绵羊绒毛的密度和厚度较高且导热性能极低,对机体有很好的保温作用,故绵羊对寒冷环境有很强的适应能力[1]。但冬季昼夜温差大、短暂强降温或长期寒冷环境还是能造成绵羊机体生理平衡破坏,导致免疫力下降,表现为抗病性差、生长缓慢、生产性能下降等[2-3]。因此,研究绵羊冷应激初期免疫指标变化和分子机制,对绵羊冷应激的早期监测和防治具有重要意义。动物对冷热环境的感受不仅受环境温度而且还受环境相对湿度和风速等因素的影响[4]。热应激环境通常用温湿指数(temperature humidity index, THI)来评价[5-6],许多学者认为,该指标不宜用于冷应激环境评价,原因是风速对动物的冷应激环境感受影响很大,认为风寒温度(wind chill temperature, WCT)更能评价冷应激环境[7-9]。有研究表明,急性与重度冷应激抑制动物免疫机能,而渐变与温和冷应激则可增强动物免疫机能[10-11]。一般来说,冷应激对动物免疫系统功能的影响主要是抑制性的[12-13]。动物遭受应激时,组织细胞会快速产生一类高度保守的保护性热休克蛋白来抵抗应激对细胞的损伤[14-15]。根据相对蛋白质量大小和同源性程度,可把热休克蛋白分为HSP110、HSP90、HSP70、HSP60、小分子HSP及泛素等6大家族[16]。研究表明,在所有热休克蛋白中HSP70对温度变化最敏感[17-18]。Banerjee等[14]研究不同季节对山羊血淋巴细胞HSP70家族基因(HSPA1A、HSPA1L、HSPA2、HSPA6和HSPA8)表达的影响,发现HSPA1A和HSPA8基因表达量在夏季和冬季都显著升高,说明HSPA1A和HSPA8基因对温度变化更敏感。Nagayach等[19]也发现,山羊肝、心等组织中HSP70基因表达量在冷热环境下会显著升高,HSP70可作为山羊冷热应激的生物标记物。目前对于绵羊冷应激的研究大多趋向于神经内分泌系统,而急性冷应激对绵羊免疫因子及HSP70家族基因表达影响的研究较少。
本试验模拟北方高寒地区羊舍内外环境温度和风速,从HSP70家族中选择更具有代表性的HSPA1A、HSPA6和HSPA8基因,采用ELISA法和实时荧光定量PCR法检测急性冷应激前后绵羊血清免疫指标和不同组织中HSP70家族基因表达量的变化,旨在从分子层面探讨绵羊在急性冷应激下免疫系统的调节功能和HSP70家族基因对机体不同组织产生的保护作用,为北方高寒地区绵羊冷应激的早期监测和防治提供理论依据。
1 材料与方法 1.1 试验动物和样品采集在小尾寒羊×湖羊杂交F1群体中随机选取健康、体况接近的(12±0.5)月龄母羊8只,单只单笼饲养。试验羊在舍内非冷应激环境(温度(-7.14±2.53)℃,风速0 m·s-1)中适应7 d。第8天晚上21:00采集8只试验羊血样,并随机屠宰3只采集肝、肾、脾、心、背最长肌和十二指肠等组织样(对照组),同时将剩下5只试验羊移至舍外冷应激环境(温度(-21.35±2.48)℃,风速4.0 m·s-1)进行急性冷应激处理12 h,第9天早上09:00采集血样,并随机屠宰3只采集对应的组织样(冷应激组)。
1.2 饲养管理试验在兰州大学临泽草地生态试验站(39.15′ N,100.02′ E)进行,羊舍为砖混结构,四周围上一层保温薄膜,使羊舍处于无风、保温性较好的环境。距地面垂直距离为1.5 m处悬挂温湿度记录仪(型号:SIN-TH512,杭州联测自动化技术有限公司)测定舍内外温湿度,每1 h自动记录1次数据;用风速仪(型号:AS856,香港希玛科技有限公司)测定风扇(型号:FS-75C,江门金羚排气扇制造有限公司)风速,通过调整风扇转速及与试验羊的距离,控制风速为4.0 m·s-1。饲喂市售育肥羊全混合日粮(总能17.42 MJ·kg-1,粗蛋白16.29%),每天10:30和16:30投料加水,自由采食、饮水。
1.3 试验羊血清免疫指标的测定采集冷应激前后试验羊非抗凝血10 mL,室温下静置,待血清析出后,在4 ℃、3 000 r·min-1离心10 min,吸取上清液,分装于1.5 mL离心管内,-20 ℃冷冻待测。白细胞介素(IL-1β、IL-2、IL-4和IL-6)、肿瘤坏死因子-α(TNF-α)、免疫球蛋白G(IgG)采用酶免法测定。试验所用试剂盒均购自北京华英生物技术研究所。
1.4 试验羊不同组织热休克蛋白70家族基因表达水平的检测 1.4.1 组织总RNA提取参照Eastp® Super总RNA试剂盒(Promega,上海)说明书提取冷应激前后试验羊肝、肾、脾、心、肌肉(背最长肌)和小肠(十二指肠)等组织的总RNA。用Micro-Spectro-photometer核酸定量仪(K5800,上海DRAWELL科学仪器有限公司)测定总RNA的纯度和浓度。用1%琼脂糖凝胶电泳检测总RNA的完整性。
