早在1979年12月,便秘就被列入世界卫生组织(WHO)推荐针灸治疗的43种病症之一。对于消化系统病谱,便秘是针灸治疗的优势病种[1]。便秘是兽医临床常见疾病,西药主要通过刺激胃肠运动进行缓解,但长期使用效果不理想。针灸治疗动物便秘取穴方便,疗效确切。根据古籍记载和临床经验,后海(GV-1)能够显著缓解动物便秘,后三里(ST-36)和大肠俞(BL-25)广泛治疗人和动物的胃肠道疾病[2-4]。虽然临床证实了这些穴位对便秘的治疗效果,但兽医针灸的基础研究非常薄弱,目前,尚不明确不同穴位缓解便秘的作用途径和作用机理是否一致,治疗便秘的优势穴位是哪个等。本试验用洛哌丁胺建立大鼠便秘模型[5],选择临床治疗动物便秘常用的GV-1、ST-36、BL-25,研究3个穴位对便秘大鼠的缓解作用,检测针灸对胃肠动力相关神经递质的影响,比较结肠组织结构的完整性及变化,比较3个穴位的异同,探索治疗便秘的优势穴位,为临床应用提供理论依据。
1 材料与方法 1.1 试验材料1.1.1 动物 选取SPF级SD大鼠60只[购自斯贝福(北京)生物科技有限公司],体质量(210±10)g,在ivc鼠房单只单笼饲养,自由饮水饮食,室内温度(21±2)℃,相对湿度(55±5)%。于垫料上放置滤网方便采集大鼠粪便和观察粪便情况。
1.1.2 药物 将盐酸洛哌丁胺(2 mg·粒-1)(上海朝晖药业有限公司)除去外面胶囊,将药粉与蒸馏水混匀,制成混悬液备用。将枸橼酸莫沙必利(5 mg·片-1)(鲁南贝特有限公司)片剂压成粉末,与蒸馏水混匀制成混悬液备用。
1.1.3 主要试剂与仪器 Go Taq®pCR Master Mix(A6001,Promega, USA)、GoScript TM Reverse Transcription System (A5001, Promega, USA)、Eastep® Super总RNA提取试剂盒(LS1040, Promega,北京)、Rat VIP ELISA KIT、Rat SS ELISA KIT、Rat SP ELISA KIT、Rat 5-HT ELISA KIT (酶联生物科技有限公司,上海)、冷冻离心机(Sigma公司)、SYNERGY HTX全波长酶标仪(Bio Tek公司)、LightCycler® 96全自动荧光定量PCR仪(罗氏诊断产品有限公司,上海)等。
1.2 试验方法1.2.1 动物分组及处理 60只SD大鼠(210±10)g适应性常规饲养后,采用抽签的方法随机分为6组,即对照A组、模型B组、西药治疗C组、GV-1D组、ST-36 E组、BL-25 F组,每组10只,均单笼饲养。A组灌服生理盐水,其他各组均灌服3 mg·(kg·d)-1洛哌丁胺混悬液,每天上午1次,建立便秘模型[5]。5 d后,粪便含水量下降,首粒黑便时间延长,确定造模成功。每天上午继续洛哌丁胺灌胃,同时在下午进行不同治疗,连续6 d。其中,C组灌服莫沙必利[1.38 mg·(kg·d)-1][6],D、E、F组大鼠分别针刺GV-1、ST-36、BL-25 3个穴位。GV-1位于尾根与肛门之间的凹陷,直刺5 mm;ST-36双侧取穴,位于膝关节下方3寸(体寸),胫骨前嵴外侧0.5寸(体寸),直刺6 mm;BL-25双侧取穴,位于背中线旁开1.5寸(体寸),第4腰椎两侧,直刺5 mm。穴位定位及针刺操作参照罗永江等[7]及Xie等[2]的方法,采用Mac脉针灸针(0.16 mm×7 mm)快速直刺入穴位,每10 min进行1次捻转提插行针,留针30 min。A组、B组和C组在大腿内侧的非穴位处针刺30 min。第7天心脏采血,根据美国兽医协会推出的动物安乐死指南颈椎脱臼安乐死。血液室温静置3 h,4 ℃ 3 500 r·min-1离心15 min,分离血清,分装后置于超低温冰箱保存备用。摘取结肠前段, 置于4%甲醛溶液固定和超低温冰箱保存备用。本研究经河北省动物保护协会批准,动物处理过程通过河北农业大学实验动物管理使用委员会的动物伦理学审查。
