| 表面活性剂对ZnWO4:Tb3+绿色荧光粉发光性能的影响 |
稀土离子具有丰富的能级结构,其发光波长可在紫外到红外之间变化,常被作为发光中心应用于发光材料中[1-4].对于稀土离子Tb3+来说,由于其在绿光波段(5D4→7F5)有个很强的发射,因而Tb3+离子掺杂的发光材料广泛用于绿色荧光粉.目前用Tb3+离子掺杂主要是铝酸盐[5]和磷酸盐[6],但用传统的铝酸盐,硼酸盐和磷酸盐掺杂稀土离子作为发光材料都有其各自的缺陷[7-9].金属钨酸盐是一种重要的无机功能材料,在众多领域都有广泛的应用,例如激光材料[10],微波陶瓷[11],及光致发光荧光材料[12].而具有钨锰矿铁石结构的ZnWO4发光材料,由于存在着W-O键,5d轨道的W及2p轨道的O分别充当导带和价带;此外,WO42-本身存在自激发荧光, 在紫外光照射下发出蓝绿光, 而且能将能量有效的传递给稀土离子,因而受到人们的广泛关注. Dai Qilin等[13]等人采用水热法合成ZnWO4:Eu荧光粉, 并研究了不同温度和pH对荧光粉发光性能的影响. He[14]成功合成ZnWO4:Sm纳米荧光粉并研究了Sm3+离子浓度对其发光的影响.
在稀土掺杂ZnWO4体系中,由于三价稀土离子Tb3+与Zn2+电荷不等价,一般在合成ZnWO4:Tb3+过程中引入Na+作为电荷补偿,其电荷补偿是通过以下式来完成:2Zn2+=Tb3++Na+.另外,Na+的引入将增强稀土离子的发光强度[2, 15].目前稀土掺杂ZnWO4的合成方法有溶胶凝胶法[16],燃烧法[17],水热法[18],微乳液法[19].然而,据我们所知,目前暂未见利用表面活性剂合成绿色荧光粉ZnWO4:Tb3+的报道.因此,本文采用不同表面活性剂,通过水热法合成ZnWO4:Tb3+荧光粉,系统考察不同表面活性剂对ZnWO4:Tb3+荧光粉结构及发光性能的影响.
1 实验方法与测试 1.1 实验原料及制备过程本实验所用药品:六水合硝酸锌(Zn(NO3)2·6H2O), 氢氧化钠(NaOH), 八水合钨酸氨(H40N10O41W12·8H2O), 氧化铽(Tb4O7),硝酸(HNO3),聚乙烯吡咯烷酮(PVP),十六烷基三甲基溴化铵(CTAB),聚乙二醇2000(PEG-2000).
PVP为表面活性剂的样品制备过程:采用HNO3和蒸馏水溶解Tb4O7粉末并配成浓度为0.05mol/L Tb(NO3)3溶液.首先称取0.0061g NaOH,0.8032g Zn(NO3)2·6H2O,0.6g PVP(聚乙烯吡咯烷酮)用蒸馏水溶解,取3mL 0.05mol/L Tb(NO3)3溶液加入至上述溶液并不断搅拌形成50mL的A混合溶液.然后称取0.7922g H40N10O41W12·8H2O溶于氨水中形成B溶液,在不断搅拌的情况下,将B液缓慢加入至A液,再用氨水调节溶液的pH=8,搅拌3h后转入高压反应釜,在453K水热18h后得沉淀物,将沉淀物离心洗涤3~4次,最后进行353K干燥4h得到最终产物.采用其它表面活性剂(只是把表面活性剂分别更换成0.448g CTAB,0.4g PEG-2000)的样品制备方法同上述.
1.2 实验测试采用Cu靶Kα1辐射的PANalytical X'Pert Pro粉末X射线衍射仪来测定物相, 扫描电子显微镜(TM-3030)测定样品形貌与尺寸,Edinburgh Instruments FLS920荧光光谱仪对样品的激发和发射以及荧光衰减曲线进行测定.所有测试均在室温下进行.