1.4.2 cDNA合成参照Thermo反转录试剂盒(ThermoScientific,USA)说明书对组织总RNA进行反转录,合成cDNA第一链。反转录体系20 μL:总RNA约4 μg(试验总RNA浓度约为1 000 ng·μL-1,加入总RNA体积约为4 μL),Oligo(dT)18 1 μL,5×Reaction Buffer 5 μL,RiboLock RNase Inhibitor (20 U·μL-1) 1 μL,10 mmol·L-1 dNTP Mix 2 μL,RevertAid M-MuLV RT(200 U·μL-1) 1 μL,RNase Free ddH2O补至20 μL。反转录PCR程序:42 ℃ 60 min;70 ℃ 5 min终止反应,cDNA产物于-20 ℃保存。
1.4.3 引物设计与合成根据NCBI (http://www.ncbi.nlm.nih.gov/)公布的绵羊目的基因(HSPA1A、HSPA6、HSPA8、HSF1)和内参基因(β-actin)的mRNA序列,使用软件Primer Premier 5.0设计特异性引物,引物序列见表 1,委托北京奥科鼎盛生物技术有限公司合成。
|
|
表 1 引物序列 Table 1 Primer sequences |
进行普通PCR扩增检测引物特异性,后续进行实时荧光定量PCR。普通PCR反应体系总体积为12.5 μL:cDNA 0.5 μL,2×Es Taq MasterMix (Dye) 5.0 μL,上、下游引物各0.5 μL,RNase Free ddH2O 6.0 μL。PCR扩增条件:95 ℃预变性3 min;95 ℃变性30 s,60 ℃退火30 s,72 ℃延伸15 s,35个循环;72 ℃延伸5 min;4 ℃保存。1.5%琼脂糖凝胶电泳检测PCR产物大小。
实时荧光定量PCR反应在BioRad iQ5实时荧光定量仪上进行,反应体系为12.5 μL:SYBR Premix Ex TaqTMⅡ(2×) 6.25 μL,cDNA 1 μL,上、下游引物各0.5 μL,RNase Free ddH2O 4.25 μL。PCR扩增条件:95 ℃预变性30 s;95 ℃变性5 s,60 ℃退火30 s,72 ℃延伸30 s,共40个循环。熔解曲线分析65~95 ℃:0.5 ℃/0.05s。
1.5 羊舍内外风寒温度(WCT)判定WCT以温度为单位表示动物冷应激的程度,根据Tucker等[20]方法计算WCT,WCT=13.127+0.621 5T-11.947V0.16+0.486TV0.16。式中,WCT为风寒指数以温度表示(℃),V为风速(m·s-1),T为气温(℃)。该指数中,-10~-25 ℃为轻度冷应激,-25~-45 ℃为中度冷应激,-45~-59 ℃为高度冷应激,-60 ℃以上为致命冷应激。当V≤1.34 m·s-1时,WCT与实际T相等[21-22]。
1.6 统计分析采用2-△△CT方法计算目的基因的相对表达量[23],免疫指标和目的基因相对表达量结果均以“平均数±标准差”表示,用SPSS22.0统计软件进行t检验,以P < 0.05作为差异显著性的判断标准。
2 结果 2.1 试验羊舍内外冷应激判定试验羊在舍内适应7 d,适应期舍内24 h温度变化如图 1所示,温度在-3.2~-11.1 ℃之间变化,最高温度在下午16:00,最低温度为早上08:00。舍内平均温度为(-7.14±2.53)℃,风速为0 m·s-1,根据Tucker等[20]判定方法,WCT平均为(-7.14±2.53)℃,试验羊在舍内处于非冷应激状态。
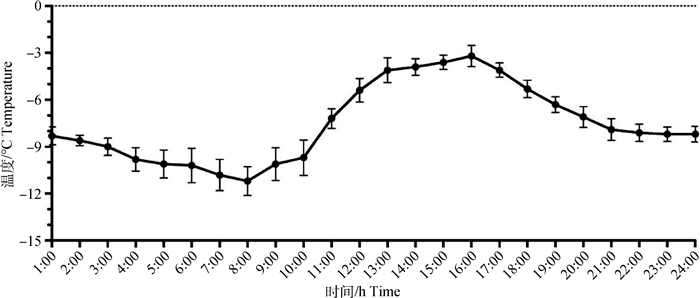
|
图 1 适应期舍内24 h的温度变化 Fig. 1 Continuous change of ambient temperature in 24 h of adaptive phase in indoor |
试验羊在舍外进行12 h冷暴露时温度变化如图 2所示,冷暴露前(0 h)的温度为-7.8 ℃,风速为0 m·s-1;冷暴露12 h内的温度在-16.5~-23.8 ℃之间变化,平均温度为(-21.35±2.48)℃,风速为4.0 m·s-1,根据Tucker等[20]判定方法,WCT平均为(-27.40±3.12)℃,试验羊在舍外处于冷应激状态。
