2. 石河子大学动物科技学院, 石河子 832000;
3. 新疆农垦科学院 省部共建绵羊遗传改良与健康养殖国家重点实验室, 石河子 832000
2. College of Animal Science and Technology, Shihezi University, Shihezi 832000, China;
3. State Key Laboratory of Sheep Genetic Improvement and Healthy Breeding, Xinjiang Academy of Agricultural and Reclamation Sciences, Shihezi 832000, China
黄体(corpus luteum,CL)是母畜卵巢在生殖生理过程中的一个内分泌腺体,是建立和维持妊娠必需的内分泌器官[1]。在正常繁殖周期内,如果卵子受精,CL将分泌孕酮等来参与调节受精卵着床、胚胎发育及妊娠维持[2]。如果卵子未受精或处于妊娠末期(或分娩后),CL将会正常退化成为启动下一个生殖周期的前提[3]。因此,CL退化在哺乳动物的生殖周期中起着重要的“关卡”作用,对于重启母畜生殖功能的周期性变化具有重要的意义。
前列腺素(prostaglandins,PGs)是一组内源性的酸性脂质,广泛分布于哺乳动物体内,涉及大量的生殖过程[4-5]。大多数内源性PGs由花生四烯酸代谢衍生而来,当花生四烯酸通过磷脂酶A2从质膜释放时,环氧合酶(COX或PTGS)1和2会将花生四烯酸转化为PGH2;在特定酶的作用下,PGH2被选择性转化为相应的PGs,包括PGF2α、PGE2、PGD2、PGI2和TXA2[6-7]。其中,PGF2α被认为是母畜CL退化的主效溶解素,而PGE2则被认为是CL维持的重要保护介质[4, 8]。通常在妊娠期间PGE2分泌水平较高,能够竞争性抑制PGH2向PGF2α的合成转化,从而降低PGF2α浓度,利于CL维持和胎儿发育;但在分娩启动后,随着胎儿保护机制的消失,CL维持则转而退化,PGE2合成下降,PGH2向PGF2α合成增加,从而促使产后或者空怀母体完成功能性(主要表现为P4下降)及结构性(主要表现为内皮细胞失去紧密的连接,黄体体积减少、完整性被破坏)的CL退化进程[9-10],继而开启新的生殖周期。但是,与PGs家族其他成员(如PGF2α、PGE1和PGE2)不同的是,PGD2在繁殖方面的相关调控作用并未引起人们足够的重视,其有关研究更是鲜有报道。
目前,大量研究证实,前列腺素家族的作用机制并不是某一种单一PGs成员的效应,而是多个PGs家族成员通过其相应受体的结合而共同发挥作用[11-12],因此,在子宫或卵巢黄体组织中不同的PGs成员之间可能会发生不同程度的协同作用或竞争性地拮抗效应。本研究选取哈萨克母羊为研究对象,探究PGD2与PGF2α在母体CL退化过程中可能存在的作用机制,为进一步优化母畜高效繁育技术、保障畜牧业的连续繁育生产提供新的理论依据。
1 材料与方法 1.1 试验动物及饲养管理2020年5月份选择购置同一个养殖场16只健康的哈萨克母羊(年龄3~4岁,体重47 kg左右,无繁殖疾病且发情周期正常),将试验羊群饲养于石河子大学动物科技学院试验站。栏内散养,每天早上和下午饲喂,自主采食苜蓿干草,配置舔砖(防应激),自由饮水、自然光照,保证羊栏里干净无杂物、管理及环境条件一致。待其饲养14 d适应圈舍环境后,再进行试验。
1.2 主要试剂PGD2与PGF2α兔源多克隆抗体,山羊抗兔IgG二抗均购自Abcam公司;PGF2α购自Macklin公司;绵羊孕酮(PROG)、雌激素(E2)、前列腺素F2α(PGF2α)和D2(PGD2)ELISA Kit均购自晶美生物工程有限公司;HE染色试剂盒、总RNA提取试剂盒、总蛋白提取试剂盒、SCA蛋白定量试剂盒、SDS-PAGE试剂盒均购自Solarbio公司;HiFiScript cDNA Synthesis Kit购自北京康为世纪有限公司;TB Green Premix Ex Taq Ⅱ(Tli RNaseH Plus)购自TaKaRa公司;蛋白Marker购自博奥森生物公司;PVDF膜购自ThermoFisher Scientific公司;ECL显色剂购自上海信裕生物科技有限公司。
1.3 试验方法1.3.1 子宫肌内注射处理 采用阴道埋孕酮栓和肌内注射氯前列腺烯醇法使母羊同期发情。为消除同期发情对试验的影响,待其同期发情处理第2个发情周期的黄体期(前期课题组已对同一批40只哈萨克母羊进行了2个发情周期的观察,发现哈萨克母羊群体中发情行为呈现较为明显的周期性变化,其发情周期为18 d左右,发情持续时间为24~48 h,为本试验选择黄体期中期即发情周期第11天进行子宫肌内注射处理提供了保障)进行试验分组处理,采用微创手术在有中期黄体的一侧子宫小弯处进行肌内注射药物,并将试验分为4组,即分别为PGD2组(1 mg·mL-1,0.2 mL)、PGF2α组(1 mg·mL-1,0.2 mL)、PGD2+PGF2α组(1 mg·mL-1,各0.2 mL)及生理盐水(0.2 mL,对照组),每组4个重复。