2 结果与讨论 2.1 添加不同表面活性剂合成ZnWO4:Tb3+的物相与形貌分析图 1所示为添加不同表面活性剂所制备的ZnWO4:Tb3+XRD图.由图 1可知,所有样品的衍射峰均与标准卡片(JCDPS NO.015-0774)相吻合,且无杂峰出现.表明制备的样品为纯的单斜相ZnWO4结构,Tb掺杂并没有改变钨酸锌结构,且结晶度好.
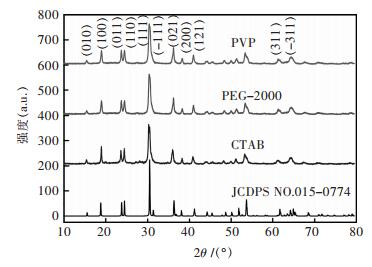 |
| 图 1 在不同表面活性剂条件下合成的ZnWO4:Tb3+样品XRD谱 Fig. 1 XRD patterns for ZnWO4:Tb3+samples with different surfactants |
图 2所示为添加不同表面活性剂所制备的ZnWO4:Tb3+SEM图,其中a, b, c分别对应PEG-2000, PVP, CTAB为表面活性剂制备样品.由图 2(a)可知,添加PEG-2000为表面活性剂制备的ZnWO4:Tb3+样品完全团聚,图 2(b)中可以看到样品团聚较为严重,但能看出几根棒状结构的ZnWO4:Tb3+,尺寸为0.9μm,图 2(c)中可以看到大量棒状结构的ZnWO4:Tb3+,平均尺寸为1.1μm, 样品部分团聚.
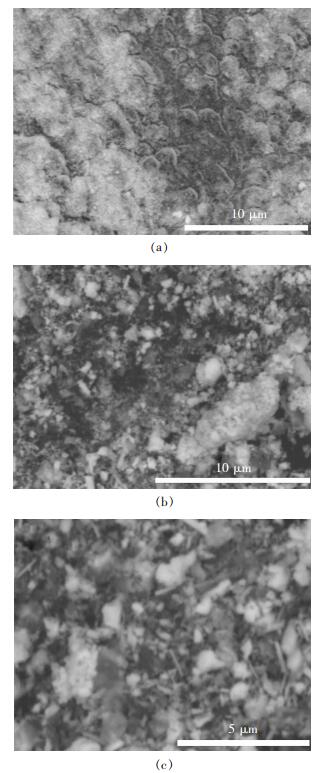 |
| 图 2 在不同表面活性剂条件下合成的ZnWO4:Tb3+样品SEM像 Fig. 2 SEM patterns for ZnWO4:Tb3+samples with different surfactants |
2.2 添加不同表面活性剂合成ZnWO4:Tb3+的荧光分析
图 3所示为添加不同表面活性剂所制备的ZnWO4:Tb3+的激发谱.激发光谱测试选用545nm(对应Tb3+的5D4-7F5能级跃迁)的发射波长作为监测波长,其中a, b, c分别对应PEG-2000, PVP, CTAB为表面活性剂制备ZnWO4:Tb3+.图中可以清楚地看到所有荧光粉的激发光谱非常相似,在230-330nm波段内出现了强而宽的峰,并且最强峰出现在272nm,这是因为在WO42+组态中O2-处于激发态2p轨道将电荷转移给W6+空轨道的中心[20].在Tb3+的激发光谱中出现WO42+组态的强激发波段表明ZnWO4:Tb3+荧光粉存在强能量传递从WO42+至Tb3+.在波长更大的区域,具有f轨道的Tb3+进行f-f电子跃迁而出现较弱的激发波段,由处于7F6基态的Tb3+激发至不同激发态而出现的激发峰:340nm (5G2), 352.2nm (5D2), 359.4nm (5G5), 369.8nm (5G6), 378.4nm (5D3), 487.8nm (5D4) [21].添加不同表面活性剂合成样品对激发光谱的位置几乎没有影响.正如我们所预料的,在ZnWO4:Tb3+荧光粉中没有发现发射峰位置移动,只是有发射强度不同.采用PEG-2000为表面活性剂的样品在230-330nm的激发最强,但由Tb3+的7F6基态激发至不同激发态的激发强度弱于采用PVP为表面活性剂的样品激发.
