寒冷是北方畜牧业常见应激源之一,常诱导禽畜产生冷应激反应。在寒冷条件下,动物机体主要通过增强自身代谢活动和骨骼肌收缩来增加产热从而维持体温恒定。因此,冷暴露中骨骼肌的功能及能量水平对维持机体运动能力起着十分重要的作用。此外,骨骼肌除了作为运动器官,还可以作为分泌器官分泌细胞因子,即肌细胞因子(myokines)[1]。其中,IL-6作为骨骼肌分泌的重要肌细胞因子之一,通过骨骼肌收缩产生、表达和释放,参与机体能量代谢[2-3]。而蛋白质O-GlcNAc糖基化修饰是细胞内一种蛋白翻译后修饰方式,O-GlcNAc糖基化修饰可以在机体应激中充当一种“应激感受器”,参与细胞信号转导,蛋白质-蛋白质相互作用或基因表达[4-5]。因此,本试验使用小鼠成肌细胞系C2C12,检测不同时长冷暴露下O-GlcNAc糖基化修饰及相关蛋白O-GlcNAc催化转移酶(O-GlcNAc transferase,OGT)、O-GlcNAc水解酶(O-GlcNAcase,OGA)和IL-6表达变化。初步探讨冷暴露下O-GlcNAc糖基化与IL-6表达之间的联系。
1 材料与方法 1.1 材料小鼠成肌细胞系C2C12为黑龙江八一农垦大学应激实验室保存。
1.2 试剂与仪器1.2.1 主要试剂 DMEM高糖培养基、双抗购自美国Gibco公司;胎牛血清购自美国Clark Bioscience公司;RIPA裂解液、BCA蛋白浓度测定试剂盒、SDS-PAGE凝胶配制试剂盒、PMSF(100 mmol·L-1)、SDS-PAGE蛋白上样缓冲液、10×TBST购自中国碧云天生物技术有限公司;ECL显色液、PVDF膜(0.22 /0.45 μm)购自美国默克密理博公司。
抗体:Anti-O-linked-N-Acetylglucosamine(RL2,Abcam),Anti-OGT/O-linked-N-Acetylglucosamine transferase(Abcam),Anti-MGEA5/OGA antibody(Abcam),Anti-IL-6 antibody(Abcam),Goat Anti-Rabbit IgG H & L(HRP,Abcam),Goat Anti-Mouse IgG H & L(HRP,Abcam),用于Western blot检测。
1.2.2 主要仪器 SHP-250恒温培养箱购自上海森信实验仪器有限公司;TDL-5-A离心机购自上海安亭科学仪器厂;电泳槽、湿转转膜仪、ChemiDoc XRS+化学发光成像系统购自美国伯乐有限公司。
1.3 细胞培养及冷暴露处理复苏C2C12细胞,细胞瓶中加入5 mL培养基(DMEM高糖培养基,含10%胎牛血清、1%双抗)置于37 ℃、5% CO2培养箱中培养。待细胞增长到汇合率为80%~90%,传代,扩增细胞。将C2C12细胞分为不做冷暴露处理的对照组(control组,37 ℃±0.5 ℃)、冷暴露(32 ℃±0.5 ℃,医学临床亚低温温度,已证实可引起体外培养细胞冷应激反应)组,其中,冷暴露组又分为冷暴露3、6、9、12 h组。待细胞增长至80%汇合率时进行试验处理,具体试验方案如图 1所示。
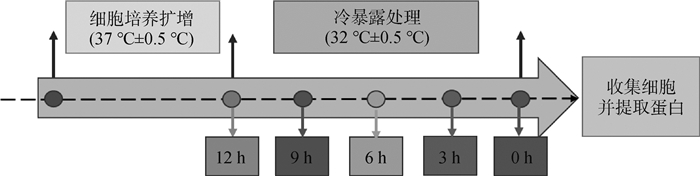
|
图 1 试验方案 Fig. 1 Experiment scheme |
C2C12细胞经过不同时长冷暴露处理后,每瓶细胞加入PMSF终浓度为0.1 mmol·L-1的RIPA裂解液100 μL,然后使用细胞刮收集细胞至1.5 mL离心管,并于冰上裂解30 min。13 000 r·min-1离心10 min,取上清。使用BCA蛋白浓度测定试剂盒按照说明书测定所提取细胞总蛋白浓度,并用RIPA裂解液进行调整,使每组样品蛋白浓度相同。将蛋白样品与SDS-PAGE蛋白上样缓冲液按照4:1的比例混合并煮沸,使蛋白变性。