1.3.2 HE染色 子宫肌内注射48 h后(避免此时黄体组织发生生理性周期性退化)进行屠宰,立即采集子宫、卵巢(黄体)组织于-80 ℃保存、备用。采集的黄体用4%多聚甲醛直接固定,待包埋切片后,利用苏木素-伊红(HE)染色,直接观察黄体组织形态。
1.3.3 血液采集、处理与激素测定 子宫肌内注射处理到屠宰间隔48 h,每隔6 h(0、6、12、18、24、30、36、42和48 h)采集一次静脉血,每次5 mL,4 000 r·min-1离心10 min,分离血清后,做好标记保存于-20 ℃备用。利用酶联免疫测定(ELISA)试剂盒检测血清中的P4、E2、PGD2及PGF2α浓度,分析不同处理组中上述激素在体内的浓度变化。
1.3.4 qRT-PCR测定 参照总RNA提取试剂盒说明书提取子宫、黄体组织中总RNA,使用Nanodrop2000检测RNA的纯度及浓度。按照HiFiScript cDNA Synthesis Kit试剂盒说明书反转录成cDNA,使用Nanodrop2000检测cDNA的纯度及浓度,保存于-20 ℃。qRT-PCR检测7个引物(表 1),由Sangon Biotech公司合成。按照TB Green Premix Ex Taq Ⅱ(Tli RNaseH Plus)试剂盒说明书操作实施qRT-PCR检测:TB Green Premix Ex Taq Ⅱ(Tli RNaseH Plus)(2×)10 μL,PCR Forward Primer(10 μmol·L-1)0.8 μL,PCR Reverse Primer(10 μmol·L-1)0.8 μL,ROX Reference Dye(50×)0.4 μL,DNA模板2 μL,灭菌水6 μL。PCR扩增程序为:95 ℃预变性30 s;2.55个循环:95 ℃ 5 s,56 ℃ 30~34 s。
|
|
表 1 引物序列信息 Table 1 Primer sequence information |
1.3.5 Western blot测定 不同组别的子宫、卵巢(黄体)组织置于液氮中研磨后,将碾碎的组织放入蛋白裂解液中裂解,离心后,提取总蛋白,并利用SCA蛋白浓度测定试剂盒检测蛋白浓度;各组提取等量蛋白后,加入5×蛋白上样缓冲液,高温变性;再制备12%分离胶和5%浓缩胶,加入样品和蛋白Marker进行SDS-PAGE,并将目的蛋白切胶转移至PVDF膜上,置于摇床室温清洗封闭,取出PVDF膜加入一抗,4 ℃摇床、孵育过夜;最后,PBST摇床洗涤,加入二抗,室温摇床孵育,再添加ECL显色剂对蛋白条带进行照相分析。
1.4 统计分析实时荧光定量数据以2-ΔΔCt法处理,Western blot蛋白结果使用Image J软件分析灰度值,使用SPSS Statistics 19软件ANOVA比较基因、蛋白表达量差异,P<0.05被认为具有统计学意义。
2 结果 2.1 处理前后黄体组织形态学观察HE染色结合物理拍照对比不同组别处理前后黄体形态变化,结果如图 1所示,各组之间的黄体组织结构形态均发生了不同程度的变化:1)PGD2组中,黄体体积未发生显著变化,但卵巢组织中出现多个卵泡,HE染色发现,黄体结构已发生空腔化并伴有CL结构退化的初期特征;2)PGF2α组中,黄体体积明显缩小,卵巢组织中出现单个较大卵泡,HE染色结果显示,黄体组织已发生显著结构性退化;3)与前两组相比,PGD2+PGF2α组中,黄体体积缩小更为明显,同时,卵巢组织中出现多个较大卵泡,HE染色发现,黄体组织已基本退化完成,并出现多个活性卵泡;4)对照组中,黄体体积未发生明显变化,同时卵巢组织呈现静止状态(无活性卵泡),HE染色结果显示,黄体组织结构完整,并未发生CL退化现象。

|
A. PGD2组;B. PGF2α组;C. PGD2+PGF2α组;D. 对照组;黑细箭头指示黄体结构,黑粗箭头指示卵泡 A. PGD2 group; B. PGF2α group; C. PGD2+PGF2α group; D. Control group; Black fine arrows indicate the corpus luteum, black thick arrows show ovarian follicle 图 1 处理前后卵巢黄体形态学变化 Fig. 1 Morphological changes in the corpus luteum before and after treatment |
2.2.1 不同组别P4水平的变化 使用ELISA法检测血清中P4水平,结果如图 2所示。PGD2组P4浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-0.212 5,在PGD2处理后24 h出现明显的单峰,峰值为8.65 ng·mL-1;PGF2α组P4浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-0.222 1,在PGF2α处理后30 h出现明显的单峰,峰值为8.37 ng·mL-1;PGD2+PGF2α组中P4浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-0.349,在PGD2结合PGF2α处理后6和36 h出现明显的双峰,峰值分别为10.69和8.70 ng·mL-1;对照组中P4浓度随时间变化不显著(P>0.05),趋势线斜率为0.021 5,在生理盐水处理后24 h出现不明显波动性变化。此外,通过组间P4激素变化趋势对比分析,发现对照组与上述3个试验组之间均存在极显著性差异(P < 0.01)。
