| 静电纺丝纳米纤维膜分离富集重金属的研究进展 |
${affiVo.labelOrder}. 2.嘉兴学院生物与化学工程学院,浙江 嘉兴 314001
${affiVo.labelOrder}. College of Biological and Chemical Engineering, Jiaxing University, Jiaxing 314001, China
静电纺丝技术在19 世纪末首次被Rayleigh[1]发现,然后在1914 年,Zeleny[2]运用静电纺丝技术在静电雾化方面做了研究.到1934 年,Formhals[3]设计了静电纺丝装置以及纺丝条件,并且申请了静电纺丝的专利.1964 年,Taylor 等[4]对静电纺丝做了更加深入的研究,提出Taylor 锥理论学说.后来随着科学技术水平的提高,促进了静电纺丝的理论研究和应用研究.静电纺丝设备主要由4 个方面组成(图 1):高压电源、注射器、推进装置和接地的接收装置.对注射器内的高分子溶液或熔体施加一定的电压,当电压大于溶液的表面张力和粘力,形成喷射纺丝流,向接收板运动,随着溶剂的挥发,在接收装置上形成纤维膜,这个过程就称为静电纺丝.得到的纳米纤维具有直径小、比表面积大的优点.在生物医学[5-7]、组织工程[8-9]、能源技术[10]、防护织物[11]、分析化学[12]和固相萃取[13]等领域具有很多潜在的应用价值,文中主要介绍静电纺丝纳米纤维膜在分离富集重金属方面的应用.
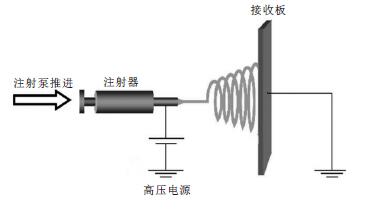 |
| 图 1 静电纺丝装置图 |
1 分离富集重金属的应用
静电纺丝纳米纤维膜对于环境水样中的重金属的分离富集,主要采用物理吸附和化学吸附原理进行.
1.1 物理吸附Keyur Desai 等[14-15]用不同脱乙酰度、不同分子量的壳聚糖与PEO 在一定比例下混合制备纳米纤维,对Cr(Ⅵ)进行吸附研究.发现壳聚糖与PEO 质量比为90∶10 时,吸附效果最好;在此配比条件下,发现在脱乙酰度为80 %的条件下,该纳米纤维的吸附量最高.
Yimin Sang 等[16]采用不同过滤方法研究了聚氯乙烯(PVC)膜在水溶液中的金属吸附性,结果表明,用胶束增强过滤法对重金属的吸附比较理想,Cu2+和Pb2+吸附率分别可以达到73 %和82 %,而Cd2+更高,达到91 %.
1.2 化学吸附化学吸附是吸附质分子(或离子)与吸附剂表面原子(或分子)发生电子的转移、交换或者共有,形成化学键的吸附.
Chang 等[17]分别用丝素以及羊毛角蛋白和丝素的混合物作为原料,通过静电纺丝制备薄膜,研究对Cu2+的吸附性能.在pH=7 时,丝素膜吸附量只有1.65 mg/g,而羊毛角蛋白和丝素混合薄膜达到了2.88 mg/g. 由于羊毛角蛋白的胱氨酸有部分氧化成-SO32-,再加上氨基酸的端基团-COO-,使得Cu2+和-SO32-、-COO-共同作用(图 2 a),提高了混合膜的吸附性能.Wu 等[18]用静电纺丝制备出一种新颖的巯基化纳米PVA/SiO2纤维膜,也研究了对Cu2+的吸附性能(图 2 b).结果表明,最大吸附容量达到489.12 mg/g,而且经过6 次脱附、再吸附循环后,该复合膜的吸附率只从93.1 %降到90.13 %.Parvin 等[19]用静电纺丝法制备聚丙烯腈(PAN) 膜,然后在表面进行胺基化修饰,对Cu2+进行吸附,饱和吸附容量达到116.522 mg/g.