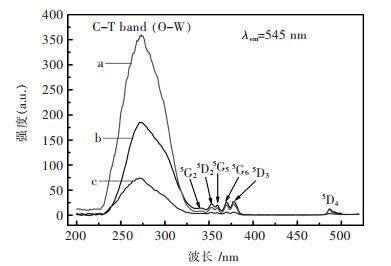 |
| 图 3 在不同表面活性剂条件下合成的ZnWO4:Tb3+样品激发谱 Fig. 3 Excitation spectra of ZnWO4:Tb samples with different surfactants |
图 4所示为添加不同表面活性剂所合成ZnWO4:Tb3+的发射光谱(a, b, c分别对应PEG-2000, PVP, CTAB制备样品).选用272nm的激发波长Tb3+的5D4-7F5电子迁移发射.在400-700nm波段均为一个宽而强的发射峰,这是由于样品中WO42+组态固有荧光发射.样品在272nm激发的发射光谱主要是由WO42+自激发的蓝光发射和Tb3+的电子跃迁发出的锐线谱组成.图 4中可以看出样品的最强发射峰在545nm处,由Tb3+的5D4-7F5电子跃迁而产生的.一系列的锐线发射谱是由4f8组态的Tb3+进行f-f电子跃迁获得.比如说绿色发射5D4→7F5 (545, 548.4 nm), 以及红色发射5D4→7F4 (583.6, 587.8 nm), 5D4→7F3 (622 nm).
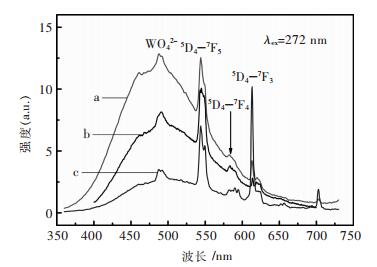 |
| 图 4 在不同表面活性剂条件下合成的ZnWO4:Tb3+样品发射谱 Fig. 4 Emission spectra of ZnWO4:Tb3+ samples with different surfactants |
2.3 添加不同表面活性剂合成的ZnWO4:Tb3+荧光寿命
图 5所示为添加不同表面活性剂所合成的ZnWO4:Tb3+的Tb3+的荧光寿命(a, b,c分别对应PEG-2000, PVP, CTAB为表面活性剂合成样品). 5D4荧光衰减曲线拟合不适用于单指数拟合,但确能用双指数拟合.通过双指数拟合数学表达式
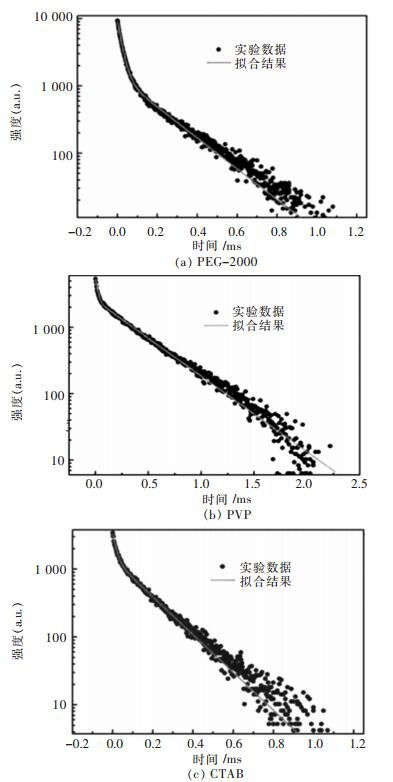 |
| 图 5 在不同表面活性剂条件下合成的ZnWO4:Tb3+样品的5D4激发态的荧光衰减曲线 Fig. 5 Luminescence decay of 5D4 state of ZnWO4:Tb3+ with different surfactants |
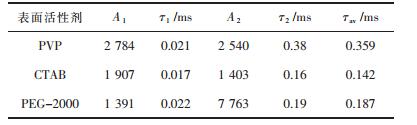
| 表1 在不同表面活性剂条件下合成的ZnWO4:Tb3+的双指数拟合参数 Table 1 The double-exponential fitting parameters of ZnWO4:Tb phosphors with different surfactants |
 |
| 点击放大 |
3 结论
1) 采用水热法,在不同表面活性剂条件下合成的ZnWO4:Tb3+绿色荧光粉样品均为纯的单斜相ZnWO4结构,Tb掺杂并没有改变钨酸锌结构,且结晶度好.