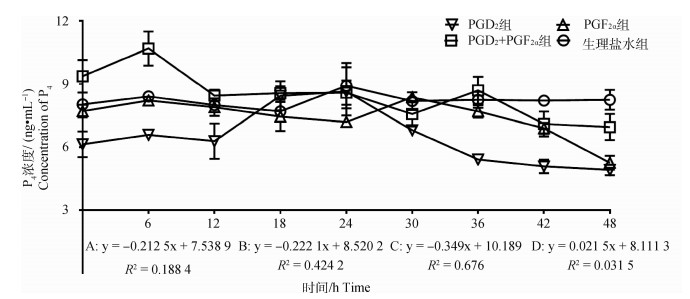
|
A. PGD2组;B. PGF2α组;C. PGD2+PGF2α组;D. 对照组。下同 A. PGD2 group; B. PGF2α group; C. PGD2+PGF2α group; D. Control group. The same as below 图 2 各组母羊血清中P4浓度变化 Fig. 2 Changes in plasma P4 concentration in the ewes in each group |
2.2.2 不同组别E2水平的变化 结果如图 3所示,PGD2组E2浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-0.953 9,在PGD2处理后24 h出现不明显的单峰,峰值为211.35 pg·mL-1;PGF2α组E2浓度随时间变化呈显著上升趋势(P < 0.05),趋势线斜率为4.538 3,在PGF2α处理后12和36 h出现明显的双峰,峰值分别为252.44和282.26 pg·mL-1;PGD2+PGF2α组中E2浓度随时间变化呈显著上升趋势(P < 0.05),趋势线斜率为0.527 7,但在PGD2结合PGF2α处理后18 h出现明显的单峰,峰值为274.82 pg·mL-1;对照组中E2浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-1.556 3,处理后48 h内未出现明显的波动性变化。此外,通过组间E2激素变化趋势对比分析,发现对照组与上述3个试验组之间均存在显著性差异(P < 0.05)。
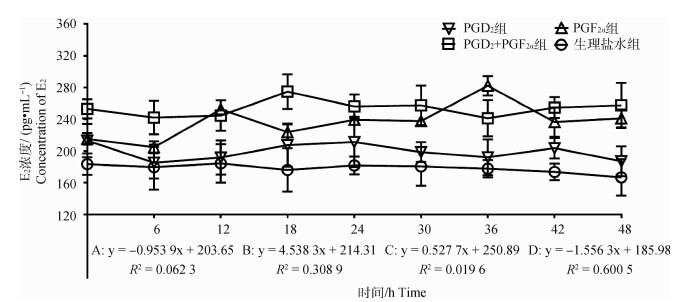
|
图 3 各组母羊血清中E2浓度变化 Fig. 3 Changes in plasma E2 concentration in the ewes in each group |
2.2.3 不同组别PGD2水平的变化 结果如图 4所示,PGD2组PGD2浓度随时间变化呈显著上升趋势(P < 0.05),趋势线斜率为2.576 1,在PGD2处理后24和36 h出现明显的双峰,峰值为77.78和101.94 pg·mL-1;PGF2α组PGD2浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-1.240 4,在PGF2α处理后6 h出现明显的单峰,峰值为83.82 pg·mL-1;PGD2+PGF2α组中PGD2浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-0.841 3,在PGD2结合PGF2α处理后6和24 h出现不明显的双峰,峰值分别为92.34和92.38 pg·mL-1;对照组中PGD2浓度随时间变化呈显著上升趋势(P < 0.05),趋势线斜率为1.204 5,处理后48 h内未出现明显的峰值。此外,通过组间PGD2激素变化趋势对比分析,发现对照组与上述3个试验组之间均存在显著性差异(P < 0.05)。
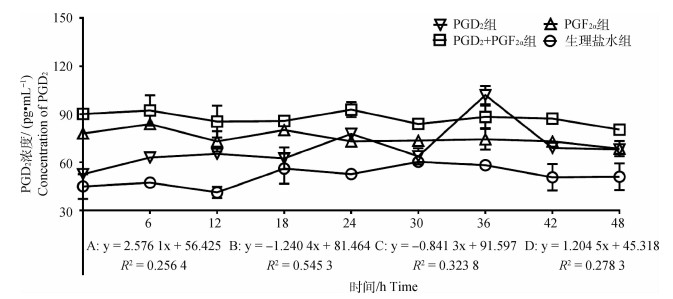
|
图 4 各组母羊血清中PGD2浓度变化 Fig. 4 Changes in plasma PGD2 concentration in the ewes in each group |
2.2.4 不同组别PGF2α水平的变化 结果如图 5所示,PGD2组PGF2α浓度随时间变化呈不显著下降趋势(P>0.05),趋势线斜率为-0.000 8,在PGD2处理后24 h出现明显的单峰,峰值为33.50 pg·mL-1;PGF2α组PGF2α浓度随时间变化呈不显著上升趋势(P>0.05),趋势线斜率为0.046,在PGF2α处理后6和18 h出现明显的双峰,峰值为34.07和33.03 pg·mL-1;PGD2+PGF2α组中PGF2α浓度随时间变化呈显著下降趋势(P < 0.05),趋势线斜率为-0.456 4,在PGD2结合PGF2α处理后18 h出现明显单峰、峰值为34.79 pg·mL-1,36 h出现明显谷峰,谷值为24.95 pg·mL-1;对照组PGF2α浓度随时间变化呈不显著上升趋势(P>0.05),趋势线斜率为0.026 2,在处理后24 h出现明显的谷峰,谷值为24.42 pg·mL-1。此外,通过组间PGF2α激素变化趋势对比分析,发现对照组与上述3个试验组之间均存在显著性差异(P < 0.05)。