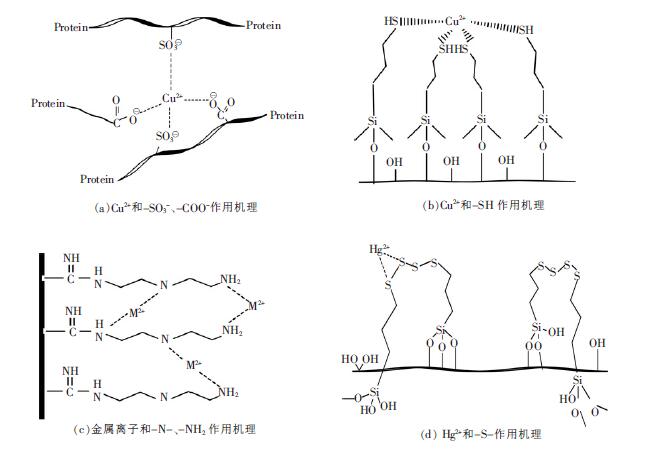 |
| 图 2 金属离子和不同基团作用机理 |
Pimolpun 等[20]采用静电纺丝技术制备了聚丙烯腈(PAN)纤维膜,并且在膜表面进行胺基化修饰,研究了在不同pH、接触时间下,吸附Cu2+、Ag+、Fe2+和Pb2+的能力(图 2 c),以及不同盐酸浓度对膜脱附能力的研究.实验表明,在pH 值为4,接触时间为10 h时,由于氮原子与金属离子的配位作用,膜对上述离子的最大吸附量达到150.6,155.5,116.5 和60.6 mg/g,盐酸浓度达到10 mol/L 时,对各重金属的脱附率均达到90 %以上.
Ye 等[21] 对纳米CA 膜表面进行聚甲基丙烯酸(PMAA)修饰,研究对Cu2+、Hg2+和Cd2+的吸附性能,研究发现PMAA 修饰CA 膜对Hg2+有很高的选择吸附性,可用于痕量Hg2+的分离富集.Stephen 等[22]采用丁二酸酐对CA 膜经行表面功能化,运用这种材料吸附水中的Cd2+、Pb2+,最大吸附容量分别达到0.59、1.21 mmol/g.
Teng 等[23]在纳米PVP/SiO2纤维膜表面进行硫醚修饰,对不同重金属进行吸附研究,发现-S-对Hg2+具有高效选择吸附性(图 2 d),而且在30 min 内达到吸附平衡,吸附容量为854 mg/g,经过3 次吸附-脱附循环以后,膜的吸附率为89.52 %,吸附容量为230.69 mg/g.Li 等[24]制备了一种新颖的巯基化二氧化硅纳米纤维,并且用来处理水中的Hg2+,在30 min 内达到吸附平衡,吸附容量达到57.49 mg/g.
Haide 等[25-26]制备了壳聚糖静电纺纳米纤维膜,得到的薄膜在K2CO3溶液中浸泡后,使得纤维上的-NH3+转化为-NH2,最终得到对重金属有吸附作用的纳米纤维膜.研究表明,这种壳聚糖纳米纤维膜在含有Cu2+和Pb2+的水溶液中吸附8 h 可达到饱和,对两种离子的最大吸附量分别达到485.44 mg/g 和263.15 mg/g.研究人员还制备了PAN 静电纺丝纳米纤维膜,然后将PAN 纳米纤维膜用偕胺肟修饰,腈基转化为偕胺肟基团,并将其用于重金属离子吸附.研究发现,偕胺肟修饰的PAN 纳米纤维对Cu2+和Pb2+的最大吸附量分别可达到52.70 mg/g 和263.45 mg/g.将吸附有金属的纤维膜放入1 mol/L 的HNO3中,1 h 后,Cu2+和Pb2+的脱附率均达到90 %以上,该纳米纤维膜具有较强的再生能力.
康学军等[27]采用聚苯乙烯(PS)和双硫腙(DZ)制备PS-DZ 共混静电纺丝纳米纤维膜,并且利用该纳米纤维膜作为固相萃取剂吸附水溶液中得Pb (Ⅱ).研究发现,PS-DZ 纳米纤维固相萃取剂吸附Pb (Ⅱ)比其他固相萃取材料效果更好.Wang 等[28]利用1,4-二羟基蒽醌(1,4-DHAQ)和醋酸纤维素(CA)制备共混纳米纤维,然后经脱乙酰化得到1,4-DHAQ @CL纳米纤维.研究表明,1,4-DHAQ 本身具有荧光性,当1,4-DHAQ @CL 纳米纤维吸附Cu2+后,得到(1,4-DHAQ)-Cu2+ @CL 纳米纤维,荧光性降低.但是(1,4-DHAQ)-Cu2+@CL 纳米纤维吸附Cr3+后,荧光增强(图 3).1,4-DHAQ @CL 纳米纤维和(1,4-DHAQ)-Cu2+@CL纳米纤维分别吸附Cu2+和Cr3+具有很强的选择性.这种具有荧光性的纳米纤维检测痕量重金属,尚属首次研究,对重金属的分析检测具有很大的实际意义.