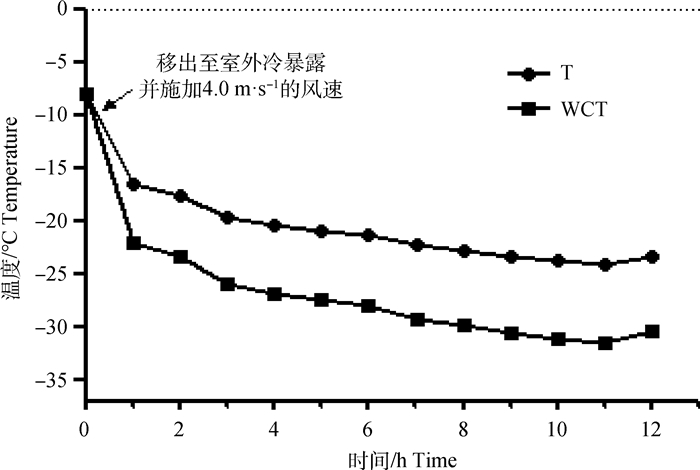
|
图 2 舍外冷暴露12 h的温度变化 Fig. 2 Continuous change of ambient temperature of 12 h cold exposure treatment in outdoor |
急性冷应激对试验羊免疫功能的影响见表 2。与应激前相比,应激后试验羊血清TNF-α、IL-1β、IL-2和IL-6浓度显著升高(P < 0.05),血清IL-4和IgG浓度显著下降(P < 0.05)。
|
|
表 2 急性冷应激对绵羊免疫功能的影响 Table 2 Effect of acute cold stress on immune function of sheep |
急性冷应激对试验羊不同组织热休克蛋白70家族基因表达的影响如图 3所示。与应激前相比,应激后试验羊HSPA1A mRNA表达量在肝、肾、心和背最长肌中显著升高(P < 0.05);HSPA6 mRNA表达量在心和背最长肌中显著升高(P < 0.05);HSPA8 mRNA表达量在背最长肌中显著升高(P < 0.05),在脾和十二指肠中显著下降(P < 0.05);HSF1 mRNA表达量在肝、心和背最长肌中显著升高(P < 0.05),在脾和十二指肠中显著下降(P < 0.05)。
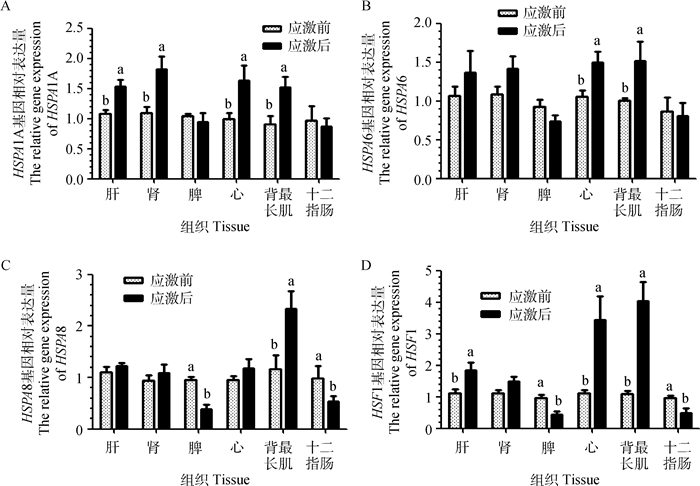
|
图柱上标不同小写字母表示差异显著(P<0.05),无字母表示差异不显著(P>0.05) The different small letters on columns mean significant difference(P < 0.05), while no letter on columns mean no significant difference (P > 0.05) 图 3 绵羊不同组织中热休克蛋白70家族基因表达量 Fig. 3 Heat shock protein 70 family genes expression in different tissues of sheep |
TNF-α、IL-1β、和IL-6等促炎细胞因子的少量分泌可以减少应激对机体造成的损伤,但分泌过多会造成组织损伤,损害机体的免疫功能[24-25]。Shini等[26]研究发现,急性冷应激能增加鸡血淋巴细胞IL-1β和IL-6 mRNA的表达量。郭爽等[27]研究发现,大鼠在急性冷应激后血清TNF-α和IL-6浓度会显著增加。本试验也发现,绵羊急性冷应激12 h后,血清TNF-α、IL-1β和IL-6浓度显著增加,说明急性冷应激可能使绵羊产生了炎症反应。IL-2又称T细胞生长因子,在启动炎症反应中起重要作用,其水平的高低是机体细胞免疫水平的重要标志[28]。Zhao等[29]研究发现,急性冷应激初期肉鸡小肠IL-2 mRNA表达量有升高趋势。计红等[30]研究发现,急性冷应激初期猪血清IL-2浓度呈升高趋势。本试验也发现,绵羊急性冷应激12 h后,血清IL-2浓度显著升高,与前人研究一致,表明机体在冷应激初期提高细胞免疫水平有利于抵抗不利环境。有研究表明,慢性冷应激下绵羊血清IL-2浓度有下降的趋势[31],这可能与冷应激的时间和强度有关。
IL-4又称B细胞生长因子-1,其主要功能是诱导B细胞成熟并刺激其产生免疫球蛋白,对于体液免疫具有重要调节作用[32]。对于应激时IL-4水平是升高还是降低,目前研究结果尚不一致。郭爽等[27]研究发现,小鼠血清IL-4浓度在急性冷应激条件下呈升高趋势,在慢性冷应激条件下呈下降趋势。Zhao等[29]研究发现,急性冷应激条件下肉鸡小肠IL-4 mRNA表达量呈先升高后降低的趋势。伊亢力[33]研究发现,急性冷应激条件下肉鸡脾、胸腺等免疫器官IL-4 mRNA表达量呈下降趋势。研究表明,急性与重度冷应激抑制动物免疫机能,而渐变与温和冷应激则可增强动物免疫机能,可能与冷应激程度、持续时间及动物种属不同有关[10-11]。本研究发现,绵羊急性冷应激12 h后,血清IL-4浓度显著下降,说明急性冷应激能够抑制绵羊的体液免疫功能,没有出现升高的趋势可能与冷应激的强度和持续时间有关。IgG是血清中主要的抗体成分,在机体免疫防御中起主要作用[34]。本研究发现,急性冷应激12 h后,绵羊血清IgG水平显著下降,说明急性冷应激能够抑制绵羊的免疫功能。
