褪黑激素(MT)是由松果体分泌,周期变化为24 h并且介导睡眠的激素,它的减少可能与失眠机制有关[1-2]。此外,位于下丘脑SCN的中枢生物钟也调控睡眠这一节律活动,其中正调节钟基因有Clock、Bmal1、Bmal2,负调节钟基因有Per1、Per2、Cry1、Cry2,其他钟基因有Per3、Reverbα/β和Rorα/β[3]。哺乳动物MT的分泌也受SCN的调控,SCN中的钟基因环路通过交感神经控制松果体MT的分泌[4]。本研究旨在探究睡眠剥夺情况下,对血浆MT与下丘脑钟基因表达变化的影响。
1 材料与方法 1.1 动物处理72只8周龄的雄性ICR-CD1小鼠(北京维通利华实验动物技术有限公司),人为提供LD:DD=14:10(7:00-21:00)光照,并随机等分为睡眠剥夺试验组(SD)和对照组(CON)。SD组利用水平台试验装置(水箱中加水至距离平台4 cm)进行睡眠剥夺72 h,CON组不进行睡眠剥夺。在睡眠剥夺72 h后的当日CT4、8、12、16、20和24时6个时间点,两组各取6只小鼠进行眼球采血并取其下丘脑组织。
1.2 qRT-PCR法测定小鼠钟基因的表达水平使用TRIzon试剂(CW0580A,康为世纪,中国)提取小鼠下丘脑总RNA,进行反转录后-20 ℃保存。以GAPDH作为内参,PCR引物序列参考文献[5]。20 μL反应体系:cDNA模板2 μL、上下游引物均0.4 μL、2 × Es Taq Master Mix 10 μL、ddH2O 7.2 μL。反应程序:95 ℃ 5 min、95 ℃变性30 s,55~60 ℃ 30 s,72 ℃ 20 s,40个循环,采用公式2-(ΔCT目的-ΔCT内参)得到结果。
1.3 ELISA测定血浆MT含量使用ELISA试剂盒检测血浆样本的MT浓度(CEA908Ge,Cloud-Clone,美国)。所有的检测按照说明书指示进行。
1.4 数据拟合使用MATLAB R2016a软件根据余弦公式f(x)=a+b×cos(x×π/12-c×π/12)得到钟基因节律曲线并判断是否有昼夜节律性,具体方法见参考文献[6]。MT分析方法与之相同。
2 结果 2.1 下丘脑正调控钟基因mRNA水平的变化对照组与试验组小鼠下丘脑的正调控钟基因(mBmal1,mClock)都出现了昼夜节律性变化(图 1A、B)。根据余弦曲线参数T检验,试验组两个钟基因转录的每日变化在睡眠剥夺后出现与对照组不同的震荡(图 1A、B)。对于mBmal1的转录,睡眠剥夺后其振幅增高57.3%(P < 0.05),且相位提前(4.75±1.52) h(P < 0.05);对于mClock的转录,睡眠剥夺后中值降低34.9%(P < 0.01),而振幅降低25.3% (P < 0.05),并且相位提前(2.39±0.29) h(P < 0.01)。
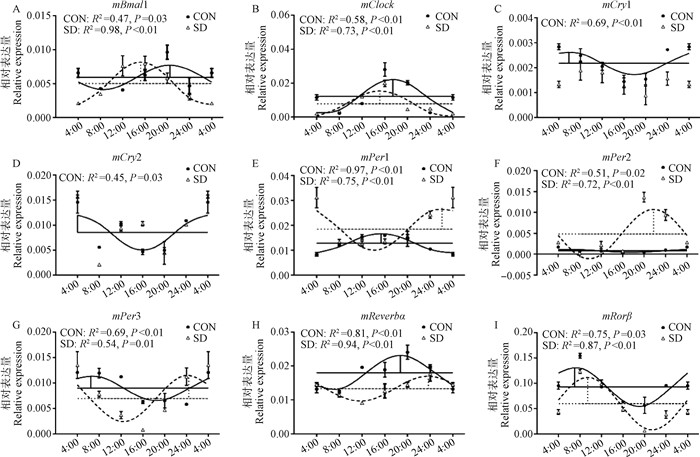
|
实线代表对照组,虚线代表睡眠剥夺组。水平横线代表中值,竖线代表相位和振幅。R2值表示实际表达与曲线相符合程度,P值代表回归分析的差异显著性,P < 0.05说明符合余弦曲线 The solid line represents the control group and the dotted line represents the sleep deprivation group. Horizontal lines represent the median value and vertical lines represent phase and amplitude. The R2 value indicates the degree of fitting. The P value represents the significance of regression analysis, with significance defined as P < 0.05 图 1 睡眠剥夺组(SD)与对照组(CON)小鼠下丘脑钟基因mRNA水平的昼夜节律 Fig. 1 Circadian expressions of clock genes in the hypothalamus of mice from CON and SD groups |
小鼠下丘脑核心负调控钟基因中,对照组mCry1和mCry2的转录表现有节律性,而试验组失去了24 h昼夜节律性(图 1C、D)。而对于mPer1~ 3对照组与试验组都具有昼夜节律性(图 1E~G)。在余弦曲线参数分析中,试验组的mPer1的中值增加了44.1%(P < 0.01),相位延后(10.84±1.21) h(P < 0.01);mPer2的中值增加了500%(P < 0.01),振幅增加了15倍(P < 0.01),相位延后(19.34±1.57)h(P < 0.01);mPer3的振幅增加了283%(P < 0.01),相位延后了(17.31±1.98) h(P < 0.05)。