2) 添加不同的表面活性剂合成的样品对激发光谱的位置几乎没有影响,但对发射强度有影响.
3) 实验证明采用PEG-2000作为表面活性剂对于荧光粉发光强度增强最有效,采用PVP为表面活性剂合成的ZnWO4:Tb3+荧光粉寿命较采用PEG-2000和CTAB为表面活性剂合成的长.综合发光强度和荧光寿命的影响,选PVP为表面活性剂最佳.
| [1] |
LI J G, LI X D, SUN X D, et al. Monodispersed colloidal spheres for uniform Y2O3:Eu3+ red-phos phor particles and greatly enhanced luminescence by simultaneous Gd3+ Doping[J].
Journal of Physical Chemistry C, 2008,112 (31):11707–11716. DOI: 10.1021/jp802383a. |
| [2] |
SU Y G, LI L P, LI G S. Self-assembly and multicolor emission of core/shell structured CaWO4:Na+/Ln3+ spheres[J].
Chemical Communications, 2008,34 :4004–4006. |
| [3] |
JUSTEL T, NIKOL H, RONDA C. New developments in the field of luminescent materials for lighting and displays[J].
Luminescence Materials, 1998,37 (22):3084–3103. |
| [4] |
WANG H, LIN C K, LIU X M, et al. Monodisperse spherical core-shell-structured phosphors obtained by functionalization of silica spheres with Y2O3:Eu3+ layers for field emission displays[J].
Applied Physics Letters, 2005,87 (18):181907-1–3. |
| [5] |
BARROS B S, MELO P S, KIMINAMI R H G A, et al. Photophysical properties of Eu3+ and Tb3+-doped ZnAl2O4 phosphors obtained by combustion reaction[J].
Journal of Materials Science, 2006,41 (15):4744–4748. DOI: 10.1007/s10853-006-0035-6. |
| [6] |
NG S L, LAM Y L, ZHOU Y, et al. Green color emission from Tb3+:LnPO4 (Ln=La, Gd or Y) phosphors for photonic applications[J].
Journal of Materials Science Letters, 2000,19 (6):495–497. DOI: 10.1023/A:1006749603191. |
| [7] |
ZHONG J, LIANG H, HAN B, et al. NaGd(PO3)4:Tb3+-A new promising green phosphor for PDPs application[J].
Chemical Physics Letters, 2008,453 (4-6):192–196. DOI: 10.1016/j.cplett.2008.01.032. |
| [8] |
LI G Z, WANG Z L, QUAN Z W, et al. Growth of highly crystalline CaMoO4:Tb3+ phosphor layers on spherical SiO2 particles via Sol-Gel process: structural characterization and luminescent properties[J].
Crystal Growth and Design, 2007,7 (9):1797–1802. DOI: 10.1021/cg0701978. |
| [9] |
SU Y G, LI L P, LI G G. Synthesis and optimum luminescence of CaWO4-based red phosphors with codoping of Eu3+and Na+[J].
