2. 重庆市高校兽医科学工程研究中心中兽药创新研发实验室, 重庆 402460
2. Chinese Veterinary Herbal Drugs Innovation Research Lab, University Veterinary Science Engineering Research Center in Chongqing, Chongqing 402460, China
我国西南部分地区夏季气候呈现高温高湿,仔猪、家禽等经济型动物易患湿热泄泻疾病。临床上常采用抗生素或抗病毒类药物治疗动物腹泻,但抗生素耐药性和兽药残留等问题已引发社会的广泛关注。随着临床用药的多样化,兽医工作者逐渐发现中药及其组方在治疗动物腹泻疾病方面展现出了有效、安全的优势[1]。笔者根据中兽医辨证论治,研发出了具有清热解毒、燥湿止痢作用的中药组方——复方术苦芩[2]。该组方能有效治疗仔猪湿热泄泻[3],且术苦芩总多糖能修复湿热泄泻仔猪小肠肠黏膜[2]。根据“不治已病治未病”的现代化养殖策略,我们采用复方术苦芩提取液预防给药小鼠,然后腹腔注射大肠杆菌(Escherichia coli,E. coli)并观察其预防效果,同时探究其对小鼠小肠上皮内淋巴细胞(intestinal intraepithelial lymphocyte,iIELs)数量及十二指肠INF-γ和IL-4 mRNA水平的影响,为复方术苦芩防治动物腹泻及调节炎症与免疫提供理论依据。
1 材料与方法 1.1 试验药物复方术苦芩[2]提取液(黄芩采用8倍量的75%乙醇加热回流提取2次,每次3 h,合并滤液,回收乙醇并浓缩得到提取液A,黄芩药渣及其余药材采用6倍量的水煎煮提取3次,第1次提取2 h,第2、3次均提取1.5 h,合并滤液,浓缩得到提取液B,合并提取液A和B,浓缩至含生药5 g·mL-1的清膏,灭菌,4 ℃保存),临用时用生理盐水将其分别稀释成每毫升含0.1、1、10 mg复方术苦芩生药的溶液(下文简称0.1、1、10 mg·mL-1复方术苦芩提取液);黄芪多糖口服液[购自河南安普生物科技有限公司,批号:兽药字(2015)16270523]。
1.2 实验动物72只健康清洁级昆明小鼠,20 g±2 g,雌雄各半,购自西南大学荣昌校区实验动物中心。
1.3 菌种猪源致病性大肠杆菌O101(菌株CVCC231),购自中国兽药监察所菌种保藏中心。
1.4 主要试剂与仪器甲醛(重庆川东化工有限公司,批号:20160501),石蜡(上海华永石蜡有限公司,批号:20161120),苏木素(上海宝曼生物科技有限公司,批号:20130405),伊红(上海试剂三厂,批号:20150304),B511321-UNIQ-10柱式Trizol总RNA抽提试剂盒(上海生物工程有限公司),定量PCR试剂盒(北京全式金生物技术有限公司),反转录试剂盒(TaKaRa公司)。KD2258切片机(浙江省金华市科迪仪器设备有限公司),CX31RTSF显微镜(日本东京OLYMPUS公司),荧光定量PCR仪(Roche生物科技公司)。
1.5 分组与处理将72只小鼠适应性饲养7 d后,随机分为空白组、感染组、阳性药物组和药物Ⅰ、Ⅱ、Ⅲ组,共6个组,每组12只。空白组和感染组灌服生理盐水,阳性药物组灌服1.2 mg·mL-1黄芪多糖口服液,药物Ⅰ、Ⅱ、Ⅲ组分别灌服0.1、1、10 mg·mL-1复方术苦芩提取液,每只小鼠按0.5 mL·(次·d)-1进行灌服,连续7 d。末次给药24 h后,除空白组外,各组小鼠腹腔注射5×107CFU的E. coli,感染24 h后统计小鼠腹泻情况。当观察到感染组小鼠精神沉郁、饮食欲下降及排稀便[4],则设定为试验0 h,并连续观察4 d,于试验6 h、4 d时采样检测。
