哺乳动物卵泡发育起源于原始生殖细胞,发育过程主要包括原始卵泡、初级卵泡、次级卵泡与成熟卵泡。与哺乳动物不同,禽类成熟卵泡拥有其独特的发育特征,当卵泡成熟突出卵巢基质,卵母细胞开始沉积卵黄[1-2],其壁层细胞也已经基本分化完毕,从内到外主要包括颗粒层、膜层(包括内膜层、外膜层)与结缔组织层。其中颗粒层与膜层是卵泡壁中的主要分泌与功能组织,它们的发育与卵泡的整体发育过程彼此高度关联[3-4],并通过营养、类固醇与生长因子的分泌等与卵母细胞协同作用,进而在调控卵泡的生长、发育、选择与排卵的过程中扮演着重要的角色[2-3, 5-6]。
早在1968年,就有研究者从组织学层面提出了定义哺乳动物卵泡发育阶段的多种标准,包括卵泡本身形态,卵母细胞形态与卵泡壁的形态等[7]。但在禽类中,最初仅仅通过外观与排卵顺序将卵泡的发育阶段分为等级前卵泡与等级卵泡(F1~F5),且这样的定义大多来源并应用于鸡(Gallus gallus)[4, 6, 8]。之后研究者们结合了鸡成熟卵泡的外观与直径尺寸进一步定义了其卵泡的发育阶段(小白卵泡(SWF)直径小于2 mm; 大白卵泡(LWF)直径为2~4 mm; 小黄卵泡(SYF)直径为4~5至9~10 mm;F1~F5等级卵泡直径为10~12至40 mm)[1, 5, 9-12],而这样的标准一直沿用至今[13-17]。然而, 对于不同体型的禽类卵泡的外观、尺寸大小与其对应的发育特点可能存在较大的差异,例如鸽子,火鸡与鸵鸟[18-20],其成熟卵泡尺寸与对应形态均与鸡明显不同。不仅如此,虽然禽类卵泡外观、大小与其功能密切相关,但卵泡由多个组分构成,卵泡本身的发育过程未必与其组分形态、功能全部一致,所以这样的分级模式对于卵泡其他重要组分的研究也未必适用,尤其是对于鸡以外禽类物种。
因此,本研究以鹅(与鸡卵泡尺寸相差较大且相对低产的家禽品种)作为研究对象,将其所有阶段成熟卵泡的重要功能结构与发育的标志性结构——卵泡壁层细胞作为探索禽类卵泡发育规律的指标,旨在通过探究鹅成熟卵泡壁层中各个结构组分的形态特点与发育规律,为禽类成熟卵泡动态发育的分级提供新的视角与依据,为深入研究禽类成熟卵泡的发育规律奠定基础。
1 材料与方法 1.1 试验动物本试验样本选自四川农业大学家禽育种实验场提供的同批孵化的天府肉鹅母系。所有试验用鹅均在相同的生长、饲养管理条件(圈养、自由采食和饮水等)下养殖,选取健康的、开产时间和体重基本一致、处于产蛋高峰期(35~45周龄左右)的母鹅5只进行卵巢组织的分离。
1.2 鹅卵泡的分离与卵泡壁切片的制作将完整取出的卵巢与卵泡转入PBS(pH为7.4,索莱宝科技有限公司)中,之后根据卵泡直径迅速将卵泡划分为:0~2、2~4、4~6、6~8、8~10 mm与F5~F1(被选择后的卵泡,直径F5<F4<F3<F2<F1)阶段,随后将对应阶段卵泡分别放入4%多聚甲醛(成都里来生物技术有限公司)中固定24 h,组织经由全自动脱水机脱水,包埋,切片。之后脱蜡至水,使用伊红和苏木精(HE)进行组织学染色、制片(成都里来生物技术有限公司)。因为不同阶段卵泡数量存在差异,在F5~F1阶段(每只鹅相应阶段的卵泡最多仅有一个)每个卵泡均随机选取3个以上不同区域进行切片制作;而在0~2、2~4、4~6、6~8与8~10 mm卵泡中,每个阶段均随机选取至少3个以上的卵泡,其中每个卵泡(除0~2 mm阶段)均随机选取3个以上不同区域进行切片制作。
1.3 鹅卵泡壁形态的观察与测量使用OLYMPUS(BX51)显微镜进行卵泡壁形态的观察,每个切片均随机选择3个以上的区域进行图像采集。使用Image-pro Plus分别在每张卵泡壁图像上随机选取3个区域,对其对应的卵泡壁、颗粒层、内膜层、外膜层与结缔层的厚度进行测量。