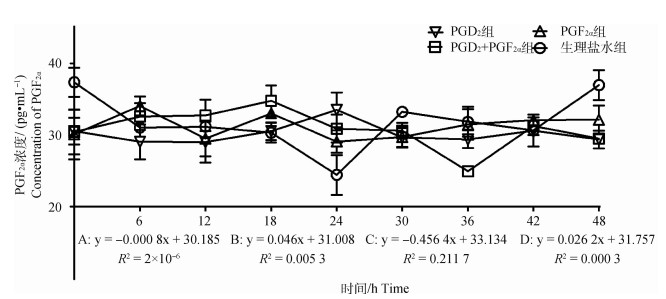
|
图 5 各组母羊血清中PGF2α浓度变化 Fig. 5 Changes in plasma PGF2α concentration in the ewes in each group |
不同方案处理后48 h分别提取总RNA和蛋白,进行qRT-PCR和Western blot检测子宫端PGs的关键合成酶表达水平。结果如图 6所示,与对照组相比,PGD2组HPGDS mRNA和蛋白表达量显著上调(P < 0.05),PGF2α组和PGD2+PGF2α组HPGDS mRNA和蛋白表达量显著下调(P < 0.05);而PGFS mRNA和蛋白表达量在上述3个试验组中均呈显著性上调表达(P < 0.05)。
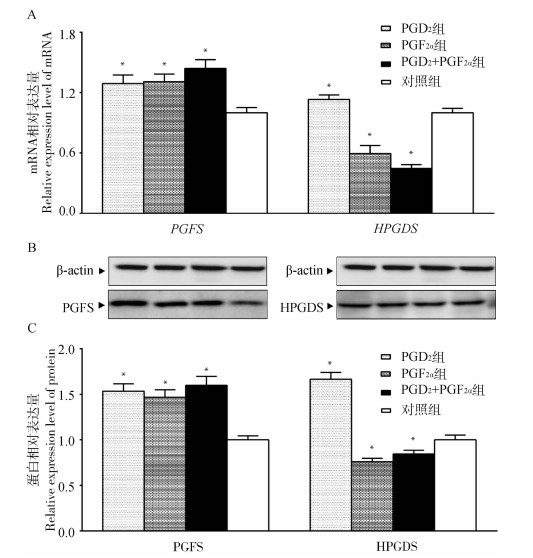
|
图 6 PGD2和PGF2α关键合成酶mRNA(A)和蛋白相对表达量(B和C) Fig. 6 Relative expression of PGD2 and PGF2α key synthetase mRNA (A) and protein (B and C) |
不同方案处理后48 h分别提取总RNA和蛋白,qRT-PCR和Western blot检测黄体端PGs的受体表达水平。结果如图 7所示,与对照组相比,PGD2组、PGF2α组中DP1 mRNA和蛋白表达量显著上调(P < 0.05),PGD2+PGF2α组中DP1 mRNA和蛋白表达量呈不显著上调(P>0.05);类似地,PGF2α组、PGD2+PGF2α组中CRTH2 mRNA和蛋白表达量显著上调(P < 0.05),PGD2组中CRTH2 mRNA和蛋白表达量呈不显著上调(P>0.05);而FP mRNA和蛋白表达量在上述3个试验组中均呈显著性上调表达(P < 0.05)。同时,通过组间的数据对比发现,PGD2单独使用对DP1受体的影响呈最大促进效应,PGF2α单独使用时,对CRTH2受体的影响呈最大促进效应,PGD2+PGF2α结合使用时,对FP受体的影响呈最大促进效应。此外,PGD2两种类型受体的表达中,发现PGD2组中DP1表达量显著高于CRTH2(P < 0.05),而PGF2α组中CRTH2表达量显著高于DP1(P < 0.05)。
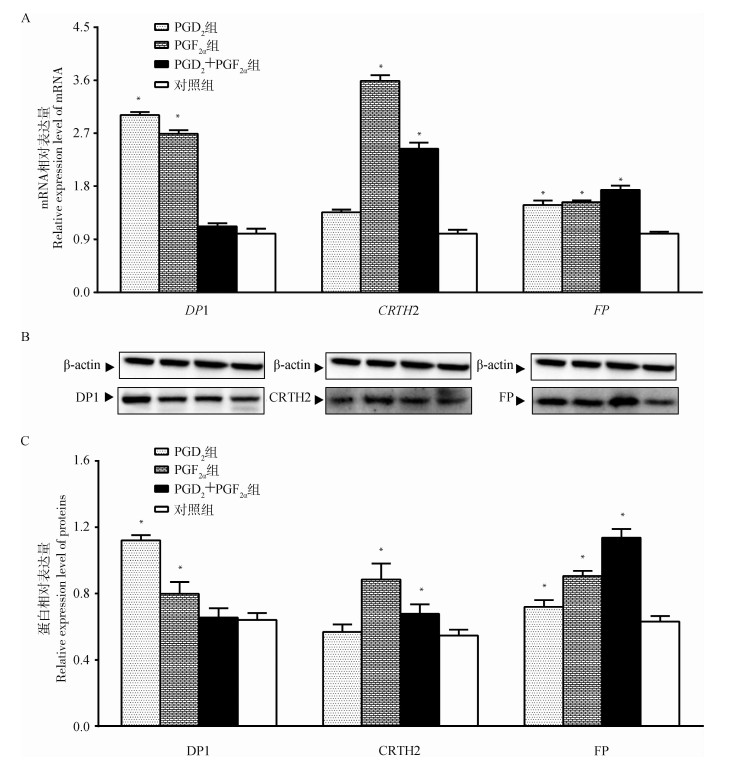
|
图 7 PGD2和PGF2α不同受体mRNA(A)和蛋白(B和C)相对表达量变化 Fig. 7 Relative expression of PGD2 and PGF2α receptor mRNA (A) and protein (B and C) |
CL是哺乳动物卵泡排空后形成的一个暂时的内分泌腺体,是建立和维持妊娠的必需内分泌器官。正常繁殖周期中,哺乳动物的CL必须有规律地退化,才能保证生殖活动的正常进行。目前,普遍认为PGF2α是引起CL退化关键因素[9, 13],PGE2是CL维持的重要保护介质[11, 14]。妊娠CL维持期间,PGE2合成酶会竞争性抑制PGH2向PGF2α的合成转化,降低体内PGF2α浓度,利于CL维持[15-16];但在CL启动退化机制后,PGE2合成的竞争性抑制效应减弱,体内PGF2α的合成增加,从而加速CL退化的进程[17-18]。因此,不难发现,PGF2α和PGE2在CL维持与退化过程中存在竞争性拮抗效应,这为本试验开展PGD2在CL退化过程中的作用机制研究提供了重要参考。
目前,大量研究显示,PGs对CL组织的调控取决于每个PGs成员之间形成的叠加效应[12, 15-17]。本研究通过HE染色结合物理拍照对比不同方案处理前后CL组织形态学变化,发现PGD2与PGF2α对母羊CL组织的作用效应一致,均能够促进CL组织的退化;但其效果呈现明显的差异,即PGF2α组>PGD2组,说明单独使用PGD2时并不能够有效的促进CL的退化。然而,PGD2不同于PGE2,它能够协同PGF2α共同促进CL的退化,其效果明显优于前两者单独使用的退化效果,这一结论与邵焱焱等[19]在黄体化细胞中分别添加PGD2、PGF2α及PGD2+PGF2α观察CL细胞退化结论一致,这提示,在CL维持与退化过程中,PGD2与PGF2α之间可能存在协同效应,而不同于PGE2与PGF2α之间的拮抗效应。