按照SDS-PAGE凝胶配制试剂盒说明书配制分离胶和浓缩胶。加入样品,50 V恒压电泳,待样品进入分离胶时改为120 V恒压电泳。电泳结束后,使用湿转转膜仪250 mA将凝胶上的蛋白条带转移至PVDF膜上,根据目的蛋白大小调整转膜时间为1.0~2.5 h。转膜结束后,PVDF膜用1×TBST溶液配制5%脱脂乳室温封闭1 h,用1×TBST溶液脱洗PVDF膜3次,每次10 min。按照抗体说明书用TBST稀释抗体,孵育过夜。次日洗膜3次,每次10 min。二抗室温下孵育1 h,然后洗膜3次,每次10 min。将ECL显色液按照1:1配制,使用ChemiDoc XRS+化学发光成像系统进行曝光观察。
1.5 数据统计分析应用凝胶成像系统配套分析软件Image J对目的条带灰度值进行分析。使用GraphPad Prism(La Jolla,CA,USA)软件对数据进行单因素方差分析(one-way ANOVA),所有数据均表示为“x±s”。P < 0.05具有统计学意义。
2 结果 2.1 不同时长冷暴露下OGT和OGA表达变化O-GlcNAc糖基化修饰受两种拮抗酶即OGT和OGA调节从而维持动态平衡。不同时长冷暴露下C2C12中OGT及OGA表达变化水平如图 2所示,不同时长冷暴露处理C2C12细胞后,OGT、OGA表达增加。与对照组比较,冷暴露3 h处理组OGT表达水平显著升高(P < 0.05),冷暴露12 h组OGT表达水平极显著升高(P < 0.01)(图 2B),而OGA表达水平在冷暴露处理6 h时显著升高(P < 0.05)(图 2C)。
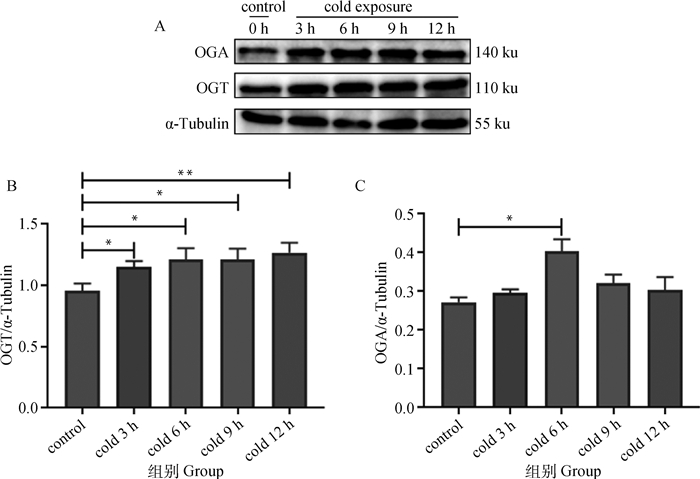
|
A. Western blot结果;B. OGT表达变化;C. OGA表达变化。数据用“x±s”表示,*. P < 0.05, **. P < 0.01 A. Western blot results; B. OGT expression changes; C. OGA expression changes. The data are expressed as "x±s", *. P < 0.05, **. P < 0.01 图 2 不同时长冷暴露下C2C12细胞OGT及OGA蛋白表达水平 Fig. 2 Expression of OGT and OGA protein in C2C12 cells to different durations of cold exposure |
不同时长冷暴露处理后,C2C12细胞O-GlcNAc糖基化修饰水平见图 3,与对照组比较,不同时长冷暴露处理后,O-GlcNAc糖基化修饰水平升高,冷暴露3 h,O-GlcNAc糖基化修饰水平极显著升高(P < 0.01)。
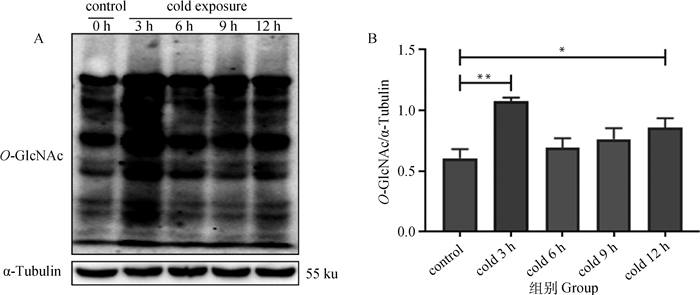
|
A. Western blot结果;B.糖基化修饰水平。数据用“x±s”表示,*. P < 0.05, **. P < 0.01 A. Western blot results; B. Glycosylation changes. The data are expressed as "x±s", *. P < 0.05, **. P < 0.01 图 3 不同时长冷暴露下C2C12细胞O-GlcNAc糖基化修饰水平 Fig. 3 Expression of O-GlcNAc glycosylation in C2C12 cells to different durations of cold exposure |
C2C12细胞经过不同时长冷暴露后,IL-6表达变化如图 4所示,与对照组相比,冷暴露处理3 h时,IL-6的表达水平极显著升高(P < 0.01),冷暴露6 h,IL-6的表达水平较对照组显著升高(P < 0.05),表达水平略低于冷暴露3 h,并且冷暴露9、12 h IL-6的表达水平持续升高。
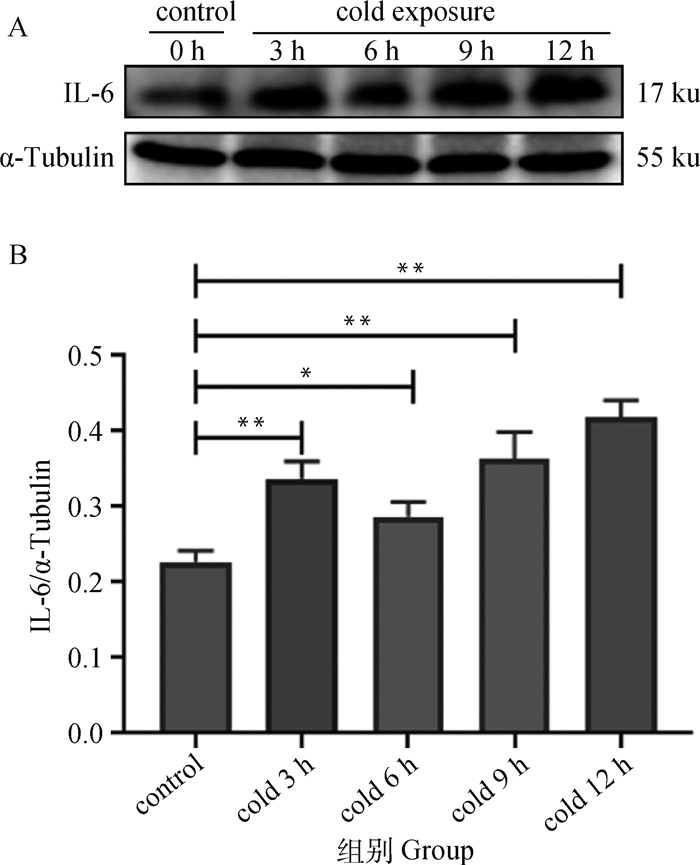
|