1.4 数据的统计分析在本试验中,直径0~2、2~4、4~6、6~8与8~10 mm卵泡每个阶段的测量数据总数均≥147,而F5~F1阶段卵泡的测量数据总数均≥48。使用SPSS 20进行数据间差异显著性统计分析,组间比较使用单因素方差分析(One-way ANOVA),用最小显著差异法(least significant difference,LSD)进行多重比较,数据以“平均值±标准差(Mean±SD)”形式表示。统计学上,P < 0.05代表差异显著,P < 0.01代表差异极显著。
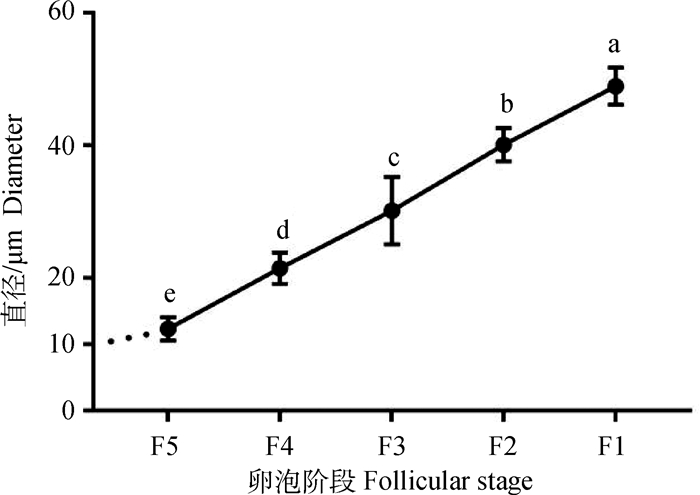
2 结果 2.1 鹅F5~F1卵泡的直径变化在卵泡被选择形成F5后,卵泡直径迅速增大,F5~F1每个阶段与相邻阶段的卵泡直径均存在显著的差异(P < 0.05)。但在卵泡刚被选择时,即被选择前(10 mm以下)到F5的发育时期,卵泡间直径的差异明显小于其他阶段(图 1)。
|
不同字母表示差异显示(P < 0.05), 图 4同 The different letters indicate significant difference(P < 0.05), the same as figure 4 图 1 F5~F1卵泡直径变化 Fig. 1 Diversification in F5-F1 follicular diameter |
|
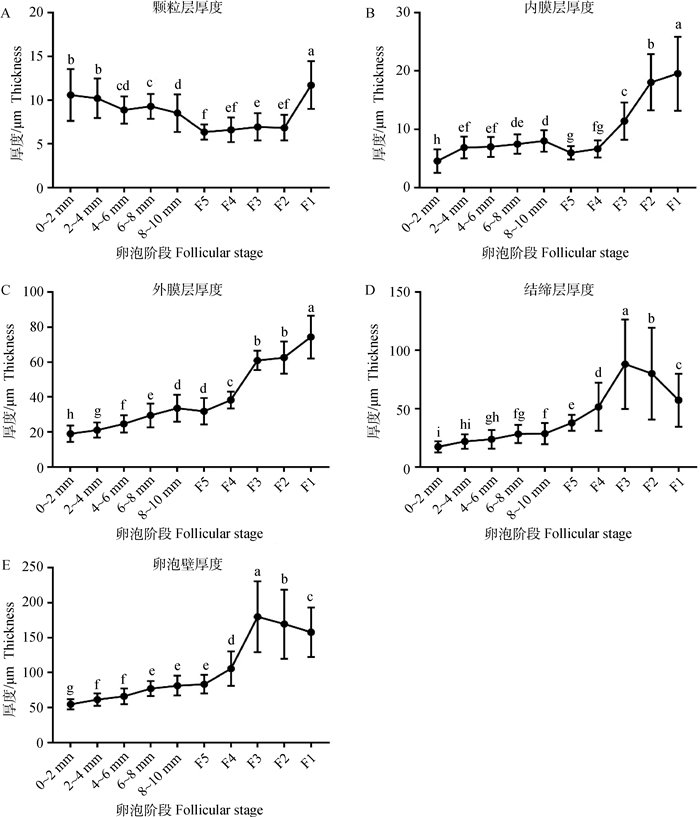
图 4 不同阶段成熟卵泡壁层及其组分的厚度变化 Fig. 4 Diversification of thickness of mature follicular wall and its components at different stages |
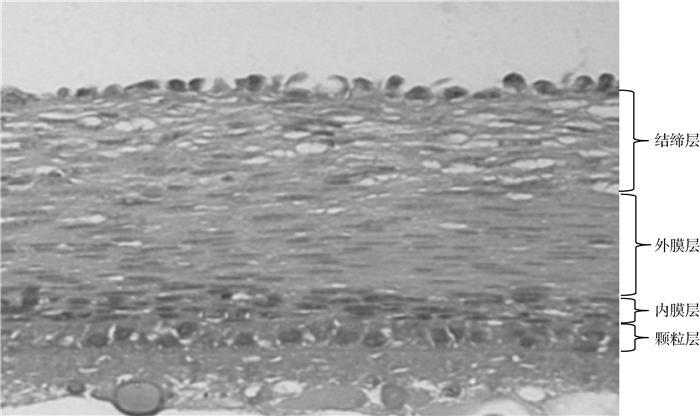
如图 2所示,鹅成熟卵泡(以直径6~8 mm卵泡为例)的卵泡壁层从内到外主要包括:颗粒层、内膜层、外膜层与结缔组织层。其中颗粒层细胞排列整齐而致密,且其细胞胞核呈立方状;而内膜、外膜与结缔层细胞核均呈梭状,其中内膜层细胞分布比较散乱,但细胞密度较高,而外膜与结缔层相对较厚,外膜层细胞分布较为稀疏,而结缔层细胞排列则十分松散。
|
图 2 6~8 mm成熟卵泡的卵泡壁层形态图(200×) Fig. 2 Morphology of mature follicular wall of 6-8 mm in diameter(200×) |
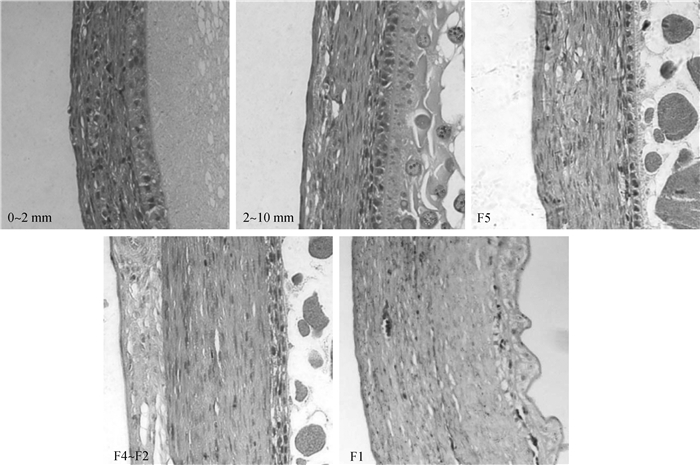
结果表明,在直径0~2 mm、2~10 mm、F5、F4~F2与F1卵泡的壁层之间存在一定的形态差异,其中在直径小于2 mm的卵泡中,卵泡壁层较薄,颗粒层细胞边缘分界较为模糊,外膜层与结缔层也相对不发达,且在部分区域的卵泡壁层中并未出现内膜层结构(图 3)。直径2~10 mm的卵泡(被选择前卵泡)与F5(刚被选择的卵泡)卵泡在形态上并未出现显著的差异(图 3),其颗粒层排列整齐而致密,内膜、外膜与结缔组织层的形态比例均比较稳定,并没有明显的发育与变化。随着被选择卵泡的进一步发育(F5之后),卵泡尺寸显著增加(P < 0.05,图 1),卵黄大量积累,其颗粒层呈现“被拉伸”状态,整体胞层厚度降低,细胞结构呈扁平立方状;其膜层形态相较于等级前变化不大,但其厚度显著增加(P < 0.05);而其结缔层迅速发育,厚度显著增加(P < 0.05,图 4)。到F1(排卵前)阶段时,细胞壁层形态发生了一些变化,其中内膜、外膜与结缔层厚度、细胞数与细胞层数均增多,颗粒层也恢复立方状,且形态较为松散(图 3)。
|
图 3 鹅不同阶段成熟卵泡壁层形态差异对比图(0~2 mm、2~10 mm、F5与F4~F2为200×;F1为100×) Fig. 3 Morphology of mature follicular wall at different stages(0-2 mm, 2-10 mm, F5 and F4-F2:200×; F1: 100×) |