1.6 复方术苦芩提取液对小鼠iIELs数量的影响在试验6 h、4 d时,各组分别取6只小鼠颈椎脱臼致死,取小肠各肠段置于10%福尔马林溶液中固定,石蜡包埋后进行切片,经苏木精-伊红染色,封片。应用Moditec照相处理软件,在光学显微镜下计数100个肠黏膜柱状上皮细胞间的iIELs数量[5];每只小鼠每个肠段各取不相邻的3张切片计数,取平均值作为iIELs数量。
1.7 复方术苦芩提取液对十二指肠IFN-γ、IL-4基因转录的影响 1.7.1 小肠的采集试验6 h和4 d时,分别取空白组、感染组、阳性药物组和药物Ⅲ组小鼠的十二指肠,每组6只,用PBS缓冲液冲洗净肠道内容物,放入无RNA酶的EP管中,于-80 ℃保存。
1.7.2 引物设计登陆GenBank,如表 1所示,根据已有的小鼠IFN-γ、IL-4和小鼠内参基因(GAPDH)的核苷酸序列设计引物。
|
|
表 1 IFN-γ、IL-4和GAPDH荧光定量PCR引物 Table 1 Primer sequence of IFN-γ, IL-4 and GAPDH mRNA |
取约50 mg十二指肠组织,参照B511321-UNIQ-10柱式Trizol总RNA抽提试剂盒说明书进行操作。
1.7.4 反转录(RT)按照反转录试剂盒GoScriptTM Reverse Transcription System说明书将提取的总RNA反转录成cDNA。
1.7.5 qRT-PCR测定20 μL反应体系:2×SYBR Premix EX-Taq Mix 10 μL,QF(10 μmol·L-1)与QR(10 μmol·L-1)各0.5 μL,Template cDNA 2 μL,RNase Free dH2O 7 μL。扩增程序:94 ℃预变性30 s;94 ℃变性5 s,退火至61 ℃延伸35 s,循环40次。溶解程序:97 ℃ 10 s,65 ℃ 60 s,97 ℃ 1 s,循环1次。采用2-△△Ct法计算各检测样本中目的基因的相对转录量。
1.8 数据分析数据以x±s表示,采用SPSS20.0统计软件对所得出数据进行单因素方差分析和Duncan多重比较,P < 0.05为差异显著,P < 0.01为差异极显著。
2 结果 2.1 复方术苦芩提取液对小鼠腹泻的保护效果感染24 h时,空白组小鼠精神状态和饮食欲俱佳,被毛整洁,呼吸正常。感染组小鼠均出现严重腹泻,被毛逆立,精神沉郁,食欲下降,呼吸加重并伴有腹式呼吸,眼睑红肿且有少量淡黄色分泌物等临床症状。阳性药物组、药物Ⅰ、Ⅱ、Ⅲ组小鼠食欲较好,但大部分小鼠仍伴有腹式呼吸,眼睑红肿等症状,少数小鼠表现轻微腹泻。各组小鼠的腹泻发生率和保护率如表 2所示。
|
|
表 2 感染24 h时小鼠腹泻发生率和保护率 Table 2 Incidence and protection rate of diarrhea in mice after infected in 24 h |