A. Western blot结果;B. IL-6表达水平。数据用“x±s”表示,*. P < 0.05, **. P < 0.01 A. Western blot results; B. IL-6 expression changes. The data are expressed as "x±s", *. P < 0.05, **. P < 0.01 图 4 不同时长冷暴露下C2C12细胞IL-6蛋白表达水平 Fig. 4 Expression of IL-6 protein in C2C12 cells to different durations cold exposure |
O-GlcNAc糖基化修饰是一种高度动态且可逆的过程。OGT和OGA两种酶催化细胞内蛋白翻译后的O-GlcNAc糖基化,对细胞内、核和线粒体信号蛋白的数千个丝氨酸或苏氨酸(Ser/Thr)残基进行修饰。OGT催化GlcNAc单糖从UDP-GlcNAc转移至底物Ser/Thr残基上,而OGA作用正好与OGT作用相反[6-7]。在冷暴露处理3 h时,OGT表达水平显著增加(P < 0.05),冷暴露处理6 h时,OGA表达水平显著增加(P < 0.05)。OGT和OGA两种调节酶共同调节O-GlcNAc糖基化修饰水平,并保持冷暴露3 h时O-GlcNAc糖基化高水平动态平衡。O-GlcNAc糖基化修饰作为“应激感受器”,当细胞感受到应激时,整体的O-GlcNAc修饰水平会增加[8]。O-GlcNAc糖基修饰动态可逆地调节细胞内代谢和信号通路,从而对各种应激作出迅速的反应[9]。Zachara等[10]用过氧化氢或亚砷酸盐处理COS7细胞或小鼠胚胎成纤维细胞,构建细胞氧化应激模型,发现O-GlcNAc糖基化水平升高。此外,在内质网应激和热应激下O-GlcNAc糖基化水平升高[10-11]。而本试验发现冷暴露处理使C2C12细胞中蛋白O-GlcNAc修饰水平升高,在冷暴露处理3 h时,表达极显著升高(P < 0.01)。O-GlcNAc修饰水平在3 h时达到高峰,冷暴露6 h时O-GlcNAc糖基化修饰水平较3 h有所下降。但冷暴露6、9、12 h呈持续升高状态。此外,Yao等[12]发现急性冷应激下小鼠整体O-GlcNAc糖基化水平升高,笔者的结果与之相符。
IL-6是一种典型的细胞因子,是具有多种生物学活性的一种糖基化蛋白。有研究表明,暴露于急性应激的人和啮齿动物的外周IL-6水平升高[13-16]。并且Yildirim等[17]发现,冷应激可以诱导大鼠肝、肺、脑和心组织中IL-6表达增加。慢性间歇性冷应激也会改变大鼠脑内IL-6的表达[18]。本试验发现冷暴露处理C2C12细胞,与对照组相比,IL-6表达水平升高,在冷暴露处理3 h时,表达极显著升高(P < 0.01)。因此,与其他研究结果相同,笔者发现冷应激引起C2C12细胞IL-6表达水平升高。Zhou等[14]研究发现束缚应激未造成大鼠组织损伤及炎症,因此, IL-6在急性应激条件下发挥非免疫学功能。此外,Wernsted等[19]发现在4 ℃时,与野生型小鼠相比,IL-6-/-的耗氧量和核心温度都较低,冷诱导产热较低。这表明内源性IL-6对于小鼠在应激和冷诱导的能量消耗中起重要作用。并且Febbraio等[20]研究结果显示,骨骼肌在收缩时分泌的IL-6引起的内源性葡萄糖产生增加,维持骨骼肌内糖原稳定。Knudsen等[21]发现骨骼肌分泌IL-6可以调节葡萄糖摄取能力以及脂肪生成和脂肪分解,影响小鼠腹股沟白色脂肪组织质量。这些研究都表明IL-6的表达影响机体能量代谢。
此外,不同时长冷暴露处理下O-GlcNAc糖基化修饰水平及IL-6表达趋势高度一致,均在冷暴露3 h时显著升高,冷暴露6、9、12 h持续升高。有研究表明,T、B淋巴细胞中OGT介导的活化T细胞1(activated T cells 1,NFATc 1)和NF-κB的O-GlcNAc糖基化修饰是淋巴细胞激活所必需的关键转录因子[22]。NFATc 1和NF-κB的O-GlcNAc糖基化修饰可以促进IL-6的转录。同时,NF-κB的RelA亚基的O-GlcNAc糖基化修饰后可以降低其与NF-κB抑制剂α(NF-κB inhibitor alpha,IκBα)的结合并增加其核转位和转录活性[22-24],进而激活下游信号。因此,笔者推测冷暴露导致C2C12细胞中O-GlcNAc糖基化修饰增加,激活NFATc 1以及NF-κB信号途径,调控IL-6表达,进而调节骨骼肌中葡萄糖、脂质代谢,实现稳态调节,最终保护机体免受冷暴露的损伤。