在成熟卵泡发育的过程中,卵泡颗粒层厚度(图 4A)整体变化幅度较小,但是在选择前后(等级前与F5)与排卵前(F1)阶段体现出了显著的差异(P<0.05)。内膜层厚度(图 4B)在等级前较为稳定,在被选择进入等级卵泡后才发生了显著的变化(P<0.05),而外膜层(图 4C)在除8~10 mm至F5,F3~F2阶段以外,均有较为显著的变化(P<0.05)。结缔层(图 4D)在卵泡壁厚度中占的比例相对较大,且选择完成后,其厚度经历了显著的变化(P<0.05)。卵泡壁层整体厚度(图 4E)在2~4 mm、6~8 mm、F4~F1阶段时发生了显著的变化(P<0.05),其中F4~F1阶段的变化趋势与结缔层厚度变化趋势较为一致。
3 讨论卵泡壁是卵泡的重要组分,在卵泡发育的过程中扮演着重要的角色,甚至被视为卵泡发育的标志与象征[21-23]。禽类卵泡的发育遵循着独特的等级制度,即卵巢中所有卵泡从小到大按排卵次序排列,因此产蛋禽类卵巢中基本含有所有阶段的卵泡[3, 24],所以对卵巢中不同阶段卵泡的分析,也从侧面反映了卵泡的动态发育信息。
从卵泡壁厚度变化趋势上来看(图 4),膜层、结缔层与卵泡壁本身组织厚度的整体变化趋势更为相似,暗示相较于颗粒层,膜层、结缔层与卵泡形态的动态变化可能有着更为密切的联系。卵泡壁层形态图表明(图 2),成熟卵泡壁层由内到外主要包括:颗粒层、内膜层、外膜层与结缔组织层。其中颗粒层细胞排列整齐而致密;而内膜层细胞分布比较散乱,但细胞密度较高;外膜与结缔层相对较厚,外膜层细胞分布较为稀疏,结缔层细胞排列十分松散。结合文献报道[1, 25]发现,卵泡壁细胞层中细胞的分布越靠外越松散、稀疏,而其血管网则越靠近卵泡壁外周则越密集。不仅如此,随着卵泡的继续发育,尤其是在被选择后的F4~F2阶段,其卵泡外膜层与结缔层的厚度(图 4 C、D)显著增加(P<0.05),这与文献报道卵泡壁血管网密度的增加趋势基本一致[1, 26]。因此,笔者推测卵泡壁中细胞的连接、分布、排列很可能与卵泡血管网的形成、营养的代谢与运输有着密切的关联。
不同阶段卵泡壁形态差异图(图 3)显示,在部分直径2 mm以下的卵泡中还无法清晰的识别、观察到内膜层的存在,也就是说在卵泡直径达到2 mm以上时,它可能才拥有完整成熟卵泡的卵泡壁层结构。结合之前的报道[24, 27-28]推测,鹅卵泡在募集过程中,卵泡壁层形成的顺序很可能依次为:颗粒层、结缔层、外膜层到内膜层。另外,本研究发现,直径2~10 mm的卵泡(卵泡成熟后,被选择前)与刚被选择的F5卵泡在卵泡尺寸(图 1)、卵泡壁形态上(图 3)并未表现出明显的差异,暗示卵泡被选择后,其卵泡壁的形态变化可能在F5阶段尚未体现。结合在鸡卵泡上的相关研究报道[3, 26, 29-30],本研究发现F5(刚被选择的卵泡)与等级前(2~10 mm卵泡)或者后续等级卵泡(F4)的性质与功能上确实存在一些差异,这表明F5卵泡很可能处于一个独立而不稳定的过渡阶段。而在F4~F2阶段卵泡中(图 3),其颗粒层呈现“被拉伸”状态,整体厚度降低,细胞呈扁平状;而膜层与结缔层厚度(图 4 B, C, D)显著增加(P<0.05),卵泡壁层整体形态发生了较大的改变。结合其功能上的巨大差异[25, 31-33],显然,此时的卵泡相较于等级前卵泡无论是形态还是功能特性都出现了明显的变化,表明卵泡的选择作用已经完成,至此卵泡已经进入等级卵泡阶段。当卵泡发育为F1后,其卵泡壁层的许多生理功能相较于其他等级卵泡均出现了较为显著的差异[28, 30, 32, 34-35],不仅如此,在图 3中发现,F1的颗粒层从“被拉伸”状态恢复为疏松的立方状,且其卵泡壁的各个组分厚度(图 4)也均出现了显著的变化(P<0.05)。因此,推测此时卵泡应该进入了一个不同的发育阶段,而此阶段中卵泡的变化应该与即将到来的排卵行为密切相关。
