfaces, 2011, 3(2): 566–572. DOI: 10.1021/am101137w. |
| [30] |
CAI J B, WU X Q, LI S X, et al. Synergistic effect of double-shelled and sandwiched TiO2@Au@C hollow spheres with enhanced visible-light-driven photocatalytic activity[J].
ACSApplied Materials andInterfaces, 2015, 7(6): 3764–3772. DOI: 10.1021/am508554t. |
| [31] |
CONG Y, LI X K, DONG Z J, et al. Core-shell structured carbon black@TiO2 microsphere with enhanced visible light photocatalytic activity[J].
Materials Letters, 2015, 138: 200–203. DOI: 10.1016/j.matlet.2014.09.061. |
| [32] |
DINH C T, YEN H, KLEITZ F, et al. Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis[J].
Angewandte Chemie International Edition, 2014, 53(26): 6618–6623. DOI: 10.1002/anie.201400966. |
| [33] |
YU C L, CAO F F, LI X, et al. Hydrothermal synthesis and characterization of novel PbWO4 microspheres with hierarchical nanostructures and enhanced photocatalytic performance in dye degradation[J].
Chemical Engineering Journal, 2013, 219: 86–95. DOI: 10.1016/j.cej.2012.12.064. |
| [34] |
YU C L, YANG K, XIE Y, et al. Novel hollow Pt-ZnO nanocomposite microspheres with hierarchical structure and enhanced photocatalytic activity and stability[J].
Nanoscale, 2013, 5(5): 2142–2151. DOI: 10.1039/c2nr33595f. |
| [35] |
YU C L, CAO F F, LI G, et al. Novel noble metal (Rh, Pd, Pt)/BiOX(Cl, Br, I) composite photocatalysts with enhanced photocatalytic performance in dye degradation[J].
Separation and Purification Technology, 2013, 120: 110–122. DOI: 10.1016/j.seppur.2013.09.036. |
| [36] |
YU C L, FAN C F, YU J C, et al. Preparation of bismuth oxyiodides and oxides and their photooxidation characteristic under visible/UV light irradiation[J].
Materials Research Bulletin, 2011, 46(1): 140–146. DOI: 10.1016/j.materresbull.2010.08.013. |
| [37] |
LI W, JIA X K, LI P T, et al. Hollow mesoporous SiO2-BiOBr nanophotocatalyst: synthesis,characterization and application in photodegradation of organic dyes under visible-light irradiation[J].
ACSSustainableChemistry and Engineering, 2015, 3(6): 1101–1110. |
| [38] | XIA J X, YIN S, LI H M, et al. Self-assembly and enhanced photocatalytic properties of BiOI hollow microspheres via a reactable ionic liquid. Langmuir, 2011, 27(3): 1200-1206. |
| [39] |
ZHANG D Q, WEN M C, JIANG B, et al. Ionothermal synthesis of hierarchical BiOBr microspheres for water treatment[J].
Journal of Hazardous Materials, 2012, 211: 104–111. |
| [40] |
DING L Y, WEI R J, CHEN H, et al. Controllable synthesis of highly active BiOCl hierarchical microsphere self-assembled by nanosheets with tunable thickness[J].
Applied Catalysis B: Environmental, 2015, 172: 91–99. |
| [41] |
DI J, XIA J X, GE Y P, et al. Reactable ionic liquid-assisted rapid synthesis of BiOI hollow microspheres at room temperature with enhanced photocatalytic activity[J].
Journal of Materials ChemistryA, 2014, 2(38): 15864–15874. DOI: 10.1039/C4TA02400A. |
| [42] |
PAN M L, ZHANG H J, GAO G D, et al. Facet-dependent catalytic activity of nanosheet-assembled bismuth oxyiodide microspheres in degradation of Bisphenol A[J].
Environmental Science and Technology, 2015, 49(10): 6240–6248. DOI: 10.1021/acs.est.5b00626. |
| [43] |
DI J, XIA J X, GE Y P, et al. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight[J].
