禽腺病毒血清4型(fowl adenovirus serotype 4,FAdV-4)是属于禽腺病毒科、禽腺病毒属的双链DNA病毒,是引起肝炎-心包积液综合征(hepatitis-hydropericardium syndrome,HHS)的主要病原[1]。HHS于1987年最早发生于巴基斯坦,随后在印度、科威特、伊拉克、日本、韩国、墨西哥等国家以及中美洲、南美洲等地区发生。自2015年5月份以来,由FAdV-4新基因型导致的HHS在我国主要养禽地区流行,给我国养禽业造成了巨大损失[2-4]。目前,FAdV-4的致病机制尚不明了。现有的研究显示,致病性FAdV-4感染后诱导鸡天然免疫应答,肝中白介素1β(IL-1β)的表达量显著升高[5-6]。
IL-1β的表达和分泌与NLRP3炎症小体密切相关。多种刺激原可以激活胞质天然免疫信号受体NLRP3,激活后的NLRP3使NLRP3炎性小体组装活化,从而引发IL-1β的分泌[7-9]。NLRP3是宿主细胞的核苷酸结合寡聚结构域(nucleotide binding oligomerization domain, NOD)样受体(NLRs)病原模式识别受体(pattern recognition receptors, PRRS)识别病原相关分子模式(pathogen-associated molecular patterns, PAMPS)或危险相关分子模式(danger associated molecular patterns, DAMPS)后,再与凋亡相关斑点样蛋白(apoptosis associated speck-like protein containing a C-terminal caspase recruitment domain, ASC)及Caspase-1(cysteine requiring aspartate protease-1, Caspase-1)共同组成的多聚蛋白复合体[10-13]。NLRP3炎性小体是天然免疫系统的重要组成部分,可响应病原刺激和细胞损伤,介导Caspase-1激活和促炎细胞因子IL-1β和IL-18等炎性因子的成熟和分泌,从而引发炎症反应[14-16]。
鉴于NLRP3炎症小体的活化与IL-1β的表达和分泌密切相关,本研究尝试建立检测鸡NLRP3的实时荧光定量PCR方法,对FAdV-4感染鸡组织中NLRP3基因的转录水平进行分析,以期为解析NLRP3在FAdV-4致病机制中的作用奠定基础。
1 材料与方法 1.1 材料致病性禽腺病毒4型(FAdV-4)毒株CH/HNJZ/2015(GenBank登录号:KU558760)由河南农业大学传染病实验室保存;总RNA提取试剂盒购自TIANGEN;反转录试剂购自TOYOBO;质粒提取和DNA胶回收试剂盒购自生工生物工程(上海)股份有限公司;常规PCR试剂、pMD-18T vector、SYBR Green Ⅰ染料和DNA Marker均购自TaKaRa。
1.2 方法1.2.1 鸡NLRP3基因的RT-PCR扩增和标准质粒的制备 根据GenBank数据库中鸡NLRP3基因序列(GenBank登录号:KF318520.1),设计鸡NLRP3荧光定量特异性引物,qNLRP3-F:5′-TGAGGATTTGGACACCTTCCACCT-3′和qNLRP3-R:5′-TGCTTGATGCAGAAGCAAAGAGCC-3′。利用总RNA提取试剂盒,提取4周龄SPF鸡肝总RNA,并反转录成cDNA。使用特异性引物进行RT-PCR扩增180 bp的NLRP3基因片段。将扩增片段连接到pMD-18T载体,构建pMD-18T-NLRP3重组质粒,测定质粒浓度并根据换算公式计算出重组质粒的拷贝数:拷贝数(copies·μL-1)=[6.02×1023×(质粒浓度ng·μL-1×10-9)]/[DNA长度(bp)×660]。
1.2.2 鸡NLRP3荧光定量PCR的建立 以标准质粒pMD-18T-NLRP3为模板,利用SYBR Green Ⅰ染料和鸡NLRP3荧光定量PCR特异性引物,进行荧光定量PCR扩增。以出现荧光的Cq值最小和相对荧光强度(RFU)最大及熔解曲线不出现非特异性扩增峰为标准,分别对引物终浓度、退火温度和循环数进行优化。将标准质粒pMD-18T-NLRP3进行10倍倍比稀释,取1.0×103~1.0×107 copies·μL-1稀释度的标准质粒作为模板,以最佳反应条件建立鸡NLRP3实时荧光定量PCR标准曲线。