1.2.2 各组大鼠便秘情况评定 禁食不禁水,动物试验处理后再用0.3 mL墨水灌胃,记录首粒黑便时间。观察记录大鼠20粒粪便长度、粪便含水量、体质量。
粪便含水量:收集粪便称取湿质量,烘干箱90 ℃烘干6 h,称取干质量:[粪便含水量=(湿质量-干质量)/湿质量×100%]
1.2.3 指标检测 酶联免疫吸附法(ELISA)检测5-羟色胺(5-HT)、生长抑素(SS)、血管活性肠肽(VIP)、P物质(SP)含量。采用聚合酶链式反应(PCR)检测结肠组织5-羟色胺3受体(5-HT3R)mRNA、5-羟色胺4受体(5-HT4R)mRNA相对表达量。结肠组织HE染色,观察肠道组织结构,计数各组隐窝-绒毛轴中杯状细胞数量。
|
|
表 1 实时定量PCR引物列表 Table 1 Sequence of primers used for real-time PCR |
1.2.4 数据统计 本试验所有结果均表示为“平均值±标准差”。试验过程中使用Excel数据库记录数据,并使用SPSS20.0分析软件(IBM Corporation,Armonk,NY,USA)对试验数据进行统计分析。方差齐性检验后,通过单因素方差分析(one way ANOVA)比较参数的差异性。LSD法进行组间多重比较。差异的显著性水平为P < 0.05或P < 0.01。
2 结果 2.1 GV-1、ST-36、BL-25对大鼠便秘指标的影响模型组大鼠首粒黑便时间延长,粪便含水量下降,粪便颗粒长度缩短,体重下降(P < 0.01或P < 0.05)。治疗后GV-1组、ST-36组的首粒黑便时间缩短(P < 0.01),与西药治疗组无显著差异,接近对照组。与模型组比较,GV-1组、ST-36组、BL-25组粪便含水量分别增加12.86%、16.67%、14.59% (P < 0.01)。GV-1组20粒粪便长度与模型组有极显著差别,并且优于西药治疗组。GV-1组和ST-36组大鼠体重比模型组显著增加(P < 0.05),详见表 2和图 1。
|
|
表 2 GV-1、ST-36、BL-25对大鼠便秘指标的影响(x±s, n=10) Table 2 The effects of GV-1, ST-36, BL-25 on constipation indexes in rats(x±s, n=10) |

|
A组. 对照;B组. 模型;C组. 西药治疗;D组. GV-1;E组. ST-36;F组. BL-25 Group A. Control; Group B. Model; Group C. Western medicine treatment; Group D. GV-1; Group E. ST-36; Group F. BL-25 图 1 GV-1、ST-36、BL-25对便秘大鼠20粒粪便颗粒长度的影响 Fig. 1 Effect of GV-1, ST-36, BL-25 on the length of 20 fecal pellets in rats with constipation |
由图 2可知,模型组大鼠血清5-HT含量升高(P < 0.01),针刺ST-36使血清中5-HT含量降低23.93%,与模型组有极显著性差异;GV-1和BL-25分别降低5.41%和7.47%,与模型组也有显著性差异(P < 0.05)。模型组血清SS含量极显著上调(P < 0.01)。针刺GV-1使SS含量降低36.34%,具有极显著差异,基本达到对照组水平。针刺ST-36使SS含量下调13.46% (P < 0.05)。模型组血清VIP含量显著降低,BL-25组VIP含量极显著升高27.48%(P < 0.01),达到对照组水平。模型组血清SP含量显著降低(P < 0.05),BL-25组SP含量显著升高22.75% (P < 0.05)。