Applied catalysis B: Environmental, 2015, 168: 51–61. |
| [44] |
HUO Y N, MIAO M, ZHANG Y, et al. Aerosol-spraying preparation of a mesoporous hollow spherical BiFeO3 visible photocatalyst with enhanced activity and durability[J].
ChemicalCommunications, 2011, 47(7): 2089–2091. |
| [45] |
XU Y S, ZHANG W D. Monodispersed Ag3PO4 nanocrystals loaded on the surface of spherical Bi2MoO6 with enhanced photocatalytic performance[J].
DaltonTransactions, 2013, 42(4): 1094–1101. DOI: 10.1039/C2DT31634J. |
| [46] |
NAKAJIMA A, KOBAYASHI T, ISOBE T, et al. Preparation and visible-light photocatalytic activity of Au-supported porous CeO2 spherical particles using templating[J].
Materials Letters, 2011, 65(19): 3051–3054. |
| [47] |
FENG L L, ZHANG C L, GAO G, et al. Facile synthesis of hollow Cu2O octahedral and spherical nanocrystals and their morphology dependent photocatalytic properties[J].
NanoscaleResearch Letters, 2012, 7(1): 1–10. |
| [48] |
LU W W, GAO S Y, WANG J J. One-Pot synthesis of Ag/ZnO self-assembled 3D hollow microspheres with enhanced photocatalytic performance[J].
Journal of Physical ChemistryC, 2008, 112(43): 16792–16800. |
| [49] |
WANG W S, ZHEN L, XU C Y, et al. Room temperature synthesis, growth mechanism, photocatalytic and photoluminescence properties of cadmium molybdate core-shell microspheres[J].
CrystalGrowthand Design, 2009, 9(3): 1558–1568. |
| [50] |
ZHANG L S, WANG W Z, ZHOU L, et al. Bi2WO6 nano-and microstructures: shape control and associated visible-light-driven photocatalytic activities[J].
Small, 2007, 3(9): 1618–1625. DOI: 10.1002/(ISSN)1613-6829. |
| [51] |
YU J G, QI L F, CHENG B, et al. Effect of calcination temperatures on microstructures and photocatalytic activity of tungsten trioxide hollow microspheres[J].
Journal of Hazardous Materials, 2008, 16(2): 621–628. |
| [52] |
ZHANG X, AI Z H, JIA F L, et al. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X=Cl, Br, I) nanoplate microspheres[J].
Journal of Physical ChemistryC, 2008, 112(3): 747–753. |
| [53] |
DI J, XIA J X, YIN S, et al. One-pot solvothermal synthesis of Cu-modified BiOCl via a Cu-containing ionic liquid and its visible-light photocatalytic properties[J].
RSC Advances, 2014, 4(27). |
| [54] |
DI J, XIA J X, YIN S, et al. Preparation of sphere-like g-C3N4/BiOI photocatalysts via a reactable ionic liquid for visible-light-driven photocatalytic degradation of pollutants[J].
Journal of Materials Chemistry A, 2014, 2(15): 5340–5351. DOI: 10.1039/c3ta14617k. |
| [55] |
DI J, XIA J X, GE Y P, et al. Facile fabrication and enhanced visible light photocatalytic activity of few-layer MoS2 coupled BiOBr microspheres[J].
Dalton Ttransactions, 2014, 43(41): 15429–15438. DOI: 10.1039/C4DT01652A. |
| [56] |
JIAO H P, YU X, LIU Z Q, et al. One-pot synthesis of heterostructured Bi2S3/BiOBr microspheres with highly efficient visible light photocatalytic performance[J].
RSC Advances, 2015, 5: 16239–16249. DOI: 10.1039/C4RA16948D. |
| [57] |
GUO X J, ZHEN M M, LIU H J, et al. BiOBr-BiOI microsphere assembled with atomthick ultrathin nanosheets and its high photocatalytic activity[J].
RSC Advances, 2015, 5(31): 24777–24782. DOI: 10.1039/C5RA01086A. |
| [58] |
GIRIJA K, THIRUMALAIRAJAN S, MASTELARO V R, et al. Photocatalytic degradation of organic pollutants by shape selective synthesis of b-Ga2O3 microspheres constituted by nanospheres for environmental remediation[J].