3.2 急性冷应激对热休克蛋白70家族基因表达的影响HSP70具有保护变性蛋白折叠、展开和复性的伴侣活性[35],赋予细胞或生物体从应激状态中恢复的能力,并保护它们免受应激的损害,是对环境和代谢应激综合反映的代表[36]。有研究发现,热休克蛋白70主要包括诱导型热休克蛋白70(主要包括HSPA1A、HSPA1B和HSPA6等3类)及结构型热休克蛋白70(主要包括HSPA1L、HSPA2和HSPA8等3类)等[15, 37]。热休克转录因子1(HSF1)是热休克蛋白表达的主要转录因子[38],在非应激条件下与热休克蛋白以无活性状态结合,被应激源刺激后激活并与热休克蛋白分离,然后被蛋白激酶磷酸化并转移到细胞核内发生三聚反应;这些HSF1三聚体复合物与HSP基因启动子区域中的热休克元件(HSE)结合,转录HSP mRNA并离开细胞核翻译成新的HSP[39]。本研究发现,不同组织中HSP70家族基因mRNA表达量无论升高还是降低,与HSF1 mRNA表达量的变化趋势一致,说明HSF1基因能直接调控HSP70基因的表达[40]。
HSP70不仅作为分子伴侣发挥作用,而且在免疫调节中起重要作用[41]。HSP70能够以胞外蛋白形式存在,并通过MAPK和NF-kB等胞内信号传导途径发挥免疫调节功能[42]。有研究表明,胞内HSP70与核因子抑制蛋白α结合,最终抑制NF-kB信号通路的激活,从而抑制TNF-α、IL-1β等促炎症因子的转录[43],提高细胞对炎症因子的耐受力;而外源性HSP70能刺激单核/巨噬细胞、血管内皮细胞、树突状细胞(DC)等合成和释放TNF-α、IL-1β、和IL-6等促炎症因子[44-45],促进DC成熟和T淋巴细胞增生,激发T细胞介导的适应性免疫反应[46-47]。本研究发现,急性冷应激12 h后,绵羊肝、心和背最长肌等组织中HSP70家族基因(HSPA1A、HSPA6和HSPA8) mRNA表达量升高,与血清中TNF-α、IL-1β、和IL-6等细胞因子的升高趋势相同,推测HSP70家族基因可能介导了急性冷应激导致绵羊炎症反应的免疫调控过程,具体机制有待进一步研究。
在寒冷条件下,动物需要通过骨骼肌和内脏(肝、心、肾等)产热来维持体温平衡[48]。张冬杰等[49]研究发现,母猪肌肉组织中背最长肌HSP70 mRNA在冷应激后会显著升高,认为背最长肌在骨骼肌颤栗性产热中占优势。杨焕民[50]也发现,肉牛背最长肌HSP70 mRNA表达量在冷应激后显著升高。肝是机体重要的能量代谢器官,肝和肌肉都与动物体温调节有关,在动物机体抵抗寒冷应激中起重要调节作用[51]。心主要功能是为肌肉、肝和大脑等组织器官提供充足的血流量,以供应氧和大量营养物质并带走代谢的终产物,使细胞正常的代谢和功能得以维持,寒冷应激中动物体温调节也需要依赖心泵血功能[52]。谢永新等[53]研究发现,肉鸡肝和心等组织HSP70 mRNA表达量在冷应激6 h后显著升高。Nagayach等[19]研究发现,山羊肝、心等组织HSP70 mRNA表达量在冷应激后会显著升高。动物冷应激时机体各个组织器官需要互相协调对抗寒冷应激[54]。本研究也发现,绵羊急性冷应激12 h后,HSPA1A mRNA表达量在肝、心、背最长肌和肾中显著升高,HSPA6 mRNA表达量在心和背最长肌中显著升高,HSPA8 mRNA表达量在背最长肌中显著升高,而在其他组织中无显著变化,这可能与热休克蛋白在各组织中的表达水平不同有关[55-56]。HSP70家族基因(HSPA1A、HSPA6和HSPA8)高水平表达有助于机体抵抗冷应激带来的不利影响[14, 31],本试验发现,多个组织中HSPA1A的表达量对温度变化更为敏感。脾是机体最大的免疫器官,是机体细胞免疫和体液免疫的中心[52]。急性冷应激会降低动物脾、胸腺等免疫器官中免疫细胞因子的表达量,进而导致机体免疫力下降[33]。赵福庆[57]研究发现,急性冷应激下HSP70 mRNA在雏鸡各器官组织中表达量整体呈上升趋势,但在脾中表达量减少,可能是急性冷应激抑制了雏鸡免疫器官的功能。于宪一等[58]发现,急性冷应激能使雏鸡肠道组织HSP70 mRNA表达量普遍降低。本研究也发现,急性冷应激12 h后,绵羊HSPA8 mRNA表达量在脾和十二指肠中显著下降,可能是急性冷应激对绵羊的脾和十二指肠造成了一定的损伤。此外,HSPA1A和HSPA6 mRNA在脾和十二指肠中表达量差异不明显,推测HSPA8可能对脾和十二指肠损伤更为敏感,具体机制有待进一步研究。
4 结论在本试验条件下,急性冷应激能抑制绵羊的免疫功能;HSP70家族基因(HSPA1A、HSPA6和HSPA8)在各组织中的表达水平不同,其中HSPA1A对温度更敏感,宜作为绵羊冷应激的生物标记物;肝、心和背最长肌等组织中HSP70家族基因表达量升高可能与其保护组织细胞维持正常产热有关。
| [1] |
赵有璋.