CL作为母体合成和释放孕激素的重要内分泌器官,其结构或功能的改变直接或间接地影响着机体卵巢-子宫轴内分泌功能的变化[20-21]。本研究通过检测外周血P4分泌水平发现,3个试验组P4浓度随时间变化均呈现下降趋势,其下降程度分别为PGD2+PGF2α组>PGF2α组>PGD2组。这一结果与上述形态学观察结果一致,提示PGD2与PGF2α单独使用或联合使用均能够引起CL发生功能性退化。但是,由于PGs作用于CL组织存在一定的时效性,导致不同组别中CL功能性退化时间出现差异,效果也不相同[22-24]。本研究发现,PGD2组中P4浓度曲线峰值出现的时间(24 h),比PGF2α组提前6 h,这可能是由于PGD2舒张了子宫-卵巢轴血管丛系统(UOP)血管,导致其血流加大、速度加快,使其PGs(内源性PGF2α和外源性PGD2)被迅速转运至黄体组织而发挥生物学效应[25-26];但是这种退化机制效应没有PGF2α强烈。不过,与对照组和上述试验组相比,PGD2结合PGF2α使用时,P4浓度曲线呈现双峰状态,峰值出现在6和36 h,说明二者结合使用能够更早地启动CL的功能性退化。P4和E2作为CL组织自分泌或旁分泌的调节剂[27],在CL退化过程中P4浓度的降低会导致LH搏动性增加,从而加快发育中卵泡的生长速率和E2生成水平[28]。这一结果与本研究中PGD2+PGF2α组和PGF2α组的结果相吻合,即在CL退化机制启动后,卵巢卵泡的抑制效应被解除,从而导致E2浓度升高[29-30]。但是,值得注意的是,PGD2+PGF2α组中E2浓度曲线单峰最大值出现的时间明显早于PGF2α组,这与本研究中P4浓度曲线变化趋势相吻合。但与前者不同,PGD2单独使用时,E2水平却呈现明显下降趋势,这一现象也与Farhat等[31]发现的PGD2能够调节卵巢颗粒细胞增殖、促进E2的分泌结果相悖;推测产生这种差异的主要原因,可能是相较于卵泡结构,PGD2更倾向于优先靶向CL组织,以参与其维持与退化过程的调节;但其具体机制仍有待于进一步研究。
CL退化过程中,不同PGs家族成员之间既相互制约、又相互促进。本研究通过ELISA法检测外周血液PGD2和PGF2α浓度发现,在PGD2组中,PGD2在处理后24和36 h出现明显的双峰,而PGF2α浓度则仅在处理后24 h出现一个明显的单峰,提示在24 h出现的CL功能性退化很可能是由于“外源PGD2+内源性PGF2α”分泌的短暂增加所致[32-33]。但与前者不同,PGF2α单独使用或联合PGD2使用时,发现PGD2浓度呈现下降趋势,说明在此时PGF2α能够抑制内源性PGD2的生成,从而导致体内PGD2浓度下降[34]。与PGD2类似,在本研究中PGD2+PGF2α组中PGF2α浓度也呈下降趋势;但产生这一下降趋势的原因与PGD2不同,在PGD2+PGF2α组中很可能是由于超生理浓度的PGD2较大程度地舒张了UOP血管[25-26],导致大部分外源和内源性PGF2α能够及时地被转运至CL组织发挥生物学效应而产生损失。这一趋势结果与HE观察结果相一致,说明当PGD2和PGF2α联合使用时,PGD2很可能发挥着双重调控作用:即一方面促进内源性PGF2α的产生、延长其衰减时长,另一方面促进UOP血管舒张、增加外源性PGF2α的有效利用,从而呈现了明显的优于单独注射PGF2α时CL退化效果。
PGs属于类二十烷酸的多不饱和脂肪酸的衍生物,它们是由特定的PGs关键合酶催化反应产生。其中,PGD2关键合成酶是HPGDS,PGF2α关键合成酶是PGFS。本研究通过检测HPGDS和PGFS的表达水平发现,无论是单独使用还是结合注射外源性PGF2α,子宫端HPGDS的表达均呈显著性下调,提示当体内PGF2α浓度升高时能够明显地抑制其内源性PGD2的合成,从而引起外周PGD2浓度的下降;这一结论与ELISA检测PGD2的结果相吻合。但与前者不同,PGD2单独或联合PGF2α使用时,子宫端PGFS的表达呈显著性上调,说明当体内PGD2浓度增加时能够明显促进其内源性PGF2α的合成,从而导致机体CL退化呈现最大化。造成这一现象的原因很可能与体内E2含量变化有关,即E2处于低水平时,能够促进PGD2的合成,而当E2水平升高时,则促进PGF2α的合成;Chaud等[35]、Acosta等[36]和Hayashi[37]的研究也证实了这一观点。同时,依据不同的受体类型,PGs在靶标组织中发挥着不同的生物学效应。本研究发现,PGD2或PGF2α单独使用时,主要影响PGD2受体(DP1和CRTH2)的表达,即PGD2促进DP1受体的高表达,而PGF2α则促进CRTH2受体的高表达。但两者不同的是,当PGD2浓度升高时,PGD2主要是通过结合DP1受体扩张UOP血管[38-39],以加速内源性PGF2α向CL组织的转运,促进CL退化;而当PGD2浓度较低时,PGD2则主要是通过结合CRTH2受体开启CL细胞的凋亡过程[40],以“外源性PGF2α促进+内源性PGD2启动凋亡”的机制形式,双重促进CL退化,这一观点也在本研究中HE染色与形态学观察结果中得到验证。此外,当PGD2结合PGF2α使用时,发现二者对PGF2α受体(FP)的作用呈最大促进效应,且此时DP1和CRTH2受体也均呈较高水平,说明PGD2结合PGF2α使用时之所以能够表现出最佳的CL退化效果,很可能是开启了两种机制:即一是促进主效激素PGF2α的合成与受体的表达,二是提高PGD2受体DP1和CRTH2受体的均衡表达,最终以“PGF2α+ PGD2”协同作用于CL退化过程。
4 结论本研究发现,PGD2无论单独使用或结合PGF2α使用,均呈现促进CL退化的效应。但单独使用时,其促进CL退化效果不明显,而当其与主效促溶素PGF2α联合使用时,呈现出最佳的促进CL退化效果,这为全面探析CL退化机制以及进一步优化哺乳动物高效繁殖技术(尤其是PGs方案)提供了新的参考依据。