Journal of Materials Chemistry A, 2015, 3(6): 2617–2627. DOI: 10.1039/C4TA05295A. |
| [59] |
XIA W W, MEI C, ZENG X H, et al. Nanoplate-built ZnO hollow microspheres decorated with gold nanoparticles and their enhanced photocatalytic and gas-sensing properties[J].
ACSApplied Materials andInterfaces, 2015, 7(22): 11824–11832. DOI: 10.1021/acsami.5b01333. |
| [60] |
YUE L F, WANG S F, SHAN G Q, et al. Novel MWNTs-Bi2WO6 composites with enhanced simulated solar photoactivity toward adsorbed and free tetracycline in water[J].
Applied Catalysis B: Environmental, 2015, 176: 11–19. |
| [61] |
FAN H M, WANG D J, LIU Z P, et al. Self-assembled BiVO4/Bi2WO6 microspheres: synthesis, photoinduced charge transfer properties and photocatalytic activities[J].
Dalton Ttransactions, 2015, 44(26): 11725–11731. DOI: 10.1039/C5DT01222H. |
| [62] |
LOU S Y, JIA X B, WANG Y Q, et al. Template-assisted in-situ synthesis of porous AgBr/Ag composite microspheres as highly efficient visible-light photocatalyst[J].
Applied Catalysis B: Environmental, 2015, 176: 586–593. |
| [63] |
GUO F, SHI W L, LIN X, et al. Novel BiVO4/InVO4 heterojunctions: Facile synthesis and efficient visible-light photocatalytic performance for the degradation of rhodamine B[J].
Separation and Purification Technology, 2015, 141: 246–255. DOI: 10.1016/j.seppur.2014.11.026. |
| [64] |
ZHONG J S, WANG Q Y, CHEN D Q, et al. Biomolecule-assisted solvothermal synthesis of 3D hierarchical Cu2FeSnS4 microspheres with enhanced photocatalytic activity[J].
Applied Surface Science, 2015, 343: 28–32. DOI: 10.1016/j.apsusc.2015.03.066. |
| [65] |
LIU D, WANG J, ZHANG M, et al. A superior photocatalytic performance of a novel Bi2SiO5 flower-like microsphereviaa phase junction[J].
Nanoscale, 2014, 6(24): 15222–15227. DOI: 10.1039/C4NR05058D. |
| [66] |
JIANG G H, WANG R J, WANG X H, et al. Novel highly active visible-light-induced photocatalysts based on BiOBr with Ti doping and Ag decorating[J].
ACS Applied Materials and Interfaces, 2012, 4(9): 4440–4444. DOI: 10.1021/am301177k. |
| [67] |
LIU X W, FANG Z, ZHANG X J, et al. Preparation and characterization of Fe3O4/CdS nanocomposites and their use as recyclable photocatalysts[J].
Crystal Growth and Design, 2009, 9(1): 197–202. DOI: 10.1021/cg800213w. |
| [68] |
LI X N, HUANG R K, HU Y H, et al. A templated method to Bi2WO6 hollow microspheres and their conversion to double-shell Bi2O3/Bi2WO6 hollow microspheres with improved photocatalytic performance[J].
Inorganic Chemistry, 2012, 51(11): 6245–6250. DOI: 10.1021/ic300454q. |
| [69] |
MURUGANANDHAM M, KUSUMOTO Y. Synthesis of N, C codoped hierarchical porous microsphere ZnS as a visible light-responsive photocatalyst[J].
Journal of Physical ChemistryC, 2009, 113(36): 16144–16150. |
| [70] |
DONG F, SUN Y J, FU M, et al. Novel in situ N-doped (BiO)2CO3 hierarchical microspheres self-assembled by nanosheets as efficient and durable visible light driven photocatalyst[J].
Langmuir, 2012, 28(1): 766–773. DOI: 10.1021/la202752q. |
| [71] |
DONG F, LI Q Y, SUN Y J, et al. Noble metal-like behavior of plasmonic Bi particles as a cocatalyst deposited on (BiO)2CO3 microspheres for efficient visible light photocatalysis[J].