4 结论从卵泡壁层组织形态和结构分析,鹅卵泡的发育历程可划分为2 mm以下、2~10 mm、F5、F4~F2与F1卵泡5个不同的阶段,这些阶段均有其独特的形态、结构特点,且这样的特点很可能与其功能的差异密切相关。
| [1] | JOHNSON A L, WOOD D C. Ovarian dynamics and follicledevelopment[M]//JAMIESON B G M.Reproductive biology and phylogeny of birds[M].Enfield, NH: Science Publishers, 2007. |
| [2] | LOVELL T M, GLADWELL R T, GROOME N P, et al. Ovarian follicle development in the laying hen is accompanied by divergent changes in inhibin A, inhibin B, activin A and follistatin production in granulosa and theca layers[J]. J Endocrinol, 2003, 177(1): 45–55. DOI: 10.1677/joe.0.1770045 |
| [3] | ONAGBESAN O, BRUGGEMAN V, DECUYPERE E. Intra-ovarian growth factors regulating ovarian function in avian species:A review[J]. Anim Reprod Sci, 2009, 111(2-4): 121–140. DOI: 10.1016/j.anireprosci.2008.09.017 |
| [4] | WILLIAMS J B, SHARP P J. Ovarian morphology and rates of ovarian follicular development in laying broiler breeders and commercial egg-producing hens[J]. Br Poult Sci, 1978, 19(3): 387–395. DOI: 10.1080/00071667808416490 |
| [5] | NITTA H, MASON J I, BAHR J M. Localization of 3β-hydroxysteroid dehydrogenase in the chicken ovarian follicle shifts from the theca layer to granulosa layer with follicular maturation[J]. Biol Reprod, 1993, 48(1): 110–116. DOI: 10.1095/biolreprod48.1.110 |
| [6] | HOCKING P M, GILBERT A B, WALKER M, et al. Ovarian follicular structure of White Leghorns fed ad libitum and dwarf and normal broiler breeders fed ad libitum or restricted until point of lay[J]. Br Poult Sci, 1987, 28(3): 493–506. DOI: 10.1080/00071668708416983 |
| [7] | PEDERSEN T P H. Proposal for a classification of oocytes and follicles in the mouse ovary[J]. J Reprod Fertil, 1968, 17(3): 555–557. DOI: 10.1530/jrf.0.0170555 |