| [1] | WILTBANK M C, MEZERA M A, TOLEDO M Z, et al. Physiological mechanisms involved in maintaining the corpus luteum during the first two months of pregnancy[J]. Anim Reprod, 2018, 15(S1): 805–821. |
| [2] | WILTBANK M C, SOUZA A H, CARVALHO P D, et al. Physiological and practical effects of progesterone on reproduction in dairy cattle[J]. Animal, 2014, 8(S1): 70–81. |
| [3] | MESEN T B, YOUNG S L. Progesterone and the luteal phase: a requisite to reproduction[J]. Obstet Gynecol Clin North Am, 2015, 42(1): 135–151. DOI: 10.1016/j.ogc.2014.10.003 |
| [4] | MEZERA M A, HAMM C S, GAMARRA C A, et al. Profiles of prostaglandin F2α metabolite in dairy cattle during luteal regression and pregnancy: implications for corpus luteum maintenance[J]. Biol Reprod, 2019, 101(1): 76–90. DOI: 10.1093/biolre/ioz074 |
| [5] | OCHOA J C, PEÑAGARICANO F, BAEZ G M, et al. Mechanisms for rescue of corpus luteum during pregnancy: gene expression in bovine corpus luteum following intrauterine pulses of prostaglandins E1 and F2α[J]. Biol Reprod, 2018, 98(4): 465–479. DOI: 10.1093/biolre/iox183 |
| [6] | PARILLO F, CATONE G, BOITI C, et al. Immunopresence and enzymatic activity of nitric oxide synthases, cyclooxygenases and PGE2-9-ketore-ductase and in vitro production of PGF2α, PGE2 and testosterone in the testis of adult and prepubertal alpaca (Lama pacos)[J]. Gen Comp Endocrinol, 2011, 171(3): 381–388. DOI: 10.1016/j.ygcen.2011.03.001 |
| [7] | DOUCETTE L P, WALTER M A. Prostaglandins in the eye: function, expression, and roles in glaucoma[J]. Ophthalmic Genet, 2017, 38(2): 108–116. DOI: 10.3109/13816810.2016.1164193 |
| [8] | STOUFFER R L, BISHOP C V, BOGAN R L, et al. Endocrine and local control of the primate corpus luteum[J]. Reprod Biol, 2013, 13(4): 259–271. DOI: 10.1016/j.repbio.2013.08.002 |
| [9] | ABOELENAIN M, KAWAHARA M, BALBOULA A Z, et al. Status of autophagy, lysosome activity and apoptosis during corpus luteum regression in cattle[J]. J Reprod Dev, 2015, 61(3): 229–236. DOI: 10.1262/jrd.2014-135 |
| [10] | CHOI J Y, JO M W, LEE E Y, et al. The role of autophagy in corpus luteum regression in the rat[J]. Biol Reprod, 2011, 85(3): 465–472. DOI: 10.1095/biolreprod.111.091314 |