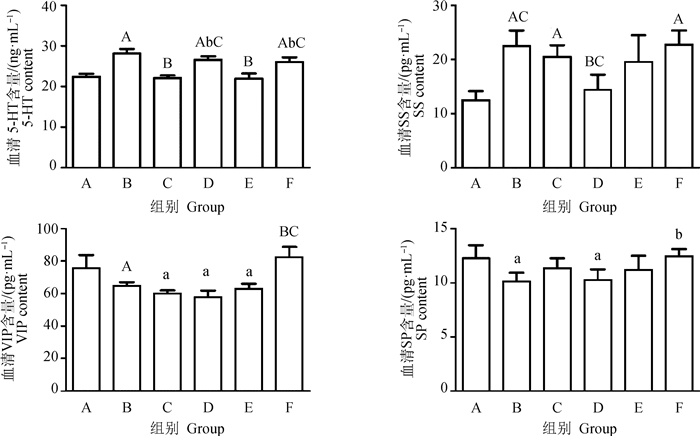
|
图 2 GV-1、ST-36、BL-25对便秘大鼠神经递质的影响 Fig. 2 The effect of GV-1, ST-36, BL-25 on neurotransmitters in constipation rats |
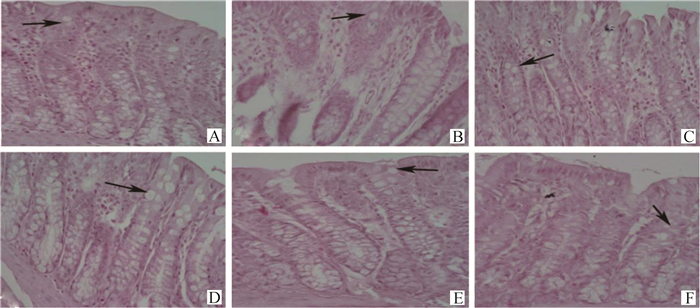
|
→表示绒毛杯状细胞 →refers to villous goblet cells 图 3 GV-1、ST-36、BL-25对大鼠结肠组织结构的影响(400×) Fig. 3 Effects of GV-1, ST-36, BL-25 on the tissue structure of rat colon (400×) |
对照组大鼠结肠组织结构清晰完整,肠腺发达,黏膜上皮细胞连接紧密,固有层无炎症或萎缩,有大量杯状细胞,杯状细胞在进行HE染色时,黏原颗粒被染料溶解,多呈现出空泡状[8]。模型组大鼠结肠组织结构模糊,肠腺萎缩、肿胀,黏膜上皮缺失,杯状细胞减少。GV-1组结肠组织结构清晰,肠腺恢复正常,杯状细胞变大、增多。ST-36和BL-25组较模型组黏膜上皮完整,肠腺增宽。此外,GV-1组结肠隐窝-绒毛轴中的杯状细胞数量(418.60±12.0)个,与模型组(381.80±8.62)个比较,差异显著(P < 0.01),优于西药治疗组,ST-36、BL-25组杯状细胞数量也有显著增加(数据略),达到西药治疗组水平。
2.4 GV-1、ST-36、BL-25对便秘大鼠结肠组织中5-HT3R、5-HT4R mRNA的影响由图 4可知,与对照组比较,模型组结肠组织中5-HT3R mRNA相对表达量降低67.96% (P < 0.01)。而针刺GV-1、ST-36,5-HT3R mRNA相对表达量比模型组分别增加36.07%、40.98% (P < 0.05),与西药组无差异。模型组结肠组织中5-HT4R mRNA相对表达量降低42%(P < 0.01)。而ST-36组5-HT4RmRNA相对表达量比模型组增加65.52%(P < 0.01),优于西药治疗组。GV-1组、BL-25组5-HT4RmRNA相对表达量分别增加32.76%、37.93%,差异显著(P < 0.05)。
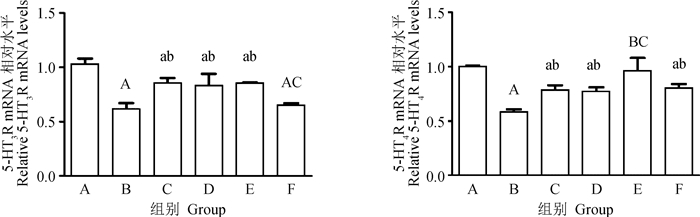
|
图 4 GV-1、ST-36、BL-25对便秘大鼠结肠组织中5-HT3R、5-HT4R mRNA相对表达量的影响 Fig. 4 The effect of GV-1, ST-36, BL-25 on the relative expression of 5-HT3R and 5-HT4R mRNA in the colon tissue of constipated rats |
兽医针灸是我国优秀的文化遗产,由于兽医针灸的基础研究相对滞后,严重影响其在动物临床上的应用。消化系统疾病是兽医临床常见病,针灸治疗快速有效,GV-1、ST-36和BL-25是治疗便秘及其他消化系统疾病的代表穴位[2.9]。
后海(GV-1)为督脉之始穴,动物的后海取穴方便,效果迅速,多用于腹泻、便秘及其他胃肠道疾病的治疗[2.9]。本试验表明,针刺GV-1能够降低血清中SS含量。SS作为肠道抑制性神经递质,当胃肠道中SS的含量高于正常水平时, 则会使肠蠕动变缓,分泌异常[10]。GV-1还可以降低血清中5-HT浓度,5-HT表达水平过高会产生兴奋性毒性[11],导致结肠部位环形肌收缩和舒张功能异常[12]。由于5-HT浓度的升高,结肠持续处于收缩状态,与粪便结合更紧密,过多吸收粪便中水分,导致粪便中水分减少引起便秘。针刺GV-1后下调了5-HT血清中的含量,缓解洛哌丁胺造成结肠环形肌收缩和舒张功能异常[13],加快粪便通过结肠时间从而缓解便秘症状。