1.2.3 致病性禽腺病毒4型(FAdV-4)感染鸡不同组织中NLRP3基因转录水平的检测 60只4周龄SPF鸡(购自山东昊泰实验动物繁育有限公司)被随机平均分成2组,FAdV-4感染组鸡用105TCID50致病性FAdV-4毒株CH/HNJZ/2015经口服途径感染,对照组口服PBS。FAdV-4感染鸡在感染后4~6 d,相继发病死亡。分别取发病/死亡鸡和对照鸡的心、肝、肺、脾、肾、腺胃和盲肠扁桃体等组织样品,立即冻存于-80 ℃。利用RNA提取试剂盒提取各组织的总RNA,定量1 μg RNA反转录成cDNA。以cDNA为模板,利用建立的NLRP3荧光定量PCR检测鸡组织中NLRP3基因的转录水平。再利用GraphPad Prism5.0软件进行单因子方差(One-way ANOVA)分析和t检验统计分析。**表示差异显著(P < 0.01),***表示差异极显著(P < 0.001)。
2 结果 2.1 鸡NLRP3基因扩增和标准质粒的制备以鸡肝总RNA反转录得到的cDNA为模板,使用常规PCR扩增NLRP3目的基因。扩增产物经2%琼脂糖凝胶进行检测,可观察到180 bp目的条带,大小与预期相符合(图 1)。将PCR产物胶回收纯化后连接至pMD-18T载体,经测序鉴定成功得到pMD-18T-NLRP3重组质粒。测定pMD-18T-NLRP3标准质粒浓度为233 ng·μL-1,利用计算公式计算出该标准品拷贝数约为7.5×1010 copies·μL-1。
|
M. DL2000 DNA相对分子质量标准;1.扩增的NLRP3基因;2.空白对照 M. DL2000 DNA marker; 1.Amplified NLRP3 gene; 2.Blank control 图 1 NLRP3基因的扩增 Fig. 1 Amplification of NLRP3 gene |
经过一系列荧光定量反应条件的筛选,最终的反应体系为总体系20 μL,其中,SYBRⓇ Prexim Ex TaqTM Ⅱ 10 μL,无菌DEPC水6 μL,qNLRP3-F/R(10 μmol·L-1)各1 μL,模板2 μL。最佳反应条件:95 ℃ 1 min;95 ℃ 5 s,60 ℃ 30 s,72 ℃ 8 s,共40个循环;95 ℃ 10 s;65 ℃ 5 s;95 ℃ 5 s。在该条件下熔解曲线单峰,表明设计的引物特异性良好(图 2)。

|
A.扩增曲线;B.标准曲线(X为拷贝数);C.熔解曲线;1~5.1.0×107~1.0×103 copies·μL-1 A. Amplification curve; B. Standard curve (X.Copies number); C. Melting curve; 1-5.1.0×107-1.0×103 copies·μL-1 图 2 鸡NLRP3 SYBR Green Ⅰ实时荧光定量PCR标准曲线建立 Fig. 2 Establishment of standard curve of chicken NLRP3 SYBR Green Ⅰ real-time PCR |
利用1.0×107、1.0×106、1.0×105、1.0×104、1.0×103 copies·μL-1稀释度的标准品为模板进行荧光定量PCR。荧光定量结果显示,以相对荧光强度(RFU)为纵坐标,循环数(cycle)为横坐标建立的扩增曲线光滑,扩增效率好;荧光定量标准曲线显示,以Cq值为Y、拷贝数为X建立的标准曲线方程为Y=-3.434lgX+37.777,相关系数R2为0.999,扩增效率E为95.5%,满足R2>0.999,110%>E>90%。标准曲线显示质粒标准品拷贝数与Cq值之间具有良好的线性关系(图 2)。
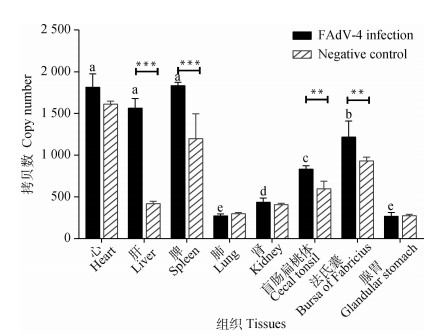
2.3 致病性FAdV-4感染鸡不同组织中NLRP3基因转录水平的检测利用建立的鸡NLRP3 SYBR Green Ⅰ荧光定量PCR方法,对致病性FAdV-4感染鸡和未感染对照鸡各组织中NLRP3的转录水平进行测定。结果显示,该方法能检测出鸡不同组织中NLRP3基因的转录,各组织中NLRP3的转录水平有所差异。FAdV-4感染鸡肝和脾中NLRP3的转录水平极显著(P < 0.001)高于对照组,盲肠扁桃体和法氏囊中NLRP3的转录水平显著(P < 0.01)高于对照组(图 3)。在致病性FAdV-4感染鸡的心、肝和脾组织中NLRP3的转录水平极显著(P < 0.001)高于法氏囊、肺、肾、腺胃和盲肠扁桃体,而心、肝、脾组织中NLRP3的转录水平差异不显著。法氏囊中NLRP3的转录水平极显著(P < 0.001)高于肺、肾、腺胃和盲肠扁桃体,且盲肠扁桃体中NLRP3的转录水平极显著(P < 0.001)高于肺、肾和腺胃,肾中NLRP3的转录水平极显著(P < 0.001)高于肺和腺胃,肺和腺胃组织中NLRP3的转录水平差异不显著。