羊生产学[M]. 3版. 北京: 中国农业出版社, 2011.
ZHAO Y Z. Sheep production[M]. 3rd ed. Beijing: China Agriculture Press, 2011. (in Chinese) |
| [2] |
杨莉, 王凤丽, 刘海燕, 等. 寒冷应激对阿勒泰羊硬脂酰辅酶A去饱和酶表达的影响以及序列分析[J]. 中国兽医学报, 2015, 35(6): 989–994.
YANG L, WANG F L, LIU H Y, et al. Effects of cold stress on the expression of stearoyl-coenzyme A desaturase of Altay sheep and its sequence analysis[J]. Chinese Journal of Veterinary Science, 2015, 35(6): 989–994. (in Chinese) |
| [3] |
刘璐.冷应激对饥饿断奶羔羊生理生化指标及内分泌的影响[D].乌鲁木齐: 新疆农业大学, 2014.
LIU L.Effect of cold stress on physiological, biochemical and endocrinological values in fasting weaned lambs[D]. Urumqi: Xinjiang Agricultural University, 2014.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10758-1015548229.htm |
| [4] | PHILLIPS C. The welfare risks and impacts of heat stress on sheep shipped from Australia to the Middle East[J]. Vet J, 2016, 218: 78–85. DOI: 10.1016/j.tvjl.2016.09.011 |
| [5] | KAUFMAN J D, SAXTON A M, RÍUS A G, et al. Short communication:relationships among temperature-humidity index with rectal, udder surface, and vaginal temperatures in lactating dairy cows experiencing heat stress[J]. J Dairy Sci, 2018, 101(7): 6424–6429. DOI: 10.3168/jds.2017-13799 |
| [6] | BERMAN A, HOROVITZ T, KAIM M, et al. A comparison of THI indices leads to a sensible heat-based heat stress index for shaded cattle that aligns temperature and humidity stress[J]. Int J Biometeorol, 2016, 60(10): 1453–1462. DOI: 10.1007/s00484-016-1136-9 |
| [7] |
高玉红, 李宏双, 郭建军, 等. 坝上寒区不同建筑类型奶牛舍冬季温热环境评价[J]. 东北农业大学学报, 2015, 46(12): 52–57, 93.
GAO Y H, LI H S, GUO J J, et al. Assessment of thermal environment of different styles of dairy houses in winter in chill region of Bashang[J]. Journal of Northeast Agricultural University, 2015, 46(12): 52–57, 93. DOI: 10.3969/j.issn.1005-9369.2015.12.008 (in Chinese) |
| [8] |
单春花, 陈伟, 李宏双, 等. 舍饲散栏有窗奶牛舍温热环境的年动态研究[J]. 中国畜牧兽医, 2017, 44(10): 3106–3112.
SHAN C H, CHEN W, LI H S, et al. Annual dynamic of thermal environment of dairy cowshed in free stall-feeding with window[J]. China Animal Husbandry & Veterinary Medicine, 2017, 44(10): 3106–3112. (in Chinese) |
| [9] | HAQUE N, SINGH M, HOSSAIN S A. Improved milk production through PG-PL system by provision of in-house shelter management in lactating Murrah buffaloes during winter season[J]. J Anim Physiol Anim Nutr (Berl), 2018, 102(1): 166–174. DOI: 10.1111/jpn.12674 |
| [10] |
刘晓丹.肉鸡冷应激相关基因的mRNA表达谱分析及差异表达基因的研究[D].哈尔滨: 东北农业大学, 2012.