后三里(ST-36)是治疗各种消化系统疾病的重要穴位,其疗效已经过千百年的验证。研究表明,针刺ST-36可以上调结肠组织5-HT3R、5-HT4R mRNA相对表达量。5-HT3R属于配体门控阳离子通道受体[14],5-HT3R可以影响细胞内Ca2+浓度增加间质细胞的活性,从而提高胃肠道的运动能力。据报道,5-HT3R拮抗剂能抑制结肠蠕动,减少消化液分泌,导致便秘发生[15-16]。5-HT4R能增强肠道平滑肌收缩,增加肠道分泌。推测5-HT3R与5-HT结合增加细胞内Ca2+浓度并刺激中枢和外周神经元的兴奋性,促进神经递质的释放,增加乙酰胆碱(ACH)含量[17]。5-HT作用于肠道5-HT4R,促进ACH分泌,增加肠道平滑肌收缩频率,增强肠道平滑肌蠕动,影响肠蠕动和分泌,缩短在结肠的时间及水分重吸收[18]。ST-36还可以下调SS缓解便秘症状。
大肠俞(BL-25)是大肠背俞穴,广泛治疗便秘及其他肠道疾病。本试验表明,针刺BL-25可以提高血清中VIP和SP含量。研究证实,VIP能够改变消化道运动功能,改善便秘症状[19],或者通过VIP-cAMP-PKA-AQP3通路治疗功能性便秘[20]。VIP可使内脏血管、胃肠道平滑肌舒张,增加胆汁、小肠水电解质的分泌[21],同时具有正性肌力的作用[22]。SP是体内最强的兴奋平滑肌的物质之一,可直接作用于肠道平滑肌[23],使平滑肌收缩、蠕动增强,促进肠道排空,从而缓解便秘症状。
另外,针刺GV-1、ST-36、BL-25均可增加结肠杯状细胞的数量,研究表明,结肠杯状细胞分泌黏蛋白,是结肠黏液层的重要成分[24]。杯状细胞分泌的黏蛋白伴随水分的扩散分泌进入肠腔,增加肠腔内水分,缓解便秘。
4 结论针刺GV-1、ST-36、BL-25穴位均能改善便秘症状。GV-1可显著降低血清中5-HT、SS含量,上调结肠组织中5-HT3R、5-HT4R mRNA表达。ST-36降低血清中5-HT含量,提高结肠组织中5-HT3R、5-HT4R mRNA表达。BL-25提高血清中VIP、SP含量,增加5-HT4R mRNA表达。3个穴位均能改善便秘大鼠结肠组织结构,增加杯状细胞数量。综上表明,GV-1治疗便秘效果优于ST-36和BL-25。
| [1] |
杜元灏, 李桂平, 林雪, 等. 消化系统针灸病谱的研究[J]. 针灸临床杂志, 2006, 22(3): 1–2.
DU Y H, LI G P, LIN X, et al. Research on disease menu of acupuncture and moxibustion therapy in digestive system[J]. Journal of Clinical Acupuncture and Moxibustion, 2006, 22(3): 1–2. DOI: 10.3969/j.issn.1005-0779.2006.03.001 (in Chinese) |
| [2] | XIE H S, WEDEMEYER L, CHRISMAN C L, et al. Practical guide to traditional Chinese veterinary medicine, Vol. 2: small animal practice[M]. Florida: Chi Institute Press, 2014: 333-344. |
| [3] |
弓素梅, 郭红斌, 王安忠. 电针犬后三里穴治疗脾虚泄泻证对其生理指标的影响[J]. 动物医学进展, 2010, 31(12): 76–78.