光学显微检查发现,空白组小鼠十二指肠绒毛、黏膜均完整,iIELs广泛分布于肠上皮基底附近,少量游离于黏膜下层(如图 1A′中箭头所示),主要是一些小型淋巴细胞,细胞质较少且细胞核深染;感染组小鼠十二指肠绒毛严重破损,大部分黏膜已经消失(如图 1B′所示);药物Ⅲ组小鼠十二指肠绒毛完整,仅有小部分黏膜出现损伤(如图 1C′所示)。
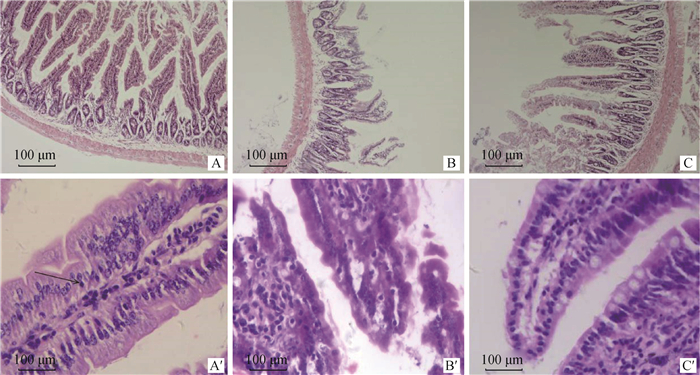
|
A和A′.空白组;B和B′.感染组;C和C′.药物Ⅲ组;A、B、C.10×10; A′、B′、C′.10×40 A and A′. Control group; B and B′: Infection group; C and C′. Drug group Ⅲ; A, B, C. 10×10; A′, B′, C′. 10×40 图 1 试验6 h时小鼠十二指肠iIELs光镜观察(HE染色) Fig. 1 The microexamination of iIELs in duodenum of mice at 6 h (HE staining) |
试验6 h时,与空白组相比,感染组小鼠十二指肠iIELs数量增多,差异极显著(P < 0.01);与感染组相比,阳性药物组和药物Ⅰ、Ⅱ、Ⅲ组小鼠十二指肠iIELs数量均降低(见图 1C′),差异极显著或显著(P < 0.01或P < 0.05)。试验4 d时,与空白组相比,感染组小鼠十二指肠iIELs数量增加,差异极显著(P < 0.01);与感染组相比,阳性药物组和药物Ⅱ、Ⅲ组小鼠十二指肠iIELs数量降低,差异均极显著(P < 0.01),药物Ⅰ组小鼠十二指肠iIELs数量增加,差异不显著(P>0.05)。十二指肠iIELs数量降低幅度与复方术苦芩提取液用药剂量呈正相关;试验4 d与6 h相比,各组小鼠十二指肠iIELs数量均有下降趋势,详见表 3。
|
|
表 3 试验6 h和4 d时小鼠十二指肠iIELs数量(x±s) Table 3 The quantity of iIELs in duodenum of mice at 6 h and 4 d (x±s) |
试验6 h时,与空白组相比,感染组小鼠空肠iIELs数量增高,差异极显著(P < 0.01)。与感染组相比,阳性药物组和药物Ⅰ、Ⅱ、Ⅲ组小鼠空肠iIELs数量均降低,差异极显著(P < 0.01)或不显著(P>0.05)。试验4 d时,与空白组相比,感染组小鼠空肠iIELs数量减少,差异极显著(P < 0.01)。与感染组相比,阳性药物组和药物Ⅰ、Ⅱ、Ⅲ组小鼠空肠iIELs数量增加,差异均极显著(P < 0.01)。试验6 h时空肠iIELs数量降低幅度与复方术苦芩提取液用药剂量呈正相关;试验4 d与6 h相比,各组小鼠空肠iIELs数量均有下降趋势,详见表 4。
|
|
表 4 试验6 h和4 d时小鼠空肠iIELs数量(x±s) Table 4 The quantity of iIELs in jejunum of mice at 6 h and 4 d (x±s) |
试验6 h时,与空白组相比,感染组小鼠回肠iIELs数量增高,差异极显著(P < 0.01)。与感染组相比,阳性药物组和药物Ⅰ、Ⅱ、Ⅲ组小鼠回肠iIELs数量均降低,差异极显著(P < 0.01)。试验4 d时,与空白组相比,感染组小鼠回肠iIELs数量增加,差异极显著(P < 0.01)。与感染组相比,阳性药物组和药物Ⅱ、Ⅲ组小鼠回肠iIELs数量降低,差异均极显著(P < 0.01)。试验4 d与6 h相比,各组小鼠回肠iIELs数量均有下降趋势,详见表 5。