LIU X D.Analysis of mRNA expression profile of cold stress gene in broilers and study of differential expression genes[D].Harbin: Northeast Agricultural University, 2012.(in Chinese) http://d.wanfangdata.com.cn/Thesis/Y2235128 |
| [11] | SU Y Y, WEI H D, BI Y J, et al. Pre-cold acclimation improves the immune function of trachea and resistance to cold stress in broilers[J]. J Cell Physiol, 2019, 234(5): 7198–7212. DOI: 10.1002/jcp.27473 |
| [12] | ZHAO F Q, ZHANG Z W, QU J P, et al. Cold stress induces antioxidants and Hsps in chicken immune organs[J]. Cell Stress Chaperones, 2014, 19(5): 635–648. DOI: 10.1007/s12192-013-0489-9 |
| [13] | ZHANG Z B, CHEN B J, YUAN L, et al. Acute cold stress improved the transcription of pro-inflammatory cytokines of Chinese soft-shelled turtle against Aeromonas hydrophila[J]. Dev Comp Immunol, 2015, 49(1): 127–137. |
| [14] | BANERJEE D, UPADHYAY R C, CHAUDHARY U B, et al. Seasonal variation in expression pattern of genes under HSP70[J]. Cell Stress Chaperones, 2014, 19(3): 401–408. DOI: 10.1007/s12192-013-0469-0 |
| [15] | DEANE C A S, BROWN I R. Knockdown of heat shock proteins HSPA6(Hsp70B') and HSPA1A (Hsp70-1) sensitizes differentiated human neuronal cells to cellular stress[J]. Neurochem Res, 2018, 43(2): 340–350. DOI: 10.1007/s11064-017-2429-z |
| [16] | DANGI S S, DANGI S K, CHOUHAN V S, et al. Modulatory effect of betaine on expression dynamics of HSPs during heat stress acclimation in goat (Capra hircus)[J]. Gene, 2016, 575(2): 543–550. DOI: 10.1016/j.gene.2015.09.031 |
| [17] | DANGI S S, GUPTA M, DANGI S K, et al. Expression of HSPs:an adaptive mechanism during long-term heat stress in goats (Capra hircus)[J]. Int J Biometeorol, 2015, 59(8): 1095–1106. DOI: 10.1007/s00484-014-0922-5 |
| [18] | DANGI S S, GUPTA M, NAGAR V, et al. Impact of short-term heat stress on physiological responses and expression profile of HSPs in Barbari goats[J]. Int J Biometeorol, 2014, 58(10): 2085–2093. DOI: 10.1007/s00484-014-0809-5 |
| [19] | NAGAYACH R, GUPTA U D, PRAKASH A, et al. Expression profiling of hsp70 gene during different seasons in goats (Capra hircus) under sub-tropical humid climatic conditions[J]. Small Rumin Res, 2017, 147: 41–47. DOI: 10.1016/j.smallrumres.2016.11.016 |
| [20] | TUCKER C B, ROGERS A R, VERKERK G A, et al. Effects of shelter and body condition on the behaviour and physiology of dairy cattle in winter[J]. Appl Anim Behav Sci, 2007, 105(1-3): 1–13. DOI: 10.1016/j.applanim.2006.06.009 |
| [21] | SHITZER A, DE DEAR R. Inconsistencies in the "New" windchill chart at low wind speeds[J]. J Appl Meteor Climatol, 2006, 45(5): 787–790. DOI: 10.1175/JAM2373.1 |
| [22] | MEKIS É, VINCENT L A, SHEPHARD M W, et al. Observed trends in severe weather conditions based on humidex, wind chill, and heavy rainfall events in Canada for 1953-2012[J]. Atmos-Ocean, 2015, 53(4): 383–397. DOI: 10.1080/07055900.2015.1086970 |
| [23] | COSTA W P, FACANHA D A E, LEITE J H G M, et al. Thermoregulatory responses and blood parameters of locally adapted ewes under natural weather conditions of Brazilian semiarid region[J]. Sem:Ciênc Agrár, 2015, 36(6Suppl 2): 4589–4600. |
| [24] |
魏凤仙, 胡骁飞, 张敏红, 等. 相对湿度和氨气应激对肉仔鸡血氨水平及细胞因子含量的影响[J]. 动物营养学报, 2013, 25(10): 2246–2253.
WEI F X, HU X F, ZHANG M H, et al. Effects of relative humidity and ammonia stress on plasma ammonia level and cytokine contents of broilers[J]. Chinese Journal of Animal Nutrition, 2013, 25(10): 2246–2253. DOI: 10.3969/j.issn.1006-267x.2013.10.008 (in Chinese) |
| [25] | YANG Z J, LIU C, ZHENG W J, et al. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens[J]. Biol Trace Elem Res, 2016, 169(2): 341–351. DOI: 10.1007/s12011-015-0407-3 |
| [26] | SHINI S, HUFF G R, SHINI A, et al. Understanding stress-induced immunosuppression:exploration of cytokine and chemokine gene profiles in chicken peripheral leukocytes[J]. Poult Sci, 2010, 89(4): 841–851. DOI: 10.3382/ps.2009-00483 |
| [27] |
郭爽, 郭景茹, 汪志, 等. 不同时间强度冷刺激对大鼠血清中炎症相关细胞因子的影响[J]. 中国兽医学报, 2013, 33(3): 352–357, 361.