GONG S M, GUO H B, WANG A Z. Effect of electro-acupuncture at housanli on physiological indexes in dogs with splenasthenic diarrhea[J]. Progress in Veterinary Medicine, 2010, 31(12): 76–78. DOI: 10.3969/j.issn.1007-5038.2010.12.019 (in Chinese) |
| [4] | WANG Y, NIU W M, YANG X H, et al. Effects of electronically stimulating Tianshu (ST 25) and Dachangshu (BL 25), Quchi (LI 11) and Shangjuxu (ST 37) on the expressions of jejunum c-kit protein and c-kit mRNA in rats with functional diarrhea[J]. JTradit Chin Med, 2016, 36(6): 779–783. |
| [5] |
谢建超, 吴国泰, 牛亭惠, 等. 便秘动物模型的复制概况及评价[J]. 实验动物科学, 2016, 33(5): 64–67, 70.
XIE J C, WU G T, NIU T H, et al. Copy before animal model of constipation and evaluation[J]. Laboratory Animal Science, 2016, 33(5): 64–67, 70. DOI: 10.3969/j.issn.1006-6179.2016.05.013 (in Chinese) |
| [6] |
张辉. 电针治疗功能性便秘的临床疗效观察与实验研究[D]. 沈阳: 辽宁中医药大学, 2019.
ZHANG H. Observation and experimental study on the clinical efficacy of electroacupuncture on functional constipation[D]. Shenyang: Liaoning University of Traditioanal Chinese Midicine, 2019. (in Chinese) |
| [7] |
罗永江, 郑继芳, 辛蕊华.
比较针灸学[M]. 2版. 北京: 中国农业出版社, 2016: 227-232.
LUO Y J, ZHENG J F, XIN R H. Comparative acupuncture[M]. 2nd ed. Beijing: China Agriculture Publishing House, 2016: 227-232. (in Chinese) |
| [8] |
邹仲之, 李继承.
组织学与胚胎学[M]. 7版. 北京: 人民卫生出版社, 2013: 10-11.
ZOU Z Z, LI J C. Histology and embryology[M]. 7th ed. Beijing: China People's Publishing House, 2013: 10-11. (in Chinese) |
| [9] |
林春丽. 不同穴位及针术对腹泻家兔胃肠动力及离子通道的影响[D]. 保定: 河北农业大学, 2020.
LIN C L. Effects of different acupoints and needle technique on gastrointestinal motility and ion channels in diarrhea rabbits[D]. Baoding: Hebei Agricultural University, 2020. (in Chinese) |
| [10] |
马腾飞, 王业秋, 张宁, 等. 菝葜治疗便秘型肠易激综合征作用机制的实验研究[J]. 中国药理学通报, 2012, 28(1): 109–114.
MA T F, WANG Y Q, ZHANG N, et al. An experimental study of Smilax treatment of constipation predominant irritable bowel syndrome and its mechanism[J]. Chinese Pharmacological Bulletin, 2012, 28(1): 109–114. DOI: 10.3969/j.issn.1001-1978.2012.026 (in Chinese) |
| [11] |
汪正芳, 贾玉, 侯亚男. 健脾调肝温肾方干预对腹泻型IBS患者血清炎性反应因子及5-羟色胺的影响[J]. 世界中医药, 2017, 12(1): 116–119.
WANG Z F, JIA Y, HOU Y N. Effects of Jianpi Tiaogan Wenshen formula on serum inflammatory factors and 5-hydroxytryptamine in diarrhea type IBS[J]. World Chinese Medicine, 2017, 12(1): 116–119. DOI: 10.3969/j.issn.1673-7202.2017.01.029 (in Chinese) |
| [12] |
杨玲, 施征, 王晓梅, 等. 电针对便秘型肠易激综合征大鼠结肠组织5-HT、5-HT4受体的调节作用[J]. 上海针灸杂志, 2014, 33(3): 266–269.