| [8] | BIFANO C, LINCK R G. The effect of nonbridging ligands on the rate of electron-transfer reactions[J]. J Am Chem Soc, 1967, 89(15): 3945–3947. DOI: 10.1021/ja00991a075 |
| [9] | CHAPEAU C, ENGELHARDT H, KING G J, et al. Alkaline phosphatase activity in the theca of ovarian follicles of the hen throughout follicular development[J]. Poult Sci, 1996, 75(12): 1536–1545. DOI: 10.3382/ps.0751536 |
| [10] | BAHR J M, WANG S C, HUANG M Y, et al. Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen[J]. Biol Reprod, 1983, 29(2): 326–334. DOI: 10.1095/biolreprod29.2.326 |
| [11] | NITTA H, OSAWA Y, BAHR J M. Immunolocalization of steroidogenic cells in small follicles of the chicken ovary:anatomical arrangement and location of steroidogenic cells change during follicular development[J]. Domest Anim Endocrinol, 1991, 8(4): 587–594. DOI: 10.1016/0739-7240(91)90028-I |
| [12] | ZADWORNY D, SHIMADA K, ISHIDA H, et al. Gonadotropin-stimulated estradiol production in small ovarian follicles of the hen is suppressed by physiological concentrations of prolactin in vitro[J]. Gen Comp Endocrinol, 1989, 74(3): 468–473. DOI: 10.1016/S0016-6480(89)80044-9 |
| [13] | LI P F, YU X J, XIE J S, et al. Expression of cocaine- and amphetamine-regulated transcript (CART) in hen ovary[J]. Biol Res, 2017, 50: 18. DOI: 10.1186/s40659-017-0123-x |
| [14] | LONG L, WU S G, YUAN F, et al. Effects of dietary octacosanol supplementation on laying performance, egg quality, serum hormone levels, and expression of genes related to the reproductive axis in laying hens[J]. Poult Sci, 2017, 96(4): 894–903. |