|
|
表 5 试验6 h和4 d时小鼠回肠iIELs数量(x±s) Table 5 The quantity of iIELs in ileum of mice at 6 h and 4 d (x±s) |
如图 2所示,与空白组相比,感染组十二指肠IFN-γ mRNA水平在试验6 h时降低,试验4 d时增高,差异均极显著(P < 0.01)。与感染组相比,阳性药物组和药物Ⅲ组十二指肠中的IFN-γ mRNA水平在试验6 h时增高,试验4 d时降低,差异均极显著(P < 0.01)。与空白组相比,试验6 h和4 d时,感染组十二指肠IL-4 mRNA水平增高,差异均极显著(P < 0.01);与感染组相比,阳性药物组和药物Ⅲ组十二指肠IL-4 mRNA水平降低,差异极显著或显著(P < 0.01或P < 0.05)。
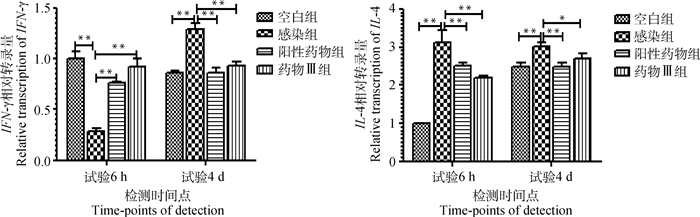
|
**表示组间差异极显著(P < 0.01);*表示组间差异显著(P < 0.05) ** means highly significant difference between groups (P < 0.01), * means significant difference between groups (P < 0.05) 图 2 小鼠十二指肠IFN-γ、IL-4 mRNA转录水平 Fig. 2 Transcription levels of IFN-γ and IL-4 mRNA in duodenum of mice |
如表 6所示,试验6 h时,与空白组相比,感染组十二指肠IFN-γ/IL-4 mRNA比值降低,差异极显著(P<0.01);与感染组相比,阳性药物组和药物Ⅲ组十二指肠IFN-γ/IL-4 mRNA比值增高,差异极显著(P<0.01)。试验4 d时,与空白组相比,感染组十二指肠IFN-γ/IL-4 mRNA比值增高,差异极显著(P<0.01);与感染组相比,阳性药物组和药物Ⅲ组十二指肠IFN-γ/IL-4 mRNA比值降低,差异极显著(P<0.01)。
|
|
表 6 试验6 h和4 d时小鼠十二指肠IFN-γ/IL-4 mRNA比值(x±s) Table 6 The ratio of IFN-γ/IL-4 mRNA in duodenum of mice at 6 h and 4 d (x±s) |
在兽医临床上,黄芪多糖可用于防治动物腹泻。本研究中,黄芪多糖口服液和10 mg·mL-1复方术苦芩提取液对E. coli腹泻小鼠的保护率分别为66.7%和75.0%,说明高剂量的复方术苦芩提取液对腹泻的防治效果好于某些市售的黄芪多糖口服液。中兽医药理论认为,复方术苦芩中黄芩、苦参和白术分别具有清热燥湿、补脾益气、燥湿利水之功效。前期研究发现,复方苦芩(以苦参和黄芩为君药的组方)亦能提高动物免疫功能[6]。现代研究表明,黄芩具有抗病原微生物、抗氧化、调节免疫和代谢的作用,尤其对E. coli的抑菌效果比较明显[7];黄芩多糖也对猪源金黄色葡萄球菌有较强的抑菌效果[8];苦参提取物能通过干预感染炎症进程来预防鸡大肠杆菌病[9],苦参水煎液灌胃大鼠,可降低小肠中E. coli数量,且对十二指肠E. coli数量的影响基本与抗生素一致[10];白术中的黄酮对E. coli有较强的抑菌作用[11]。本研究中,复方术苦芩提取液呈剂量依赖地保护了E. coli腹泻小鼠,说明其防治腹泻的能力可能与黄芩、苦参和白术的抗菌活性及该复方的剂量有关。