4 结论不同时长冷暴露下,C2C12细胞中O-GlcNAc糖基化修饰水平及IL-6表达水平显著升高,冷暴露3 h下达到高峰。并且O-GlcNAc糖基化修饰水平及IL-6表达趋势高度相似,因此提示冷暴露下O-GlcNAc糖基化修饰可能参与IL-6表达的调控,但O-GlcNAc糖基化修饰水平与IL-6表达之间的具体联系还需进一步研究。
| [1] | FEBBRAIO M A, PEDERSEN B K. Contraction-induced myokine production and release:is skeletal muscle an endocrine organ?[J]. Exerc Sport Sci Rev, 2005, 33(3): 114–119. DOI: 10.1097/00003677-200507000-00003 |
| [2] | STEENSBERG A. The role of IL-6 in exercise-induced immune changes and metabolism[J]. Exerc Immunol Rev, 2003, 9: 40–47. |
| [3] | PEDERSEN B K, STEENSBERG A, KELLER P, et al. Muscle-derived interleukin-6:lipolytic, anti-inflammatory and immune regulatory effects[J]. Pflügers Arch, 2003, 446(1): 9–16. DOI: 10.1007/s00424-002-0981-z |
| [4] | HART G W, HOUSLEY M P, SLAWSON C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins[J]. Nature, 2007, 446(7139): 1017–1022. DOI: 10.1038/nature05815 |
| [5] | TORRES C R, HART G W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc[J]. J Biol Chem, 1984, 259(5): 3308–3317. DOI: 10.1016/S0021-9258(17)43295-9 |
| [6] | HALTIWANGER R S, HOLT G D, HART G W. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide β-N-acetylgluco-saminyltransferase[J]. J Biol Chem, 1990, 265(5): 2563–2568. DOI: 10.1016/S0021-9258(19)39838-2 |
| [7] | DONG L Y, HART G W. Purification and characterization of an O-GlcNAc selective N-acetyl-β-D-glucosaminidase from rat spleen cytosol[J]. J Biol Chem, 1994, 269(30): 19321–19330. DOI: 10.1016/S0021-9258(17)32170-1 |
| [8] | LIU S, SHENG H X, YU Z, et al. O-linked β-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice:implications for impaired functional recovery from ischemic stress[J]. J Cereb Blood Flow Metab, 2016, 36(2): 393–398. DOI: 10.1177/0271678X15608393 |