| [15] | HU S Q, DUGGAVATHI R, ZADWORNY D, et al. Regulatory mechanisms underlying the expression of prolactin receptor in chicken granulosa cells[J]. PLoS One, 2017, 12(1): e0170409. DOI: 10.1371/journal.pone.0170409 |
| [16] | XIA W G, FOUAD A M, CHEN W, et al. Estimation of dietary arginine requirements for Longyan laying ducks[J]. Poult Sci, 2017, 96(1): 144–150. |
| [17] | ZHANG Z C, LAI S, WANG Y G, et al. Rhythmic expression of circadian clock genes in the preovulatory ovarian follicles of the laying hen[J]. PLoS One, 2017, 12(6): e0179019. DOI: 10.1371/journal.pone.0179019 |
| [18] | PORTER T E, HARGIS B M, SILSBY J L, et al. Enhanced progesterone and testosterone secretion and depressed estradiol secretion in vitro from small white follicle cells of incubating turkey hens[J]. Gen Comp Endocrinol, 1989, 74(3): 400–405. DOI: 10.1016/S0016-6480(89)80037-1 |
| [19] | BRONNEBERG R G G, TAVERNE M A M. Ultrasonography of the female reproductive organs in farmed ostriches (Struthio camelus spp.)[J]. Theriogenology, 2003, 60(4): 617–633. DOI: 10.1016/S0093-691X(03)00083-9 |
| [20] | GOERLICH V C, DIJKSTRA C, GROOTHUIS T G G. No evidence for selective follicle abortion underlying primary sex ratio adjustment in pigeons[J]. Behav Ecol Sociobiol, 2010, 64(4): 599–606. DOI: 10.1007/s00265-009-0877-4 |
| [21] | ALLAHBADIA G N, MORIMOTO Y. Ovarian stimulation protocols[M]. New Delhi: Springer, 2016. |
| [22] | ZHANG L N, FENG T, SPICER L. The role of tight junction proteins in ovarian follicular development and ovarian cancer[J]. Reproduction, 2018, 155(4): R183–R198. DOI: 10.1530/REP-17-0503 |
| [23] |
李琴. microRNA对哺乳动物卵泡发育的影响[J]. 畜牧兽医学报, 2018, 49(12): 2558–2566.