2.3 下丘脑其他钟基因mRNA水平的变化对照组与试验组小鼠下丘脑中的mReverbα与mRorβ表达量都有昼夜节律(图 1H、I)。在余弦参数的分析中,mReverbα表达量的中值减少了26.2%(P < 0.01);mRorβ中值减少了35.5%(P < 0.01),相位延后(2.92±0.81) h(P < 0.05)。
2.4 血浆中MT含量的变化小鼠的血浆MT对照组有24 h的昼夜节律性,但试验组丧失了昼夜节律性。T检验进行分析发现,试验组相比于对照组小鼠血浆MT在CT4、20和24时降低(P < 0.05),但在CT12时显著增高(P < 0.05)(图 2)。
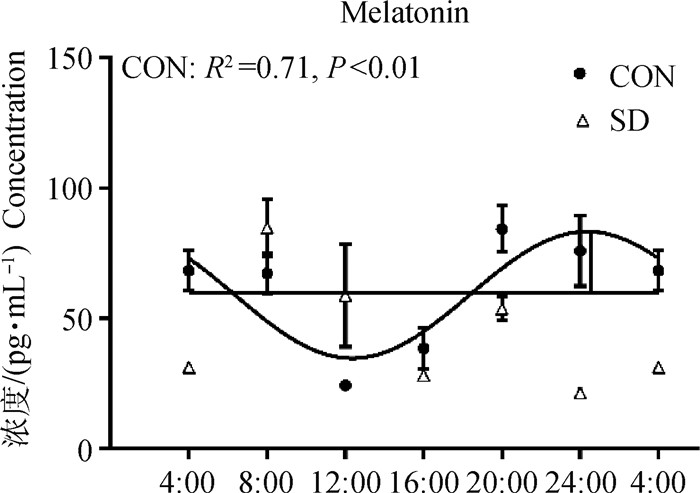
|
图 2 CON组与SD组中小鼠血浆中MT的昼夜节律 Fig. 2 The circadian rhythm of MT in the plasma of mice from CON group and SD group |
本实验室前期已经发现3 d睡眠剥夺后小鼠血浆MT显著减少[7],本试验进一步探究了3 d睡眠剥夺对于小鼠MT的影响。对24 h血浆中的MT含量进行曲线拟合后发现,试验组小鼠血浆MT水平的昼夜节律消失,并且与对照组相比,在CT4、20和24各时间点含量均显著下降,但在CT12时出现了含量的显著上升,而MT应该在白天/光照阶段受到抑制[8]。有试验发现,在野生型小鼠中,ZT22处的急性光脉冲可显著抑制MT的浓度,而在Cry1-/-/Cry2-/-小鼠中则没有出现[9]。所以下丘脑中MT升高可能与钟基因的紊乱有关。
睡眠剥夺对于钟基因mRNA的影响已有报道。Wisor等[10]发现,对AKR/J小鼠进行不同程度的睡眠剥夺后在CT6时处死,结果显示,大脑皮层钟基因表达量增加,但是这些现象并没有在下丘脑中被明显观察到。本研究对钟基因的mRNA进行曲线拟合,发现睡眠剥夺后下丘脑中的mBmal1和mPer3中值与对照组无显著差异,mClock、mRorβ和mReverbα中值显著下降(P < 0.05), 而mPer1和mPer2中值显著增加(P < 0.01),mCry1和mCry2则失去昼夜节律。与前人结果不同,可能是因为他们试验中睡眠剥夺的时间少于本试验(72 h),并且只取某一时间点小鼠的组织测定mRNA,而本试验是采用测定6个时间点下丘脑组织并进行曲线拟合来比较中值的方法。本试验关于睡眠剥夺后钟基因mRNA时相方面的变化如下:mBmal1和mClock相位都有不同程度的提前,而mRorβ和mPer1~3相位延后。而在大鼠衰老时,SCN的钟基因mRNA时相会出现不同程度的改变[11]。
在3 d睡眠剥夺后,小鼠血浆的MT昼夜节律消失,9个钟基因中的mCry1、mCry2昼夜节律也消失。有试验发现,在Cry1-/-/Cry2-/-的C3H小鼠松果体的乙酰基转移酶(AANAT)的表达减少,并且认为和从SCN输入到松果体的昼夜节律性去甲肾上腺素信号缺失有关[12]。而AANAT是MT合成过程中的关键酶[13]。因此,3 d睡眠剥夺后下丘脑中的mCry1、mCry2的昼夜节律紊乱可能也会影响到松果体的信号,最终导致松果体释放入血的MT节律也出现了紊乱。
4 结论3 d睡眠剥夺后造成小鼠的MT分泌紊乱,钟基因mCry1与mCry2的节律丧失,提示睡眠不足诱导MT的昼夜节律分泌紊乱可能与下丘脑钟基因mCry1与mCry2的节律性表达丧失有关。
| [1] | KUN X, HONG H C, SUBRAMANIAN P. Melatonin and sleep[J]. Biol Rhythm Res, 2019, 50(3): 490–493. DOI: 10.1080/09291016.2018.1443554 |
| [2] | TAKAESU Y, FUTENMA K, KOBAYASHI M, et al. A preliminary study on the relationships between diurnal melatonin secretion profile and sleep variables in patients emergently admitted to the coronary care unit[J]. Chronobiol Int, 2015, 32(6): 875–879. DOI: 10.3109/07420528.2015.1048869 |
| [3] | HASTINGS M H, MAYWOOD E S, BRANCACCIO M. Generation of circadian rhythms in the suprachiasmatic nucleus[J]. Nat Rev Neurosci, 2018, 19(8): 453–469. DOI: 10.1038/s41583-018-0026-z |