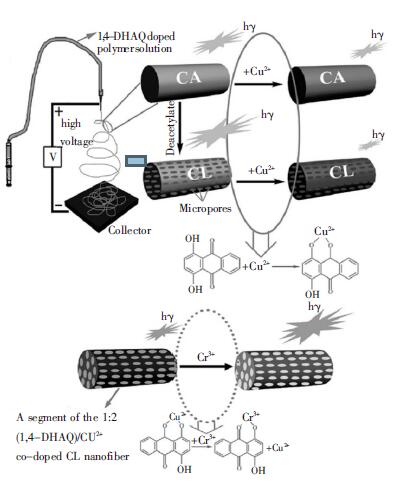 |
| 图 3 1,4-DHAQ @CL 纳米纤维和(1,4-DHAQ)-Cu2+@CL 纳米纤维吸附Cu2+和Cr3+ |
2 结论与展望
综上所述,静电纺丝作为一种简便的生产纳米纤维膜的技术,在重金属分离富集方面具有广阔的应用前景,但由于其制备所需时间长、产量低,分离也相当麻烦,因此,目前主要研究静态吸附特性,自动化程度不高,给实际的应用带来了很大困难.如果这个问题不能得到有效解决,进一步研究的意义就变得不大.虽然静电纺丝纳米纤维膜用于环境中金属离子的分离富集的研究逐渐增加,但是对某一特定金属或不同形态的选择性吸附的研究比较少,开展对于环境中某一特定金属的分离富集静电纺丝纳米纤维膜的研究具有更大的挑战性.
| [1] |
Lord Rayleigh. On the equilibrium of liquid conducting masses charged with electricity[J].
Edinburgh and Dublin Philosophical Magazine and Journal, 1882, 44: 184–186. |
| [2] |
John Zeleny. The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces[J].
Physical Review, 1914, 3: 69–91. DOI: 10.1103/PhysRev.3.69. |
| [3] |
Anton, Formhals. Process and apparatus for preparing artificial threads[J].
US Patent, 1,975,504, 1934, 504–1934. |
| [4] |
Geoffrey Taylor. Electrically driven jets[J].
Proceedings of the Royal Society of London A: Mathematical Physical and Engineering Sciences, 1969, 313: 453–475. DOI: 10.1098/rspa.1969.0205. |
| [5] |
WANG Bo-chu, WANG Ya-zhou, YIN Tie-ying, et al. Applications of electrospinning technique in drug delivery[J].
Chemical Engineering Communications, 2010, 197: 1315–1338. DOI: 10.1080/00986441003625997. |
| [6] |
Victor Leung, Frank Ko. Biomedical applications of nanofibers[J].
Polymers for Advanced Technologies, 2011, 22: 350–365. DOI: 10.1002/pat.1813. |
| [7] |
Aditya Kulkarnia, Bamboleb V A, Mahanwara P A. Electrospinning of polymers, their modeling and applications[J].
Polymer-Plastics Technology and Engineering, 2010, 49(5): 427–441. DOI: 10.1080/03602550903414019. |
| [8] |
Seema Agarwal, Joachim H Wendorff, Andreas Greiner. Progress in the field of electrospinning for tissue engineering applications[J].
Advanced Materials, 2009, 21: 3343–3351. DOI: 10.1002/adma.v21:32/33. |
| [9] |
Anca-Dana Bendrea, Luminita Cianga, Ioan Cianga. Review paper: progress in the field of conducting polymers for tissue engineering applications[J].
Journal of Biomaterials Applications, 2011, 26: 3–84. DOI: 10.1177/0885328211402704. |
| [10] |
Sara Cavaliere, Surya Subianto, Iuliia Savych, et al. Electrospinning: designed architectures for energy conversion and storage devices[J].
Energy and Environmental Science, 2011, 4: 4761–4785. DOI: 10.1039/c1ee02201f. |
| [11] |
HUANG Chao-bo, CHEN Shui-liang, Darrell H Reneker, et al. Highstrength mats from electrospun poly (p-phenylene biphenyltetracarboximide) nanofibers[J].
Advanced Materials, 2006, 18: 668–671. DOI: 10.1002/(ISSN)1521-4095. |
| [12] |
Samuel Chigome, Nelson Torto. A review of opportunities for electrospun nanofibers in analytical chemistry[J].
Analytica Chimica Acta, 2011, 706: 25–36. DOI: 10.1016/j.aca.2011.08.021. |
| [13] |
Samuel Chigome, Godfred Darko, Nelson Torto. Electrospun nanofibers as sorbent material for solid phase extraction[J].
