2. 贵州省种畜禽种质测定中心, 贵阳 550018;
3. 贵州省农科院畜牧兽医研究所, 贵阳 550000;
4. 黔东南民族职业技术学院, 凯里 556000;
5. 泰国苏兰拉里理工大学, 呵叻 30000
2. Guizhou Testing Center for Livestock and Poultry Germplasm, Guiyang 550018, China;
3. Institute of Animal Husbandry and Veterinary Medicine, Guizhou Academy of Agricultural Sciences, Guiyang 550000, China;
4. Qiandongnan Vocational and Technical College for Nationalities, Kaili 556000, China;
5. Suranaree University of Technology, Nakhon 30000, Thailand
动物体内时刻会产生各种自由基(free radicals, FR)与其他活性氧物质(reactive oxygen species, ROS);同时,机体可通过广泛的天然抗氧化酶,如超氧化物歧化酶(superoxide dismutase, SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase, GPX)和过氧化氢酶(catalase, CAT)清除体内过多FR,改善动物健康[1]。然而,当这种平衡被破坏时,易导致机体代谢紊乱,动物进入氧化应激(oxidative stress, OS)状态[2]。反刍动物汗腺不发达,且为了维持和生产密集代谢的需求,更易产生OS[3]。Sharma等[4]发现,在日粮中补饲一定水平的抗氧化微量营养素可在一定程度上缓解高产奶牛OS状态,提高生产性能。因此,在饲粮中添加抗氧化剂是减缓机体OS的重要途径之一。
目前,新型天然抗氧化剂因较高的安全性而深受消费者的欢迎[5]。花青素(anthocyanin, AC)属植物中的一类黄酮化合物,是黑醋栗、野蓝莓、紫葡萄、紫甘薯、紫玉米花瓣或果实的主要水溶性天然色素之一[6]。已有文献报道,AC有极强清除FR的能力和增强抗氧化的作用[7]。Han等[8]试验表明,饲喂富含AC的紫薯片提取物可显著提高小鼠肝SOD与GPX mRNA基因的表达量。Wu等[9]在断奶仔猪中也有类似报道,喂食冻干黑树莓提取物可提高机体的总抗氧化能力(total antioxidant capacity, T-AOC)。
据笔者查阅文献,AC目前主要局限于单胃动物代谢途径[10]、抗氧化性[11]及人保健品[12]、抗癌[13]等方面的研究,迄今未见AC提升反刍动物抗氧化机制的报道。此外,反刍动物独特的瘤胃发酵环境,势必会进一步影响AC在体内的吸收代谢效率。据此,本文旨在阐述AC在反刍动物中可能的代谢途径及增强抗氧化作用的机理,探讨其作为新型抗氧化剂的可行性。
1 花青素的结构与分类AC是植物体内重要的二级代谢产物,广泛存在于自然界中,因具有广谱的生物活性(抗炎、抗肿瘤、抗菌和抗氧化应激)逐渐受到人们的重视[14-15]。花色苷(anthocyanidin),或称糖苷配基(aglycon),由一个芳香环[A](aromatic ring)和一个含氧杂环[C](heterocyclic ring that containing oxygen)通过碳-碳键与第3个芳香环[B]结合,构成2-苯基-苯并毗喃阳离子[C6(A环)-C3(C环)-C6(B环)]基本结构[16]。AC由花色苷与葡萄糖或其他糖类物质通过糖苷键形成[17](图 1)。