| [9] | HART G W. Minireview series on the thirtieth anniversary of research on O-GlcNAcylation of nuclear and cytoplasmic proteins:nutrient regulation of cellular metabolism and physiology by O-GlcNAcylation[J]. J Biol Chem, 2014, 289(50): 34422–34423. DOI: 10.1074/jbc.R114.609776 |
| [10] | ZACHARA N E, O'DONNELL N, CHEUNG W D, et al. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress:a survival response of mammalian cells[J]. J Biol Chem, 2004, 279(29): 30133–30142. DOI: 10.1074/jbc.M403773200 |
| [11] | NGOH G A, HAMID T, PRABHU S D, et al. O-GlcNAc signaling attenuates ER stress-induced cardiomyocyte death[J]. Am J Physiol Heart Circ Physiol, 2009, 297(5): H1711–H1719. DOI: 10.1152/ajpheart.00553.2009 |
| [12] | YAO R Z, YANG Y Y, LIAN S, et al. Effects of acute cold stress on liver O-GlcNAcylation and glycometabolism in mice[J]. Int J Mol Sci, 2018, 19(9): 2815. DOI: 10.3390/ijms19092815 |
| [13] | LIU Y L, BI H, CHI S M, et al. The effect of compound nutrients on stress-induced changes in serum IL-2, IL-6 and TNF-α levels in rats[J]. Cytokine, 2007, 37(1): 14–21. DOI: 10.1016/j.cyto.2007.02.009 |
| [14] | ZHOU D, KUSNECOV A W, SHURIN M R, et al. Exposure to physical and psychological stressors elevates plasma interleukin 6:relationship to the activation of hypothalamic-pituitary-adrenal axis[J]. Endocrinology, 1993, 133(6): 2523–2530. DOI: 10.1210/endo.133.6.8243274 |
| [15] | STEPTOE A, HAMER M, CHIDA Y. The effects of acute psychological stress on circulating inflammatory factors in humans:a review and meta-analysis[J]. Brain, Behav, Immun, 2007, 21(7): 901–912. DOI: 10.1016/j.bbi.2007.03.011 |