| [11] | SAKUMOTO R, HAYASHI K G, TAKAHASHI T. Different expression of PGE synthase, PGF receptor, TNF, Fas and oxytocin in the bovine corpus luteum of the estrous cycle and pregnancy[J]. Reprod Biol, 2014, 14(2): 115–121. DOI: 10.1016/j.repbio.2013.12.003 |
| [12] | KASHIWAGI H, YUHKI K I, IMAMICHI Y, et al. Prostaglandin F2α facilitates platelet activation by acting on prostaglandin E2 receptor subtype EP3 and thromboxane A2 receptor TP in mice[J]. Thromb Haemost, 2019, 119(8): 1311–1320. DOI: 10.1055/s-0039-1688906 |
| [13] | JONCZYK A W, PIOTROWSKA-TOMALA K K, SKARZYNSKI D J. Effects of prostaglandin F2α (PGF2α) on cell-death pathways in the bovine corpus luteum (CL)[J]. BMC Vet Res, 2019, 15(1): 416. DOI: 10.1186/s12917-019-2167-3 |
| [14] | KUMAGAI A, YOSHIOKA S, SAKUMOTO R, et al. Auto-amplification system for prostaglandin F2α in bovine corpus luteum[J]. Mol Reprod Dev, 2014, 81(7): 646–654. DOI: 10.1002/mrd.22332 |
| [15] | SURESH A, REDDY I J, MISHRA A, et al. Suppression of COX-2 mRNA abundance in in vitro cultured goat (Capra hircus) endometrial cells by RNA interference and effect on PGF2α and PGE2 concentrations[J]. Anim Reprod Sci, 2019, 209: 106146. DOI: 10.1016/j.anireprosci.2019.106146 |
| [16] | LEE J H, MCCRACKEN J A, STANLEY J A, et al. Intraluteal prostaglandin biosynthesis and signaling are selectively directed towards PGF2alpha during luteolysis but towards PGE2 during the establishment of pregnancy in sheep[J]. Biol Reprod, 2012, 87(4): 97. |
| [17] | GALVÃO A, SKARZYNSKI D, FERREIRA-DIAS G. Nodal promotes functional luteolysis via down-regulation of progesterone and prostaglandins E2 and promotion of PGF2α synthetic pathways in mare corpus luteum[J]. Endocrinology, 2016, 157(2): 858–871. DOI: 10.1210/en.2015-1362 |
| [18] | SHIRASUNA K, AKABANE Y, BEINDORFF N, et al. Expression of prostaglandin F2α (PGF2α) receptor and its isoforms in the bovine corpus luteum during the estrous cycle and PGF2α-induced luteolysis[J]. Domest Anim Endocrinol, 2012, 43(3): 227–238. DOI: 10.1016/j.domaniend.2012.03.003 |
| [19] |
邵焱焱, 杨恒, 牟健, 等. PGF2α和PGD2对体外绵羊卵巢颗粒黄体化细胞溶解及相关基因差异表达的影响[J]. 石河子大学学报(自然科学版), 2021, 39(2): 171–176.