GUO S, GUO J R, WANG Z, et al. Effects of differentduration of cold stress on production of cytokines in serum of SPF wistar rats[J]. Chinese Journal of Veterinary Science, 2013, 33(3): 352–357, 361. (in Chinese) |
| [28] | CHEN J, ZHOU Y X, JIN X D, et al. Expression of interleukin-2 in Candidal balanoposthitis and its clinical significance[J]. Chin Med J (Engl), 2011, 124(17): 2776–2778. |
| [29] | ZHAO F Q, ZHANG Z W, YAO H D, et al. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers[J]. Res Vet Sci, 2013, 95(1): 146–155. DOI: 10.1016/j.rvsc.2013.01.021 |
| [30] |
计红, 杨焕民, 李士泽, 等. 冷应激对仔猪血浆相关细胞因子和IgG水平的影响[J]. 应用与环境生物学报, 2010, 16(5): 632–636.
JI H, YANG H M, LI S Z, et al. Changes in correlated cytokines and IgG of blood plasma of piglets under cold stress[J]. Chinese Journal of Applied & Environmental Biology, 2010, 16(5): 632–636. (in Chinese) |
| [31] |
杨莉, 张莉, 齐亚银, 等. 冷应激对湖羊血清因子及热休克蛋白70 mRNA表达的影响[J]. 中国畜牧兽医, 2015, 42(4): 890–895.
YANG L, ZHANG L, QI Y Y, et al. Effect of cold stress on serum factor and Hsp70 mRNA in Hu sheep[J]. China Animal Husbandry & Veterinary Medicine, 2015, 42(4): 890–895. (in Chinese) |
| [32] | BOD L, DOUGUET L, AUFFRAY C, et al. IL-4-induced gene 1:a negative immune checkpoint controlling B cell differentiation and activation[J]. J Immunol, 2018, 200(3): 1027–1038. DOI: 10.4049/jimmunol.1601609 |
| [33] |
伊亢力.免疫细胞因子基因及相关miRNAs在肉鸡急性冷、热应激中的表达调控研究[D].杨凌: 西北农林科技大学, 2017.
YI K I.The expression mechanism of immune cytokine gene and associated miRNAs in acute heat/cold stressed broilers[D]. Yangling: Northwest A& F University, 2017.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10712-1017091400.htm |
| [34] | VAN DEBOVENKAMP F S, HAFKENSCHEID L, RISPENS T, et al. The emerging importance of IgG Fab glycosylation in immunity[J]. J Immunol, 2016, 196(4): 1435–1441. DOI: 10.4049/jimmunol.1502136 |
| [35] | ARCHANA P R, ALEENA J, PRAGNA P, et al. Role of heat shock proteins in livestock adaptation to heat stress[J]. J Dairy, Vet Anim Res, 2017, 5(1): 00127. |
| [36] | LIEW P K, ZULKIFLI I, HAIR-BEJO M, et al. Effects of early age feed restriction and heat conditioning on heat shock protein 70 expression, resistance to infectious bursal disease, and growth in male broiler chickens subjected to heat stress[J]. Poult Sci, 2003, 82(12): 1879–1885. DOI: 10.1093/ps/82.12.1879 |
| [37] |
刘嘉敏, 赵元莙. HSP70的研究进展及其在生物医学中的应用[J]. 教育教学论坛, 2017(50): 61–62.
LIU J M, ZHAO Y J. Research progressfor HSP70 and its application in biomedicine study[J]. Education Teaching Forum, 2017(50): 61–62. DOI: 10.3969/j.issn.1674-9324.2017.50.028 (in Chinese) |
| [38] | BHARATI J, DANGI S S, CHOUHAN V S, et al. Expression dynamics of HSP70 during chronic heat stress in Tharparkar cattle[J]. Int J Biometeorol, 2017, 61(6): 1017–1027. DOI: 10.1007/s00484-016-1281-1 |
| [39] | GUPTA M, DANGI S S, MAURYA D, et al. Expression profile of cold shock protein genes in goats (Capra hircus) during different seasons[J]. Iran J Vet Res, 2014, 15(1): 7–12. |
| [40] | KRAKOWIAK J, ZHENG X, PATEL N, et al. Hsf1 and Hsp70 constitute a two-component feedback loop that regulates the yeast heat shock response[J]. eLife, 2018, 7: e31668. DOI: 10.7554/eLife.31668 |
| [41] |
高雅君.炎症条件下草鱼HSP70的表达及HSF1对其调控的初步研究[D].成都: 电子科技大学, 2018.