| [16] | NUKINA H, SUDO N, AIBA Y, et al. Restraint stress elevates the plasma interleukin-6 levels in germ-free mice[J]. J Neuroimmunol, 2001, 115(1-2): 46–52. DOI: 10.1016/S0165-5728(01)00260-0 |
| [17] | YILDIRIM N C, YUREKLI M. The effect of adrenomedullin and cold stress on interleukin-6 levels in some rat tissues[J]. Clin Exp Immunol, 2010, 161(1): 171–175. |
| [18] | GIROTTI M, DONEGAN J J, MORILAK D A. Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain[J]. Psychoneuroendocrinology, 2011, 36(8): 1164–1174. DOI: 10.1016/j.psyneuen.2011.02.008 |
| [19] | WERNSTEDT I, EDGLEY A, BERNDTSSON A, et al. Reduced stress- and cold-induced increase in energy expenditure in interleukin-6-deficient mice[J]. Am J Physiol Regul Integr Comp Physiol, 2006, 291(3): R551–R557. DOI: 10.1152/ajpregu.00514.2005 |
| [20] | FEBBRAIO M A, HISCOCK N, SACCHETTI M, et al. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction[J]. Diabetes, 2004, 53(7): 1643–1648. DOI: 10.2337/diabetes.53.7.1643 |
| [21] | KNUDSEN J G, BERTHOLDT L, JOENSEN E, et al. Skeletal muscle interleukin-6 regulates metabolic factors in iWAT during HFD and exercise training[J]. Obesity, 2015, 23(8): 1616–1624. DOI: 10.1002/oby.21139 |
| [22] | GOLKS A, TRAN T T T, GOETSCHY J F, et al. Requirement for O-linked N-acetylglucosaminyltrans-ferase in lymphocytes activation[J]. EMBO J, 2007, 26(20): 4368–4379. DOI: 10.1038/sj.emboj.7601845 |
| [23] | RAMAKRISHNAN P, CLARK P M, MASON D E, et al. Activation of the transcriptional function of the NF-κB protein C-rel by O-GlcNAc glycosylation[J]. Sci Signal, 2013, 27(290): ra75. |
| [24] | YANG W H, PARK S Y, NAM H W, et al. NFκB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions[J]. Procee Natl Acad Sci U S A, 2008, 105(45): 17345–17350. DOI: 10.1073/pnas.0806198105 |