ACS Catalysis, 2014, 4(12): 4341–4350. DOI: 10.1021/cs501038q. |
| [72] |
CHEN Z X, LI D Z, ZHANG W J, et al. Low-temperature and template-free synthesis of ZnIn2S4 microspheres[J].
Inorganic Chemistry, 2008, 47(21): 9766–9772. DOI: 10.1021/ic800752t. |
| [73] |
RENGARAJ S, VENKATARAJ S, TAI C W, et al. Self-assembled mesoporous hierarchical-like In2S3 hollow microspheres composed of nanofibers and nanosheets and their photocatalytic activity[J].
Langmuir, 2011, 27(9): 5534–5541. DOI: 10.1021/la104780d. |
| [74] |
ZHANG J, ELSANOUSI A, LIN J, et al. Aerosol-assisted self-assembly of aluminum borate (Al18B4O33) nanowires into three dimensional hollow spherical architectures[J].
Crystal Growth and Design, 2007, 7(12): 2764–2767. DOI: 10.1021/cg070492s. |
| [75] |
AI Z H, ZHANG L Z, LEE S C. Efficient visible light photocatalytic oxidation of NO on aerosol flow-synthesized nanocrystalline InVO4 hollow microspheres[J].
Journal of Physical ChemistryC, 2010, 114(43): 18594–18600. |
| [76] |
HUANG Y, AI Z H, HO W K, et al. Ultrasonic spray pyrolysis synthesis of porous Bi2WO6 microspheres and their visible-light -induced photocatalytic removal of NO[J].
Journal of Physical ChemistryC, 2010, 114(14): 6342–6349. |
| [77] |
DONG F, XIONG T, SUN Y J, et al. Synergistic integration of thermocatalysis and photocatalysis on black defective (BiO)2CO3 microspheres[J].
Journal of Materials ChemistryA, 2015, 3(36): 18466–18474. DOI: 10.1039/C5TA05099E. |
| [78] |
LIU G, PAN J, YIN L C, et al. Heteroatom-modulated switching of photocatalytic hydrogen and oxygen evolution preferences of anatase TiO2 microspheres[J].
Advanced Functional Materials, 2012, 22(15): 3233–3238. DOI: 10.1002/adfm.v22.15. |
| [79] |
MA Y, WANG X, JIA Y, X ., et al. Titanium dioxide-based nanomaterials for photocatalytic fuel generations[J].
Chemical Reviews, 2014, 114(19): 9987–10043. DOI: 10.1021/cr500008u. |
| [80] |
BACHMEIER A, WANG C, WOOLERTON T W, et al. How light-harvesting semiconductors can alter the bias of reversible electrocatalysts in favor of H2 production and CO2 reduction[J].
Journal of the American Chemical Society, 2013, 135(40): 15026–15032. DOI: 10.1021/ja4042675. |
| [81] |
PROTTI S, ALBINI A, SERPONE N. Photocatalytic generation of solar fuels from the reduction of H2O and CO2: A look at the patent literature[J].
Physical Chemistry Chemical Physics, 2014, 16(37): 19790–19827. DOI: 10.1039/C4CP02828G. |
| [82] |
PARIDA K. Facile fabrication of S-TiO2/β-SiC nanocomposite photocatalyst for hydrogen evolution under visible light irradiation[J].
ACS Sustainable Chemistry and Engineering, 2015, 3(2): 245–253. DOI: 10.1021/sc500570k. |
| [83] |
ZHOU W, LI W, WANG J Q, et al. Ordered mesoporous black TiO2 as highly efficient hydrogen evolution photocatalyst[J].
Journal of the American Chemical Society, 2014, 136(26): 9280–9283. DOI: 10.1021/ja504802q. |
| [84] |
YAN K, WU G S. Titanium dioxide microsphere-derived materials for solar fuel hydrogen generation[J].
ACS Sustainable Chemistry and Engineering, 2015, 3(5): 779–791. DOI: 10.1021/acssuschemeng.5b00154. |
| [85] |
ZHENG Z K, HUANG B B, LU J B, et al. Hierarchical TiO2 microspheres: synergetic effect of {001} and {101} facets for enhanced photocatalytic activity[J].