研究证明,iIELs可双向调节肠上皮细胞屏障功能,其作为一种组织驻留记忆T细胞主要参与早期的细胞免疫应答[12-13],但过量增生的iIELs也会降低肠黏膜屏障的功能[14]。所以一定范围内的iIELs数量方能体现肠黏膜屏障功能。本研究结果显示,试验6 h时,小鼠iIELs数量明显增加,与李焰等[15]的研究结果相反。但有研究认为,iIELs数量的变化可能与抗原种类、剂量以及机体的固有免疫途径和应答能力有关[16],Qiu等[17]研究表明炎性因子也能促使iIELs增生,提示E. coli可能通过造成小鼠小肠广泛的炎性损伤,进而损坏小肠黏膜屏障。本研究表明,试验4 d与6 h相比,小鼠iIELs数量均有下降趋势,复方术苦芩提取液呈剂量依赖性地降低了iIELs数量,说明复方术苦芩提取液起到了保护小肠黏膜屏障的作用,推测可能与其拮抗炎性损伤有关。但试验4 d时,腹泻小鼠空肠iIELs急剧减少,笔者推测可能是空肠绒毛严重损坏,肠上皮细胞大量增生,所以iIELs数量出现相对减少[18-19]。复方术苦芩提取液促进了空肠iIELs相对数量增加,说明其可能对空肠免疫功能具有一定调节作用,具体机制仍待进一步研究。
研究表明,iIELs中的CD4+T和CD8+T细胞参与肠黏膜免疫调节[20-21];CD4+T细胞能极化为Th1和Th2细胞,继而分泌INF-γ和IL-4。在免疫调节中,IFN-γ主要针对细胞内感染,起到呈递抗原并促进MHC分子表达的作用;IL-4主要针对细胞外感染,起到促进浆细胞形成和抗体产生的作用[22]。在炎症反应中,INF-γ/IL-4分别作为促/抗炎因子,其含量增高可分别被认为是Th1和Th2型炎症的特别指征[23]。研究认为,检测Th1/Th2的平衡可作为诊断是否感染病原微生物的重要依据[24];Bhardwaj等[25]发现肉鸡在感染病毒的中后期,INF-γ/IL-4的表达从失衡演变为平衡,也证实了Th1/Th2平衡在调节免疫与炎症过程中的重要作用。本研究表明,试验6 h时,腹泻小鼠十二指肠INF-γ和IL-4转录水平分别降低和升高,提示其十二指肠Th1/Th2表达失衡(Th1减少,Th2增多),说明腹泻期间可能产生了Th2型炎性损伤,而IL-4表达升高是抗炎的重要反应;IFN-γ表达降低则可能与CD4+T细胞极化产生Th1的过程受阻有关,但具体机制尚待进一步研究。复方术苦芩提取液给药后小鼠十二指肠INF-γ和IL-4表达逐渐平衡,说明其能提高肠黏膜Th1型免疫应答并拮抗Th2型炎性损伤[26]。本研究表明,试验4 d时,复方术苦芩提取液给药后腹泻小鼠十二指肠INF-γ/IL-4转录比值相对降低,与正常小鼠相比无明显差别;说明其能调控十二指肠黏膜Th1/Th2平衡,以达到调节炎症与免疫的作用。
4 结论复方术苦芩提取液能保护腹泻小鼠(保护率为50%~75%),维持肠黏膜免疫屏障功能,拮抗十二指肠炎性损伤;其机制可能与调控小鼠小肠iIELs数量,平衡十二指肠INF-γ/IL-4的表达有关。
| [1] |
吴山楠, 何翠华, 孙启明, 等. 中草药在畜禽常见疾病防治中的应用[J]. 黑龙江畜牧兽医, 2015(20): 146–147.