Analyst, 2011, 136: 2879–2889. DOI: 10.1039/c1an15228a. |
| [14] |
Keyur Desai, Kevin Kit. Morphological and surface properties of electrospun chitosan nanofibers[J].
Biomacromolecules, 2008, 9: 1000–1006. DOI: 10.1021/bm701017z. |
| [15] |
Keyur Desai, Kevin Kit. Nanofibrous chitosan non-wovens for filtration applications[J].
Polymer, 2009, 50: 3661–3669. DOI: 10.1016/j.polymer.2009.05.058. |
| [16] |
SAN Yi-min, LI Fa-sheng. Heavy metal-contaminated groundwater treatment by a novel nanofiber membrane[J].
Desalination, 2008, 223: 349–360. DOI: 10.1016/j.desal.2007.01.208. |
| [17] |
Chang Seok Ki, Eun Hee Gang, et al. Nanofibrous membrane of wool keratose/silk fibroin blend for heavy metal ion adsorption[J].
Journal of Membrane Science, 2007, 302: 20–26. DOI: 10.1016/j.memsci.2007.06.003. |
| [18] |
WU Sheng-ju. Effects of poly (vinyl alcohol) (PVA) content on preparation of novel thiol -functionalized mesoporous PVA/SiO2 composite nanofiber membranes and their application for adsorption of heavy metal ions from aqueous solution[J].
Polymer, 2010, 51: 6203–6211. DOI: 10.1016/j.polymer.2010.10.015. |
| [19] |
Parvin Karimi Neghlani. Preparation of aminated -polyacrylonitrile nanofiber membranes for the adsorption of metal ions:comparison with microfibers[J].
Journal of Hazardous Materials, 2011, 186: 182–189. DOI: 10.1016/j.jhazmat.2010.10.121. |
| [20] |
Pimolpun Kampalanonwat, Pitt Supaphol. Preparation and adsorption behavior of aminated electrospun polyacrylonitrile nanofiber mats for heavy metal ion removal[J].
Applied Materials and Interfaces, 2010, 2: 3619–3627. DOI: 10.1021/am1008024. |
| [21] |
TIAN Ye, WU Min. Electrospun membrane of cellulose acetate for heavy metal ion adsorption in water treatment[J].
Carbohydrate Polymers, 2011, 83: 743–748. DOI: 10.1016/j.carbpol.2010.08.054. |
| [22] |
Musyoka Stephen, Ngila Catherinea, Moodley Brend, et al. Oxolane-2, 5-dione modified electrospun cellulose nanofibers for heavy metals adsorption[J].
Journal of Hazardous Materials, 2011, 192: 922–927. DOI: 10.1016/j.jhazmat.2011.06.001. |
| [23] |
TENG Min-min, WANG Hong-tao. Thioether-functionalized mesoporous fiber membranes: Sol-gel combined electrospun fabrication and their applications for Hg2+ removal[J].
Journal of Colloid and Interface Science, 2011, 355: 23–28. DOI: 10.1016/j.jcis.2010.11.008. |
| [24] |
LI Shou-zhu, YUE Xiu-li, JING Yuan-miao, et al. Fabrication of zonal thiol-functionalized silica nanofibers for removal of heavy metal ions from wastewater[J].
Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2011, 380: 229–233. |
| [25] |
Sajjad Haider, Soo-Young Park. Preparation of the electrospun chitosan nanofibers and their applications to the adsorption of Cu (Ⅱ) and Pb(Ⅱ) ions from an aqueous solution[J].
Journal of Membrane Science, 2009, 328: 181–190. |
| [26] |
Saeed K, Haider S, OH T J, et al. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption[J].
Journal of Membrane Science, 2008, 322: 400–405. DOI: 10.1016/j.memsci.2008.05.062. |
| [27] |
DENG Jian-jun, KANG Xue-jun, CHEN Li-qin, et al. A nanofiber functionalized with dithizone by co-electrospinning for lead (II) adsorption from aqueous media[J].
Journal of Hazardous Materials, 2011, 196: 187–193. DOI: 10.1016/j.jhazmat.2011.09.016. |
| [28] |
WANG Mei-ling, MENG Guo-wen, HUANG Qing, et al. Electrospun 1, 4-DHAQ-doped cellulose nanofiber films for reusable fluorescence detection of trace Cu2+ and further for Cr3+[J].
Environmental Science and Technology, 2012, 46: 367–37. DOI: 10.1021/es202137c. |
 2012, Vol. 3
2012, Vol. 3