|
组间比较,**.差异显著(P < 0.01);***.差异极显著(P < 0.001)。感染组组织间比较,不同字母表示差异极显著(P < 0.001). Comparison between groups, **. Significant difference (P < 0.01); ***. Extremely significant difference (P < 0.001). Comparison among organizations of infected group, different letters indicate significant differences (P < 0.001) 图 3 SYBR Green Ⅰ实时荧光定量PCR检测FAdV-4感染鸡组织中的NLRP3基因转录水平 Fig. 3 SYBR Green I real-time PCR for quantitative detection of NLRP3 gene in FAdV-4-infected chicken tissues |
炎症小体既是宿主天然免疫应答的重要组成部分,同时也在病毒致病机制中发挥重要作用[17]。NLRP3炎性小体是天然免疫系统的重要调节剂,在正常细胞中保持较低的水平,然而,一些病原的刺激会引起NLRP3炎性小体的过度活化,从而诱发细胞炎性因子风暴导致机体的病理损伤[18-19],NLRP3分子是NLRP3炎症小体复合物的重要组成成分[20-21],很多病毒感染与NLRP3炎症小体的激活相关[22]。参与激活过程的病毒组分主要包括病毒核酸、离子通道蛋白和非结构蛋白等。如人腺病毒感染巨噬细胞可以导致IL-1β的快速释放,这种反应依赖于NLRP3炎症小体的形成和Caspase-1的活化[23-24];流感病毒的RNA和M2通道蛋白均可激活炎症小体[25-26];脑心肌炎病毒的通道蛋白2B可以激活NLRP3炎症小体[27];猪瘟病毒的p7通道蛋白可激活猪巨噬细胞炎症小体[28];猪繁殖与呼吸综合征病毒通过NLRP3炎症小体活化诱导巨噬细胞表达IL-1β[29-30]。丙型肝炎病毒感染会刺激肝巨噬细胞中NLRP3炎症小体活化产生IL-1β进而造成肝炎症[31]。迄今为止,与病毒感染相关的NLRP3炎症小体的研究报道主要集中在哺乳动物模型,而与禽类病毒感染相关的禽NLRP3炎症小体的研究相对较少,主要原因是受限于禽类NLRP3炎症小体相关分子的检测方法和研究材料。Ye等[32]首次研究了中国三黄鸡体内NLRP3的组织特异性表达图谱和组织分布。陶志云等[33]开展了鸡小肠上皮细胞的分离培养和NLRP3在该细胞中的表达研究。Wang等[34]证明新城疫病毒(NDV)可以激活小鼠和人巨噬细胞中的NLRP3和增加IL-1β的表达。Gao等[35]首次证实了NDV在感染的鸡细胞中通过活化NLRP3/Caspase-1炎症小体诱导IL-1β的高表达。建立鸡NLRP3分子的检测技术有助于研究其在禽病发生中的作用。目前国内外尚未见有关鸡NLRP3分子实时荧光定量PCR检测方法的报道。
自2015年5月份以来,由FAdV-4导致的鸡HHS给我国养禽业造成了巨大损失。研究显示,致病性FAdV-4感染鸡的肝、肺、脾和法氏囊等组织存在严重的淋巴细胞变性、坏死等炎症损伤,而且这些组织中IL-1β的表达量与未感染对照鸡相比显著升高[5-6]。鉴于NLRP3炎症小体的活化与IL-1β的表达和分泌密切相关,本研究设计鸡NLRP3特异性引物,建立了检测鸡NLRP3的SYBR Green Ⅰ荧光定量PCR方法,并利用该方法对致病性FAdV-4感染鸡组织中NLRP3基因的转录水平进行了分析。结果显示,所建立方法对鸡NLRP3标准质粒的扩增曲线良好,标准品的拷贝数与Cq值呈现良好的线性关系,所设计的NLRP3引物可特异性扩增鸡NLRP3基因。