WU S N, HE C H, SUN Q M, et al. Application of Chinese herbal medicine in prevention and treatment of common diseases of livestock and poultry[J]. Heilongjiang Animal Science and Veterinary Medicine, 2015(20): 146–147. (in Chinese) |
| [2] |
林春发, 郝永峰, 刘娟. 术苦芩总多糖对湿热泄泻仔猪小肠杯状细胞数量以及MUC-2和ITF-3 mRNA转录的影响[J]. 畜牧兽医学报, 2019, 50(6): 1301–1311.
LIN C F, HAO Y F, LIU J. Effect of polysaccharides from ZhuKuQin on the number of intestinal goblet cell and the transcription of MUC-2 and ITF-3 mRNA in the small intestine of damp-heat diarrhea piglets[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(6): 1301–1311. (in Chinese) |
| [3] |
曾宇君, 赖建彬, 朱兆荣, 等. 术苦芩颗粒对湿热泻痢仔猪血清D-木糖、淀粉酶及脂肪酶的影响[J]. 中国兽医杂志, 2015, 51(1): 57–59.
ZENG Y J, LAI J B, ZHU Z R, et al. The effect of Zhukuqin Ganules on serum D-xylose, amylase and lipase in piglets of damp-heat and diarrhea syndrome[J]. Chinese Journal of Veterinary Medicine, 2015, 51(1): 57–59. DOI: 10.3969/j.issn.0529-6005.2015.01.021 (in Chinese) |
| [4] |
张赛奇.葛芪复方对仔猪大肠杆菌性腹泻作用效果研究[D].北京: 中国农业科学院, 2015.
ZHANG S Q. The study of the effect of "Ge Qi" Chinese herb compound on the piglets diarrhea induced by the E. coli[D]. Beijing: Chinese Academy of Agricultural Sciences, 2015. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-82101-1015376283.htm |
| [5] |
闫金坤, 陈耀星, 王子旭. 雌激素对大鼠肠黏膜结构及上皮内淋巴细胞和杯状细胞数量、分布的影响[J]. 畜牧兽医学报, 2008, 39(9): 1267–1271.
YAN J K, CHEN Y X, WANG Z X. Effect of estrogen on structure of mucosal epithelium and number of intraepithelial lymphocytes and goblet cells in small intestine of rat[J]. Acta Veterinaria et Zootechnica Sinica, 2008, 39(9): 1267–1271. DOI: 10.3321/j.issn:0366-6964.2008.09.021 (in Chinese) |
| [6] |
朱兆荣, 刘娟, 杜林林, 等. 复方苦芩对小鼠免疫功能及犬IL-4 mRNA和IFN-γ mRNA表达的影响[J]. 中国兽医科学, 2012, 42(8): 875–880.
ZHU Z R, LIU J, DU L L, et al. Effect of Kuqin compound on immune function of experimental animals and on expressions of canine IL-4 mRNA and IFN-γ mRNA[J]. Chinese Veterinary Science, 2012, 42(8): 875–880. (in Chinese) |
| [7] |
龚妍春, 李玉萍. 黄芩在动物体内药理作用的研究进展[J]. 黑龙江畜牧兽医, 2018(1): 86–89.
GONG Y C, LI Y P. Advances in pharmacological effects of Scutellaria baicalensis in animals[J]. Heilongjiang Animal Science and Veterinary Medicine, 2018(1): 86–89. (in Chinese) |
| [8] |
陈希文, 赖守勋, 尹苗, 等. 22种中药多糖对猪源金黄色葡萄球菌的体外抑菌活性[J]. 江苏农业科学, 2016, 44(9): 264–266, 267.