Chemistry-A European Journal, 2011, 17(52): 15032–15038. DOI: 10.1002/chem.201101466. |
| [86] |
CHENG H F, HUANG B B, LIU Y Y, et al. An anion exchange approach to Bi2WO6 hollow microspheres with efficient visible light photocatalytic reduction of CO2 to methanol[J].
Chemical Communications, 2012, 48(78): 9729–9731. DOI: 10.1039/c2cc35289c. |
| [87] |
YAN K, WU G S, JARVIS C, et al. Facile synthesis of porous microspheres composed of TiO2 nanorods with high photocatalytic activity for hydrogen production[J].
Applied Catalysis B: Environmental, 2014, 148: 281–287. |
| [88] |
WEI P, LIU J W, LI Z H. Effect of Pt loading and calcination temperature on the photocatalytic hydrogen production activity of TiO2 microspheres[J].
Ceramics International, 2013, 39(5): 5387–5391. DOI: 10.1016/j.ceramint.2012.12.045. |
| [89] |
ZHENG L, XU Y, SONG Y, et al. Nearly monodisperse CuInS2 hierarchical microarchitectures for photocatalytic H2 evolution under visible light[J].
Inorganic Chemistry, 2009, 48(9): 4003–4009. DOI: 10.1021/ic802399f. |
| [90] |
SHEN S H, ZHAO L, ZHOU Z H, et al. Enhanced photocatalytic hydrogen evolution over Cu-doped ZnIn2S4 under visible light irradiation[J].
Journal of Physical ChemistryC, 2008, 112(41): 16148–16155. |
| [91] |
LIU Q, WU D, ZHOU Y, et al. Single-crystalline, ultrathin ZnGa2O4 nanosheet scaffolds to promote photocatalytic activity in CO2 reduction into methane[J].
ACSApplied Materials andInterfaces, 2014, 6(4): 2356–2361. DOI: 10.1021/am404572g. |
| [92] |
FANG B Z, BONAKDARPOUR A, REILLY K, et al. Large-Scale synthesis of TiO2 microspheres with hierarchical nanostructure for highly efficient photodriven reduction of CO2 to CH4[J].
ACSApplied Materials andInterfaces, 2014, 6(17): 15488–15498. DOI: 10.1021/am504128t. |
| [93] |
SHI F F, CHEN L L, XING C S, et al. ZnS microsphere /g-C3N4 nanocomposite photocatalyst with greatly enhanced visible light performance for hydrogen evolution: synthesis and synergistic mechanism study[J].
RSCAdvances, 2014, 4(107): 62223–62229. |
| [94] |
WU T T, XIE Y P, YIN L C, et al. Witching photocatalytic H2 and O2 generation preferences of rutile TiO2 microspheres with dominant reactive facets by boron doping[J].
Journal of Physical ChemistryC, 2015, 119(1): 84–89. |
| [95] |
GUA Q, LIAO Y S, YIN L S, et al. Template-free synthesis of porous graphitic carbon nitride microspheres for enhanced photocatalytic hydrogen generation with high stability[J].
Applied Catalysis B: Environmental, 2015, 165: 503–510. DOI: 10.1016/j.apcatb.2014.10.045. |
| [96] |
WANG S B, HOU Y D, WANG X C. Development of a stable MnCo2O4 cocatalyst for photocatalytic CO2 reduction with visible light[J].
ACSApplied Materials andInterfaces, 2015, 7(7): 4327–4335. DOI: 10.1021/am508766s. |
| [97] |
LI H, ZHANG X, CUI X. A facile and waste-free strategy to fabricate Pt-C/TiO2 microspheres: Enhanced photocatalytic performance for hydrogen evolution[J].
International Journal ofPhotoenergy, 2014, 2014: 1–9. |
| [98] |
LIU B, LIU L M, LANG X F, et al. Doping high-surface-area mesoporous TiO2 microspheres with carbonate for visible light hydrogen production[J].
Energyand Environmental Science, 2014, 7(8): 2592–2597. DOI: 10.1039/C4EE00472H. |
 2016, Vol. 7
2016, Vol. 7