应用所建立的SYBR Green Ⅰ荧光定量PCR对致病性FAdV-4感染鸡和正常鸡组织中NLRP3基因的转录水平进行了检测。结果显示,该方法能检测出不同组织中NLRP3基因的转录。与对照组比对发现,NLRP3分子在致病性FAdV-4感染鸡肝、脾中的转录水平极显著高于对照组,在盲肠扁桃体和法氏囊的表达显著高于对照组,提示这些器官是致病性FAdV-4的主要靶器官。致病性FAdV-4感染发病鸡的心、肝、脾和法氏囊组织中NLRP3的转录水平显著高于其他组织,这也与笔者前期研究结果显示的致病性FAdV-4感染鸡的心、肝、脾和法氏囊组织中IL-1β的高水平表达,以及这些组织的严重炎症损伤病变相一致。鉴于NLRP3炎症小体在IL-1β成熟和分泌过程中扮演重要角色,而IL-1β又是炎症反应的重要介质,笔者推测致病性FAdV-4的致病过程是通过激活NLRP3炎症小体,进而导致炎症因子IL-1β的过量表达和分泌,最终导致机体组织的组织炎症损伤。
4 结论本研究建立了一种检测禽腺病毒血清4型(FAdV-4)感染鸡组织中NLRP3基因转录水平的实时荧光定量PCR方法,该方法能检测鸡不同组织中NLRP3基因的转录,检测结果表明各组织中NLRP3的转录水平有所差异。致病性FAdV-4感染发病鸡肝、脾、盲肠扁桃体和法氏囊中NLRP3的转录水平显著高于未感染对照组(P < 0.01);NLRP3分子在致病性FAdV-4感染鸡的心、肝、脾和法氏囊组织中的转录水平极显著高于其他组织(P < 0.001)。本研究可为进一步研究鸡NLRP3分子在FAdV-4致病机制中的作用提供方法和技术支持。
| [1] | MAZAHERI A, PRUSAS C, VOŁ M, et al. Some strains of serotype 4 fowl adenoviruses cause inclusion body hepatitis and hydropericardium syndrome in chickens[J]. Avian Pathol, 1998, 27(3): 269–276. DOI: 10.1080/03079459808419335 |
| [2] | YE J Q, LIANG G C, ZHANG J J, et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China[J]. Emerg Microbes Infect, 2016, 5(5): e50. |
| [3] | ZHANG T, JIN Q Y, DING P Y, et al. Molecular epidemiology of hydropericardium syndrome outbreak-associated serotype 4 fowl adenovirus isolates in central China[J]. Virol J, 2016, 13(1): 188. DOI: 10.1186/s12985-016-0644-x |
| [4] | LIU Y K, WAN W Y, GAO D S, et al. Genetic characterization of novel fowl aviadenovirus 4 isolates from outbreaks of hepatitis-hydropericardium syndrome in broiler chickens in China[J]. Emerg Microbes Infect, 2016, 5(11): e117. |
| [5] | NIU Y J, SUN Q Q, LIU X P, et al. Mechanism of fowl adenovirus serotype 4-induced heart damage and formation of pericardial effusion[J]. Poult Sci, 2019, 98(3): 1134–1145. DOI: 10.3382/ps/pey485 |
| [6] | NIU Y J, SUN Q Q, ZHANG G H, et al. Fowl adenovirus serotype 4-induced apoptosis, autophagy, and a severe inflammatory response in liver[J]. Vet Microbiol, 2018, 223: 34–41. DOI: 10.1016/j.vetmic.2018.07.014 |
| [7] |
潘徐彪, 李向玉, 王志鑫, 等. NLRP3-(Caspase-1)/IL-1β信号通路的研究进展[J]. 中国医药导报, 2019, 16(1): 41–44.