CHEN X W, LAI S X, YIN M, et al. Antibacterial activity of 22 kinds of Chinese traditional medicine polysaccharides against Staphylococcus aureus from swine in vitro[J]. Jiangsu Agricultural Sciences, 2016, 44(9): 264–266, 267. (in Chinese) |
| [9] |
张瀚元, 张秀英, 施路一. 中药提取物干预鸡大肠杆菌病发病过程的研究[J]. 黑龙江畜牧兽医, 2016(3): 213–215.
ZHANG H Y, ZHANG X Y, SHI L Y. Study on the effect of Chinese herbal extracts on the pathogenesis of chicken Escherichia coli[J]. Heilongjiang Animal Science and Veterinary Medicine, 2016(3): 213–215. (in Chinese) |
| [10] |
段智璇, 张柏岳, 瞿慧琴, 等. 实时荧光定量PCR法分析苦参对大鼠胃肠道大肠杆菌数量的影响[J]. 贵阳中医学院学报, 2016, 38(6): 1–8.
DUAN Z X, ZHANG B Y, QU H Q, et al. Analysis the effect of Sophora flavescens alt on the number of Escherichia coli in the gastrointestinal tract of rats by method of real-time fluorescent quantitative PCR[J]. Journal of Guiyang College of Traditional Chinese Medicine, 2016, 38(6): 1–8. (in Chinese) |
| [11] |
武宇芳, 刘小宝, 赵二劳. 白术中黄酮的提取及其抑菌和抗氧化活性的研究[J]. 黑龙江畜牧兽医, 2017(12): 148–150.
WU Y F, LIU X B, ZHAO E L. Study on the extraction of flavonoids from Atractylodes atractylodes and the activities of antibacterial and antioxidant[J]. Heilongjiang Animal Science and Veterinary Medicine, 2017(12): 148–150. (in Chinese) |
| [12] |
李任军, 马艳粉, 卢一松, 等. 口服黄芪多糖增强肠道黏膜免疫及其对口蹄疫疫苗免疫的影响[J]. 中国兽医学报, 2017, 37(3): 491–496.
LI R J, MA Y F, LU Y S, et al. Enhancement of the gut mucosal immunity and immune response to the foot-and-mouth disease vaccine by oral administration of Astragalus polysaccharide[J]. Chinese Journal of Veterinary Science, 2017, 37(3): 491–496. (in Chinese) |
| [13] | VITALE S, PICASCIA S, GIANFRANI C. The cross-talk between enterocytes and intraepithelial lymphocytes[J]. Mol Cell Pediatr, 2016, 3(1): 20. DOI: 10.1186/s40348-016-0048-4 |
| [14] | HAYDAY A, THEODORIDIS E, RAMSBURG E, et al. Intraepithelial lymphocytes:exploring the Third Way in immunology[J]. Nat Immunol, 2001, 2(11): 997–1003. DOI: 10.1038/ni1101-997 |
| [15] |
李焰, 兰天香, 邱龙新, 等. 银杏叶复方对LPS应激断奶仔猪小肠黏膜形态及上皮内淋巴细胞和杯状细胞数量的影响[J]. 中国畜牧杂志, 2018, 54(12): 109–113.
LI Y, LAN T X, QIU L X, et al. Effects of Yinxingye compounds on the morphology of small intestinal mucosa and the quantity of intraepithelial lymphocytes and goblet cells in weaned piglets stressed by LPS[J]. Chinese Journal of Animal Science, 2018, 54(12): 109–113. (in Chinese) |
| [16] |
谢遵江, 刘文庆, 贺业春, 等. 小鼠肠上皮内淋巴细胞在粘膜免疫应答中的形态学研究[J]. 解剖学报, 1997, 28(3): 309–313.