LI Q. Roles of microRNA on mammalian follicle development[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(12): 2558–2566. DOI: 10.11843/j.issn.0366-6964.2018.12.004 (in Chinese) |
| [24] |
俞萍.鸡早期卵巢发育和原始卵泡形成激素调节的研究[D].杭州: 浙江大学, 2014.
YU P.Hormonal regulation of early ovarian development and formation of primordial follicles in the chicken[D].Hangzhou: Zhejiang University, 2014.(in Chinese) http://d.wanfangdata.com.cn/Thesis/Y2559381 |
| [25] | PERRY M M, GILBERT A B. Yolk transport in the ovarian follicle of the hen (Gallus domesticus):lipoprotein-like particles at the periphery of the oocyte in the rapid growth phase[J]. J Cell Sci, 1979, 39: 257–272. |
| [26] | KIM D, LEE J, JOHNSON A L. Vascular endothelial growth factor and angiopoietins during hen ovarian follicle development[J]. Gen Comp Endocrinol, 2016, 232: 25–31. DOI: 10.1016/j.ygcen.2015.11.017 |
| [27] | LI H, CHIAN R C.Follicular development and oocyte growth[M]//CHIAN R C, NARGUND G, HUANG J Y J.Development of in vitro Maturation for Human Oocytes: Natural and Mild Approaches to Clinical Infertility Treatment.Cham: Springer, 2017. |
| [28] | JOHNSON A L. The avian ovary and follicle development:some comparative and practical insights[J]. Turk J Vet Anim Sci, 2014, 38: 660–669. DOI: 10.3906/vet-1405-6 |
| [29] | WOODS D C, JOHNSON A L. Regulation of follicle-stimulating hormone-receptor messenger RNA in hen granulosa cells relative to follicle selection[J]. Biol Reprod, 2005, 72(3): 643–650. DOI: 10.1095/biolreprod.104.033902 |
| [30] | KIM D, JOHNSON A L. Differentiation of the granulosa layer from hen prehierarchal follicles associated with follicle-stimulating hormone receptor signaling[J]. Mol Reprod Dev, 2018, 85(8-9): 729–737. DOI: 10.1002/mrd.23042 |
| [31] | DENG Y, GAN X, CHEN D, et al. Comparison of growth characteristics of in vitro cultured granulosa cells from geese follicles at different developmental stages[J]. Biosci Rep, 2018, 38(2): BSR20171361. DOI: 10.1042/BSR20171361 |
| [32] | WOODS D C, SCHOREY J S, JOHNSON A L. Toll-like receptor signaling in hen ovarian granulosa cells is dependent on stage of follicle maturation[J]. Reproduction, 2009, 137(6): 987–996. DOI: 10.1530/REP-08-0320 |
| [33] | KIM D, JOHNSON A L. Vasoactive intestinal peptide promotes differentiation and clock gene expression in granulosa cells from prehierarchal follicles[J]. Mol Reprod Dev, 2016, 83(5): 455–463. DOI: 10.1002/mrd.22641 |
| [34] | ZHU G Y, CHEN X, MAO Y, et al. Characterization of annexin A2 in chicken follicle development:Evidence for its involvement in angiogenesis[J]. Anim Reprod Sci, 2015, 161: 104–111. DOI: 10.1016/j.anireprosci.2015.08.011 |
| [35] | SEGAWA T, TERAMOTO S, OMI K, et al. Changes in estrone and estradiol levels during follicle development:a retrospective large-scale study[J]. Reprod Biol Endocrinol, 2015, 13: 54. DOI: 10.1186/s12958-015-0051-y |