Chemistry of Materials, 2008,20 (19):6060–6067. DOI: 10.1021/cm8014435. |
| [10] |
WANG Z P, HU D W, FANG X, et al. Eye-Safe Raman Laser at μm Based on BaWO4 Crystal[J].
Phys Lett, 2008,25 (1):122–124. |
| [11] |
KIM J S, KIM J W, CHEON C I, et al. Effect of chemical element doping and sintering atmosphere on the microwave dielectric properties of barium zinc tantalates[J].
Journal of the European Ceramic Society, 2001,21 (15):2599–2604. DOI: 10.1016/S0955-2219(01)00323-5. |
| [12] |
OAKI Y, IMAI H. Room-temperature aqueous synthesis of highly luminescent BaWO4-polymer nanohybrids and their spontaneous conversion to hexagonal WO3 nanosheets[J].
Advanced Materials, 2006,18 (14):1807–1811. DOI: 10.1002/(ISSN)1521-4095. |
| [13] |
DAI Q L, SONG H W, BAI X, et al. Photoluminescence properties of ZnWO4:Eu3+ nanocrystals prepared by a hydrothermal method[J].
Journal of Physical Chemistry C, 2007,111 (21):7586–7592. DOI: 10.1021/jp066712e. |
| [14] |
HE H Y. Luminescence property of ZnWO4:Sm nanopowders synthesized with wet chemical methods[J].
Metallurgical and Materials Transactions B, 2012,43 (3):662–666. DOI: 10.1007/s11663-012-9635-5. |
| [15] |
PUCHALSKA M, ZYCH E. The effect of charge compensation by means of Na+ ions on the luminescence behavior of Sm3+-doped CaAl4O7 phosphor[J].
Journal of Luminescence, 2012,132 (3):826–831. DOI: 10.1016/j.jlumin.2011.11.015. |
| [16] |
WU Y, ZHANG S C, ZHANG L W. Photocatalytic activity of nanosized ZnWO4 prepared by the Sol-gel method[J].
Chemical Research, 2007,23 (4):465–468. |
| [17] |
LOU X M, CHEN D H. Synthesis of CaWO4:Eu3+ phosphor powders via a combustion process and its optical properties[J].
Materials Letters, 2008,62 (10-11):1681–1684. DOI: 10.1016/j.matlet.2007.09.066. |
| [18] |
LIAO J S, YOU H Y, ZHANG S A, et al. Synthesis and luminescence properties of BaWO4:Pr3+microcrystal[J].
Journal of Rare Earths, 2011,29 (7):623–627. DOI: 10.1016/S1002-0721(10)60510-8. |
| [19] |
MAI M, FELDMANN C. Microemulsion-based synthesis and luminescence of nanoparticulate CaWO4, ZnWO4, CaWO4:Tb, and CaWO4:Eu[J].
Journal of Materials Science, 2011,47 (3):1427–1435. |
| [20] |
KODAIRA C A, BRITO H F, FELINTO M C F C. Luminescence investigation of Eu3+ ion in the RE2(WO4)3 matrix (RE=La and Gd) produced using the pechini method[J].
Journal of Solid State Chemistry, 2003,171 (1-2):401–407. DOI: 10.1016/S0022-4596(02)00221-9. |
| [21] | THOMASK S, SINGHS, DIEKEG H.Energy Levels of Tb3+ in LaCl3 and other chlorides[J]. Journal of Chemical Physics,1963,38 (9):2180–2190. |
| [22] |
YU M, LIN J, FANG J. Silica spheres coated with YVO4:Eu3+ layers via Sol-gel process: a simple method to obtain spherical core-shell phosphors[J].
Chemistry of Materials, 2005,17 (7):1783–1791. DOI: 10.1021/cm0479537. |
 2016, Vol. 7
2016, Vol. 7