SHAO Y Y, YANG H, MOU J, et al. Effect of exogenous PGF2α and PGD2 on the lysis time and differential expression of related genes in sheep ovarian lutealization cells[J]. Journal of Shihezi University (Natural Science), 2021, 39(2): 171–176. (in Chinese) |
| [20] | ROCHA C C, MARTINS T, CARDOSO B O, et al. Ultrasonography-accessed luteal size endpoint that most closely associates with circulating progesterone during the estrous cycle and early pregnancy in beef cows[J]. Anim Reprod Sci, 2019, 201: 12–21. DOI: 10.1016/j.anireprosci.2018.12.003 |
| [21] | SHIRASUNA K, NITTA A, SINEENARD J, et al. Vascular and immune regulation of corpus luteum development, maintenance, and regression in the cow[J]. Domest Anim Endocrinol, 2012, 43(2): 198–211. DOI: 10.1016/j.domaniend.2012.03.007 |
| [22] | SMALLMAN M A, FILTZ T M, STORMSHAK F. Mifepristone and PGF2α activate phosphatidylinositol hydrolysis in the ovine corpus luteum[J]. Prostag Other Lipid Med, 2021, 153: 106538. DOI: 10.1016/j.prostaglandins.2021.106538 |
| [23] | CAMACHO M, GARZA D, GAULY M, et al. Superovulation of Boer goats with different synchronization regimens at different times of the year in the northern temperate zone[J]. Small Rumin Res, 2019, 177: 106–110. DOI: 10.1016/j.smallrumres.2019.06.022 |
| [24] | PIOTROWSKA-TOMALA K K, JONCZYK A W, KORDOWITZKI P, et al. The effect of basic fibroblast growth factor 2 on the bovine corpus luteum depends on the stage of the estrous cycle and modulates prostaglandin F2α action[J]. Animal, 2021, 15(1): 100048. DOI: 10.1016/j.animal.2020.100048 |
| [25] | SZARY C, WILCZKO J, ZAWADZKI M, et al. Hemodynamic and radiological classification of ovarian veins system insufficiency[J]. J Clin Med, 2021, 10(4): 646. DOI: 10.3390/jcm10040646 |
| [26] | AROSH J A, BANU S K, MCCRACKEN J A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants[J]. J Dairy Sci, 2016, 99(7): 5926–5940. DOI: 10.3168/jds.2015-10335 |
| [27] | SCHAMS D, BERISHA B. Steroids as local regulators of ovarian activity in domestic animals[J]. Domest Anim Endocrinol, 2002, 23(1-2): 53–65. DOI: 10.1016/S0739-7240(02)00145-5 |
| [28] | MLYNARCZUK J J, KOTWICA J. Effect of polychlorinated biphenyls on the secretion of oxytocin from luteal and granulosa cells in cow: possible involvement of glucocorticoid receptors[J]. Vet Med (Praha), 2006, 51(7): 391–398. |
| [29] | KOWALEWSKI M P. Luteal regression vs. prepartum luteolysis: regulatory mechanisms governing canine corpus luteum function[J]. Reprod Biol, 2014, 14(2): 89–102. DOI: 10.1016/j.repbio.2013.11.004 |
| [30] | KAYA S, KAÇAR C, POLAT B, et al. Association of luteal blood flow with follicular size, serum estrogen and progesterone concentrations, and the inducibility of luteolysis by PGF2α in dairy cows[J]. Theriogenology, 2017, 87: 167–172. DOI: 10.1016/j.theriogenology.2016.08.022 |
| [31] | FARHAT A, PHILIBERT P, SULTAN C, et al. Hematopoietic-prostaglandin D2 synthase through PGD2 production is involved in the adult ovarian physiology[J]. J Ovarian Res, 2011, 4(1): 3. DOI: 10.1186/1757-2215-4-3 |
| [32] | GINTHER O J, BASHIR S T, HOFFMAN M M, et al. Endocrinology of number of follicular waves per estrous cycle and contralateral or ipsilateral relationship between corpus luteum and preovulatory follicle in heifers[J]. Domest Anim Endocrinol, 2013, 45(2): 64–71. DOI: 10.1016/j.domaniend.2013.05.002 |
| [33] | BRESSAN F F, MEMBRIVE C M B, GOISSIS M D, et al. Endometrial prostaglandin F2α in vitro production and its modulation regarding dominant follicle position in cattle[J]. Braz J Vet Res Anim Sci, 2018, 55(2): e133937. DOI: 10.11606/issn.1678-4456.bjvras.2018.133937 |
| [34] | SINREIH M, ANKO M, KENE N H, et al. Expression of AKR1B1, AKR1C3 and other genes of prostaglandin F2α biosynthesis and action in ovarian endometriosis tissue and in model cell lines[J]. Chem-Biol Interact, 2015, 234: 320–331. DOI: 10.1016/j.cbi.2014.11.009 |
| [35] | CHAUD M, FALETTI A, DE ESTRADA M B, et al. Synthesis and release of prostaglandins D2 and E2 by rat uterine tissue throughout the sex cycle.Effects of 17-β-estradiol and progesterone[J]. Prostaglandins Leukot Essent Fatty Acids, 1994, 51(1): 47–50. DOI: 10.1016/0952-3278(94)90177-5 |
| [36] | ACOSTA T J, HAYASHI K G, OHTANI M, et al. Local changes in blood flow within the preovulatory follicle wall and early corpus luteum in cows[J]. Reproduction, 2003, 125(5): 759–767. DOI: 10.1530/rep.0.1250759 |
| [37] | HAYASHI R H.Pharmacological application of prostaglandins, their analogues, and their inhibitors in obstetrics[M]//CARSTEN M E, MILLER J D.Uterine Function: Molecular and Cellular Aspects.Boston, MA: Springer, 2013: 449-469. |
| [38] | MARONE G, GALDIERO M R, PECORARO A, et al. Prostaglandin D2 receptor antagonists in allergic disorders: safety, efficacy, and future perspectives[J]. Expert Opin Inv Drug, 2019, 28(1): 73–84. DOI: 10.1080/13543784.2019.1555237 |
| [39] | BAYS H E, RADER D J. Does nicotinic acid (niacin) lower blood pressure?[J]. Int J Clin Pract, 2009, 63(1): 151–159. DOI: 10.1111/j.1742-1241.2008.01934.x |
| [40] | YUE L, DURAND M, JACOB M C L, et al. Prostaglandin D2 induces apoptosis of human osteoclasts by activating the CRTH2 receptor and the intrinsic apoptosis pathway[J]. Bone, 2012, 51(3): 338–346. DOI: 10.1016/j.bone.2012.06.003 |