GAO Y J.Preliminary study on the expression of HSP70 and its regulation by HSF1 under inflammatory conditions in grass carp[D].Chengdu: University of Electronic Science and Technology of China, 2018.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10614-1018708257.htm |
| [42] | ZHANG A Y, GUO Y F, ZHANG S N, et al. Cytokine effects and cellular signaling pathways of grass carp HSP70 in head kidney leukocytes[J]. Fish Shellfish Immunol, 2015, 46(2): 550–556. DOI: 10.1016/j.fsi.2015.07.016 |
| [43] | CAO X, YUE L, SONG J Y, et al. Inducible HSP70 antagonizes IL-1β cytocidal effects through inhibiting NF-kB activation via destabilizing TAK1 in HeLa cells[J]. PLoS One, 2012, 7(11): e50059. DOI: 10.1371/journal.pone.0050059 |
| [44] | HULINA A, RAJKOVIC M G, DESPOT D J, et al. Extracellular Hsp70 induces inflammation and modulates LPS/LTA-stimulated inflammatory response in THP-1 cells[J]. Cell Stress Chaperones, 2018, 23(3): 373–384. DOI: 10.1007/s12192-017-0847-0 |
| [45] | HECK T G, SCOMAZZON S P, NUNES P R, et al. Acute exercise boosts cell proliferation and the heat shock response in lymphocytes:correlation with cytokine production and extracellular-to-intracellular HSP70 ratio[J]. Cell Stress Chaperones, 2017, 22(2): 271–291. DOI: 10.1007/s12192-017-0771-3 |
| [46] | REDZOVIC A, GULIC T, LASKARIN G, et al. Heat-shock proteins 70 induce pro-inflammatory maturation program in decidual CD1a+ dendritic cells[J]. Am J Reprod Immunol, 2015, 74(1): 38–53. DOI: 10.1111/aji.12374 |
| [47] | VAN EDEN W, BROERE F, VAN DER ZEE R.HSP70 is a major contributor to the MHCⅡ ligandome and inducer of regulatory T cells[M]//ASEA A A A, KAYR P.HSP70 in Human Diseases and Disorders.Cham: Springer, 2018: 163-171. |
| [48] |
金天明.
动物生理学[M]. 北京: 清华大学出版社, 2012.
JIN T M. Animal physiology[M]. Beijing: Tsinghua University Press, 2012. (in Chinese) |
| [49] |
张冬杰, 李忠秋, 汪亮, 等. 低温对猪肌肉和肝脏组织线粒体数量的影响[J]. 黑龙江畜牧兽医, 2018(1): 100–102, 253.
ZHANG D J, LI Z Q, WANG L, et al. The effect of low temperature on mitochondrial numbers in pig muscle and liver tissues[J]. Heilongjiang Animal Science and Veterinary Medicine, 2018(1): 100–102, 253. (in Chinese) |
| [50] |
杨焕民.应激肉牛几种组织中HSPs表达的研究[D].长春: 中国人民解放军军需大学, 2002.
YANG H M.Studies on the expression of HSPs in tissues of the stressed cattle[D].Changchun: Heping Campus Jilin University, 2002.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-90029-2003060466.htm |
| [51] | SHI H Z, YAO R Z, LIAN S, et al. Regulating glycolysis, the TLR4 signal pathway and expression of RBM3 in mouse liver in response to acute cold exposure[J]. Stress, 2019, 22(3): 366–376. DOI: 10.1080/10253890.2019.1568987 |
| [52] |
计红, 詹雪龙, 薛琳琳, 等. 急性冷刺激对大鼠骨骼肌和7个内脏器官的组织结构影响[J]. 中国兽医学报, 2019, 39(1): 105–112.
JI H, ZHAN X L, XUE L L, et al. Effects of cold stimulus on the histology structure of skeletal muscle and seven visceral organs in rats[J]. Chinese Journal of Veterinary Science, 2019, 39(1): 105–112. (in Chinese) |
| [53] |
谢永新, 史延, 黄利华, 等. 急性冷、热应激对肉鸡肝脏和心脏组织中HSPs表达的影响[J]. 中国家禽, 2018, 40(1): 7–11.
XIE Y X, SHI Y, HUANG L H, et al. Effects of acute heat/cold stress on the expression of HSPs in liver and heart of broiler[J]. China Poultry, 2018, 40(1): 7–11. (in Chinese) |
| [54] |
刘颖, 任文陟, 李苏楠, 等. 冷应激对猪脂肪、肝脏、心脏与肾脏组织中PPARγ2 mRNA及蛋白表达的影响[J]. 中国兽医学报, 2012, 32(4): 592–597.
LIU Y, REN W Z, LI S N, et al. PPARγ2 gene expression of swine adipose tissue, liver, heart and kidney in cold stress[J]. Chinese Journal of Veterinary Science, 2012, 32(4): 592–597. (in Chinese) |
| [55] | AFSAL A, BAGATH M, SEJIAN V, et al. Effect of heat stress on HSP70 gene expression pattern in different vital organs of Malabari goats[J]. Biol Rhythm Res, 2019. DOI: 10.1080/09291016.2019.1600270 |
| [56] | DANG W, XU N, ZHANG W, et al. Differential regulation of Hsp70 expression in six lizard species under normal and high environmental temperatures[J]. Pak J Zool, 2018, 50(3): 1043–1051. |
| [57] |
赵福庆.冷应激对雏鸡组织热休克蛋白和小肠免疫功能的影响[D].哈尔滨: 东北农业大学, 2013.
ZHAO F Q.Effect of cold stress on heat shock proteins and immune function of small intestine in chicken[D].Harbin: Northeast Agricultural University, 2013.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10224-1013207424.htm |
| [58] |
于宪一, 李术, 陈蕾, 等. 冷应激对雏鸡肠组织中HSP70 mRNA表达的影响[J]. 东北农业大学学报, 2011, 42(3): 90–93.
YU X Y, LI S, CHEN L, et al. Effects of cold stress on HSP70 mRNA expression in intestines of chickens[J]. Journal of Northeast Agricultural University, 2011, 42(3): 90–93. (in Chinese) |