| [4] | MATTAM U, JAGOTA A. Differential role of melatonin in restoration of age-induced alterations in daily rhythms of expression of various clock genes in suprachiasmatic nucleus of male Wistar rats[J]. Biogerontology, 2014, 15(3): 257–268. DOI: 10.1007/s10522-014-9495-2 |
| [5] | DKHISSI-BENYAHYA O, COUTANSON C, KNOBLAUCH K, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light[J]. Cell Mol Life Sci, 2013, 70(18): 3435–3447. DOI: 10.1007/s00018-013-1338-9 |
| [6] | SINGH D, RANI S, KUMAR V. Daily expression of six clock genes in central and peripheral tissues of a night-migratory songbird:evidence for tissue-specific circadian timing[J]. Chronobiol Int, 2013, 30(10): 1208–1217. DOI: 10.3109/07420528.2013.810632 |
| [7] | GAO T, WANG Z X, DONG Y L, et al. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice[J]. J Pineal Res, 2019, 67(1): e12574. |
| [8] | SAHA S, SINGH K M, GUPTA B B P. Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals:Similarities and differences[J]. Gen Comp Endocrinol, 2019, 279: 27–34. |
| [9] | YAMANAKA Y, SUZUKI Y, TODO T, et al. Loss of circadian rhythm and light-induced suppression of pineal melatonin levels in Cry1 and Cry2 double-deficient mice[J]. Genes Cells, 2010, 15(10): 1063–1071. DOI: 10.1111/j.1365-2443.2010.01443.x |
| [10] | WISOR J P, PASUMARTHI R K, GERASHCHENKO D, et al. Sleep deprivation effects on circadian clock gene expression in the cerebral cortex parallel electroencephalographic differences among mouse strains[J]. J Neurosci, 2008, 28(28): 7193–7201. DOI: 10.1523/JNEUROSCI.1150-08.2008 |
| [11] | DEBOER T, DÉTÁRI L, MEIJER J H. Long term effects of sleep deprivation on the mammalian circadian pacemaker[J]. Sleep, 2007, 30(3): 257–262. DOI: 10.1093/sleep/30.3.257 |
| [12] | YAMANAKA Y, YAMADA Y, HONMA K I, et al. Cryptochrome deficiency enhances transcription but reduces protein levels of pineal Aanat[J]. J Mol Endocrinol, 2018, 61(4): 219–229. DOI: 10.1530/JME-18-0101 |
| [13] | BINKLEY S. Pineal gland biorhythms:N-acetyl-transferase in chickens and rats[J]. Fed Proc, 1976, 35(12): 2347–2352. |