PAN X B, LI X Y, WANG Z X, et al. Progress in the research of NLRP3-(Caspase-1)/IL-1β signal pathway[J]. China Medical Herald, 2019, 16(1): 41–44. (in Chinese) |
| [8] | MANGAN M S J, OLHAVA E J, ROUSH W R, et al. Targeting the NLRP3 inflammasome in inflammatory diseases[J]. Nat Rev Drug Discov, 2018, 17(9): 688. |
| [9] | SHAO B Z, XU Z Q, HAN B Z, et al. NLRP3 inflammasome and its inhibitors:a review[J]. Front Pharmacol, 2015, 6: 262. |
| [10] | SCHRODER K, ZHOU R B, TSCHOPP J. The NLRP3 inflammasome: a sensor for metabolic danger?[J]. Science, 2010, 327(5963): 296–300. DOI: 10.1126/science.1184003 |
| [11] | KIM J K, JIN H S, SUH H W, et al. Negative regulators and their mechanisms in NLRP3 inflammasome activation and signaling[J]. Immunol Cell Biol, 2017, 95(7): 584–592. DOI: 10.1038/icb.2017.23 |
| [12] | GROSLAMBERT M, PY B F. Spotlight on the NLRP3 inflammasome pathway[J]. J Inflamm Res, 2018, 11: 359–374. DOI: 10.2147/JIR.S141220 |
| [13] | ELLIOTT E I, SUTTERWALA F S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly[J]. Immunol Rev, 2015, 265(1): 35–52. DOI: 10.1111/imr.12286 |
| [14] | LAROCK C N, NIZET V. Inflammasome/IL-1β responses to streptococcal pathogens[J]. Front Immunol, 2015, 6: 518. |
| [15] | JO E K, KIM J K, SHIN D M, et al. Molecular mechanisms regulating NLRP3 inflammasome activation[J]. Cell Mol Immunol, 2016, 13(2): 148–159. DOI: 10.1038/cmi.2015.95 |
| [16] | KELLEY N, JELTEMA D, DUAN Y H, et al. The NLRP3 Inflammasome: an overview of mechanisms of activation and regulation[J]. Int J Mol Sci, 2019, 20(13): 3328. DOI: 10.3390/ijms20133328 |
| [17] | LATZ E, XIAO T S, STUTZ A. Activation and regulation of the inflammasomes[J]. Nat Rev Immunol, 2013, 13(6): 397–411. DOI: 10.1038/nri3452 |
| [18] | COMPAN V, BAROJA-MAZO A, LÓPEZ-CASTEJÓN G, et al. Cell volume regulation modulates NLRP3 inflammasome activation[J]. Immunity, 2012, 37(3): 487–500. DOI: 10.1016/j.immuni.2012.06.013 |
| [19] | ZHANG X N, XU A N, LV J H, et al. Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases[J]. Eur J Med Chem, 2020, 185: 111822. DOI: 10.1016/j.ejmech.2019.111822 |
| [20] | RANSON N, KUNDE D, ERI R. Regulation and sensing of inflammasomes and their impact on intestinal health[J]. Int J Mol Sci, 2017, 18(11): 2379. DOI: 10.3390/ijms18112379 |
| [21] | ZHAO C Y, ZHAO W. NLRP3 inflammasome— a key player in antiviral responses[J]. Front Immunol, 2020, 11: 211. DOI: 10.3389/fimmu.2020.00211 |
| [22] | HAMILTON C, ANAND P K. Right place, right time: localisation and assembly of the NLRP3 inflammasome[J]. F1000Res, 2019, 8: 676. DOI: 10.12688/f1000research.18557.1 |