XIE Z J, LIU W Q, HE Y C, et al. Morphologic studies on intestinal intraepithelial lymphocytes of mice in mucosal immune response[J]. Acta Anatomica Sinica, 1997, 28(3): 309–313. (in Chinese) |
| [17] | QIU Y, WANG W S, XIAO W D, et al. Role of the intestinal cytokine microenvironment in shaping the intraepithelial lymphocyte repertoire[J]. J Leukocyte Biol, 2015, 97(5): 849–857. DOI: 10.1189/jlb.3RU1014-465R |
| [18] |
石玉祥, 闫金坤, 王雪敏, 等. 中药提取物对应激小鼠肠道上皮内淋巴细胞和杯状细胞数量及IL-2水平的影响[J]. 中国兽医科学, 2011, 41(1): 85–88.
SHI Y X, YAN J K, WANG X M, et al. Effect of the Chinese herbal extract on the number of intraepithelial lymphocytes and goblet cells and the level of IL-2 in the intestine of stressed mice[J]. Chinese Veterinary Science, 2011, 41(1): 85–88. (in Chinese) |
| [19] | YU G J, OU W H, LIAO Z B, et al. Intestinal homeostasis of juvenile tiger puffer Takifugu rubripes was sensitive to dietary arachidonic acid in terms of mucosal barrier and microbiota[J]. Aquaculture, 2019, 502: 97–106. DOI: 10.1016/j.aquaculture.2018.12.020 |
| [20] | HE S, KAHLES F, RATTIK S, et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease[J]. Nature, 2019, 566(7742): 115–119. DOI: 10.1038/s41586-018-0849-9 |
| [21] | REQUENA P, DADDAOUA A, GUADIX E, et al. Bovine glycomacropeptide induces cytokine production in human monocytes through the stimulation of the MAPK and the NF-κB signal transduction pathways[J]. Br J Pharmacol, 2009, 157(7): 1232–1240. DOI: 10.1111/j.1476-5381.2009.00195.x |
| [22] |
刘开云, 郭刚, 解庆华, 等. 沙鼠感染幽门螺杆菌后胃粘膜INF-γ、IL-4及IL-12p40表达水平的分析[J]. 第三军医大学学报, 2005, 27(13): 1320–1322.
LIU K Y, GUO G, XIE Q H, et al. INF-γ, IL-4 and IL-12p40 expressions in gastric mucosa of Helicobacter pylori infected Mongolian gerbil[J]. Acta Academiae Medicinae Militaris Tertiae, 2005, 27(13): 1320–1322. DOI: 10.3321/j.issn:1000-5404.2005.13.004 (in Chinese) |
| [23] |
王旭丹, 袁学勤. T淋巴细胞与炎症性肠病[J]. 细胞与分子免疫学杂志, 2015, 21(S1): 95–97, 100.
WANG X D, YUAN X Q. T lymphocytes and inflammatory bowel diseases[J]. Chinese Journal of Cellular and Molecular Immunology2015, 2015, 21(S1): 95–97, 100. (in Chinese) |
| [24] | ABO-AZIZA F A M, HENDAWY S H M, EL NAMAKY A H, et al. Th1/Th2 balance and humoral immune response to potential antigens as early diagnostic method of equine Strongylus nematode infection[J]. Vet World, 2017, 10(6): 679–687. DOI: 10.14202/vetworld.2017.679-687 |
| [25] | BHARDWAJ R, VERMA R, DEKA D, et al. Validation of immunomodulatory effects of lipopolysaccharide through expression profiling of Th1 and Th2 biased genes in Newcastle disease virus vaccinated indigenous chicken[J]. Vet World, 2018, 11(4): 437–445. DOI: 10.14202/vetworld.2018.437-445 |
| [26] |
王婵, 左之才, 余树民, 等. 预防用自拟复方中药对大肠杆菌感染早期小鼠免疫功能的影响试验[J]. 中国兽医杂志, 2016, 52(3): 66–68.
WANG C, ZUO Z C, YU S M, et al. Effect of self-prepared traditional Chinese compounds on immune function of mice infected with Escherichia coli in early stage[J]. Chinese Journal of Veterinary Medicine, 2016, 52(3): 66–68. DOI: 10.3969/j.issn.0529-6005.2016.03.026 (in Chinese) |