YANG L, SHI Z, WANG X M, et al. Regulating effect of electroacupuncture on colonic 5-HT and 5-HT4 receptors in rats with irritable bowel syndrome of constipation type[J]. Shanghai Journal of Acupuncture and Moxibustion, 2014, 33(3): 266–269. (in Chinese) |
| [13] | LI T, HU M M, JIANG C H, et al. Laxative effect and mechanism of Tiantian Capsule on loperamide-induced constipation in rats[J]. J Ethnopharmacol, 2021, 266: 113411. DOI: 10.1016/j.jep.2020.113411 |
| [14] | SIKANDER A, RANA S V, PRASAD K K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome[J]. Clin Chim Acta, 2009, 403(1-2): 47–55. DOI: 10.1016/j.cca.2009.01.028 |
| [15] | LIU H N, OHYA S, NISHIZAWA Y, et al. Serotonin augments gut pacemaker activity via 5-HT3 receptors[J]. PLoS One, 2011, 6(9): e24928. DOI: 10.1371/journal.pone.0024928 |
| [16] |
汪小莉, 赵春妮. 5-HT3受体拮抗剂相关便秘的研究进展[J]. 天津药学, 2016, 28(3): 54–57.
WANG X L, ZHAO C N. Advances related constipation about 5-HT3 receptor antagonists[J]. Tianjin Pharmacy, 2016, 28(3): 54–57. (in Chinese) |
| [17] |
曹佳男. 隔药饼灸对腹泻型肠易激综合征大鼠结肠5-HT、5-HT3R、5-HT4R表达的影响[D]. 长沙: 湖南中医药大学, 2019.
CAO J N. Effect of herb-partitioned moxibustion on expression of 5-HT, 5-HT3R and 5-HT4R in colon of rat with diarrhea-predominant irritable bowel syndrome[D]. Changsha: Hunan University of Chinese Medicine, 2019. (in Chinese) |
| [18] | MARTEAU P. Probiotics in functional intestinal disorders and IBS: proof of action and dissecting the multiple mechanisms[J]. Gut, 2010, 59(3): 285–286. DOI: 10.1136/gut.2008.173690 |
| [19] |
杨淑萍, 胡运莲, 刘俊琼. 易激胶囊对腹泻型肠易激综合征模型大鼠血管活性肠肽和肥大细胞表达的影响[J]. 湖北中医药大学学报, 2011, 13(1): 14–16.
YANG S P, HU Y L, LIU J Q. Influences of Yiji capsule on vasoactive intestinal polypeptide and mast cells expression in diarrhea-predominant irritable bowel syndrome rats[J]. Journal of Hubei University of Chinese Medicine, 2011, 13(1): 14–16. (in Chinese) |
| [20] |
王郁金. 基于VIP-cAMP-PKA-AQP3通路的硝菔通结方治疗功能性便秘的作用机制研究[D]. 成都: 成都中医药大学, 2015.
WANG Y J. Study on the mechanism of the treatment of functional constipation in Xiao Fu Tong Jie Fang based on the VIP-cAMP-PKA-AQP3 pathway[D]. Chengdu: Chengdu University of Traditional Chinese Medicine, 2015. (in Chinese) |
| [21] |
徐伟, 赵亚华, 孔洁. 血管活性肠肽研究进展[J]. 药物生物技术, 2002, 9(6): 364–368.
XU W, ZHAO Y H, KONG J. Progress in vasoactive intestinal peptide[J]. Pharmaceutical Biotechnology, 2002, 9(6): 364–368. (in Chinese) |
| [22] |
李冬华, 李春森, 李伍善, 等. 痛泻要方对肠易激综合征模型大鼠血管活性肠肽的影响[J]. 时珍国医国药, 2007, 18(9): 2098–2099.
LI D H, LI C S, LI W S, et al. Effects of Tongxieyaofang on vasoactive intestinal polypeptide in rats of Irritable Bowel syndrome[J]. Lishizhen Medicine and Materia Medica Research, 2007, 18(9): 2098–2099. (in Chinese) |
| [23] |
顾尽晖, 何羽, 汤灵娇, 等. 济川煎对结肠慢传输型便秘模型大鼠血浆SP、肠组织ICC与肠推动力等因素影响的研究[J]. 北京中医药, 2018, 37(5): 410–414.
GU J H, HE Y, TANG L J, et al. Study of effects of Jichuan Decoction on plasma SP, ICC of intestinal tissue and intestinal motility of model rats with colon slow transit constipation[J]. Beijing Journal of Traditional Chinese Medicine, 2018, 37(5): 410–414. (in Chinese) |
| [24] | GEREMIA A, BIANCHERI P, ALLAN P, et al. Innate and adaptive immunity in inflammatory bowel disease[J]. Autoimmun Rev, 2014, 13(1): 3–10. |