| [23] | MURUVE D A, PÉTRILLI V, ZAISS A K, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response[J]. Nature, 2008, 452(7183): 103–107. DOI: 10.1038/nature06664 |
| [24] | BARLAN A U, GRIFFIN T M, MCGUIRE K A, et al. Adenovirus membrane penetration activates the NLRP3 inflammasome[J]. J Virol, 2011, 85(1): 146–155. DOI: 10.1128/JVI.01265-10 |
| [25] | ICHINOHE T, PANG I K, IWASAKI A. Influenza virus activates inflammasomes via its intracellular M2 ion channel[J]. Nat Immunol, 2010, 11(5): 404–410. DOI: 10.1038/ni.1861 |
| [26] | ALLEN I C, SCULL M A, MOORE C B, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA[J]. Immunity, 2009, 30(4): 556–565. DOI: 10.1016/j.immuni.2009.02.005 |
| [27] | ITO M, YANAGI Y, ICHINOHE T. Encephalo-myocarditis virus viroporin 2B activates NLRP3 inflammasome[J]. PLoS Pathog, 2012, 8(8): e1002857. DOI: 10.1371/journal.ppat.1002857 |
| [28] | LIN Z, LIANG W L, KANG K, et al. Classical swine fever virus and p7 protein induce secretion of IL-1β in macrophages[J]. J Gen Virol, 2014, 95(Pt 12): 2693–2699. |
| [29] | BI J, SONG S, FANG L R, et al. Porcine reproductive and respiratory syndrome virus induces IL-1β production depending on TLR4/MyD88 pathway and NLRP3 inflammasome in primary porcine alveolar macrophages[J]. Mediators Inflamm, 2014, 2014: 403515. |
| [30] | CHEN X X, GUO Z H, JIN Q Y, et al. Porcine reproductive and respiratory syndrome virus induces interleukin-1β through MyD88/ERK/AP-1 and NLRP3 inflammasome in microglia[J]. Vet Microbiol, 2018, 227: 82–89. DOI: 10.1016/j.vetmic.2018.10.030 |
| [31] | SHRIVASTAVA S, MUKHERJEE A, RAY R, et al. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages[J]. J Virol, 2013, 87(22): 12284–12290. DOI: 10.1128/JVI.01962-13 |
| [32] | YE J H, YU M, ZHANG K Z, et al. Tissue-specific expression pattern and histological distribution of NLRP3 in Chinese yellow chicken[J]. Vet Res Commun, 2015, 39(3): 171–177. DOI: 10.1007/s11259-015-9641-6 |
| [33] |
陶志云, 朱春红, 徐文娟, 等. 鸡小肠上皮细胞分离培养及NLRP3在该细胞中的表达[J]. 中国兽医杂志, 2016, 52(4): 22–25.
TAO Z Y, ZHU C H, XU W J, et al. Isolation and culture of chicken intestinal epithelial cells and the expression of NLRP3 in these cells[J]. Chinese Journal of Veterinary Medicine, 2016, 52(4): 22–25. (in Chinese) |
| [34] | WANG B B, ZHU J, LI D D, et al. Newcastle disease virus infection induces activation of the NLRP3 inflammasome[J]. Virology, 2016, 496: 90–96. DOI: 10.1016/j.virol.2016.05.023 |
| [35] | GAO P, CHEN L B, FAN L, et al. Newcastle disease virus RNA-induced IL-1β expression via the NLRP3/caspase-1 inflammasome[J]. Vet Res, 2020, 51(1): 53. DOI: 10.1186/s13567-020-00774-0 |