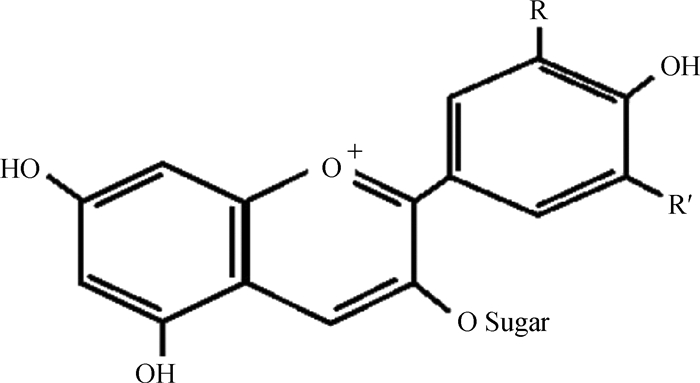
|
图 1 花青素的结构[18] Fig. 1 The structure of anthocyanin |
目前,自然界发现了23种花色苷和500多种AC[19],但植物中主要存在天竺葵素(pelargonidin, Pel)、芍药素(peonidin, Peo)、矢车菊色素(cyanidin, Cya)、锦葵素(malvidin, Mal)、牵牛花素(petunidin, Pet)和飞燕草素(delphinidin, Del)[20]。Aoki等[21]发现,秘鲁紫玉米主要含有矢车菊素-3-β-D-葡萄糖苷(cyanidin-3-O-β-D-glucoside)、天竺葵素-3-O-β-D-葡萄糖苷(pelargonidin-3-O-β-D-glucoside)、芍药素-3-O-β-D-葡萄糖苷(peonidin-3-O-β-D-glucoside)、矢车菊素-3-β-D-6-丙二酰基葡萄糖苷(cyanidin-3-O-β-D-(6-malonyl-glucoside))、天竺葵素-3-O-β-D-6-丙二酰葡萄糖苷(pelargonidin-3-O-β-D-(6-malonyl-glucoside))和芍药素-3-O-β-D-6-丙二酰葡萄糖苷(peonidin-3-O-β-D-(6-malonyl-glucoside))。Hou等[22]在黑米中检测出了矢车菊素-3-葡萄糖苷(cyanidin-3-glucoside)、芍药素-3-葡萄糖苷(peonidin-3-glucoside)、矢车菊素-3, 5-二葡萄糖苷(cyanidin-3, 5-diglucoside)和矢车菊素-3-芸香糖苷(cyanidin-3-rutinoside)。上述研究表明,虽然植物中常见的花色苷类型较少,但AC的种类和浓度差异较大。因此,饲予不同来源植物AC或提取物对反刍动物抗氧化性能提升的程度,势必会因AC结构中糖类物质部分的差异而有所不同,这也是将来探索的热点之一。
2 花青素的安全性植物天然来源的AC作为着色剂在食品和饮料中已被欧洲、日本、美国和其他国家广泛运用[23]。联合国粮食与农业组织/世界卫生组织(FAO/WHO)和食品添加剂专家委员会(JECFA)从致突变性、生殖毒性及致畸作用分析AC的安全性得出,口服富含AC的果实或提取物对人和动物未发现任何副作用[24]。究其原因可能与AC极低的消化利用效率有关,这也是今后研究亟需待解决的问题。Hosoda等[25]试验结果显示,在绵羊日粮中补饲1.52 g·d-1(25.48 mg·kg-1 BW)的紫玉米AC提取物不影响羊只的干物质采食量(dry matter intake, DMI),可改善机体血浆SOD活性。Hosoda等[26]在泌乳奶牛中也有相似报道,饲喂24.05 g·d-1(42.80 mg·kg-1 BW)富含AC的紫玉米青贮对奶产量和奶成分均无显著性影响,有降低天冬氨酸转氨酶(aspartate aminotransferase, AST)和提升SOD活性的功效。
3 花青素在反刍动物体内可能的消化、吸收及转运途径Song等[27]利用瘤胃体外发酵技术研究表明,彩色大麦的AC含量在瘤胃发酵中具有极高的稳定性。不同的是,Leatherwood[28]报道紫肉甘薯提取物中的AC易被瘤胃液降解。通过上述文献报道结果假设:瘤胃液微生物不易降解植物中的AC,大部分过瘤胃进入肠道进行吸收代谢;提取物中的部分AC(约20%)降解于瘤胃液中,影响机体瘤胃内环境。因此,小肠是AC主要消化吸收的场所[29]。综上,AC在反刍动物中可能的吸收代谢途径如下:1)植物中的AC主要过瘤胃到达小肠进行消化吸收,提取物中部分AC降解于瘤胃;小肠菌群可分解AC,脱去糖基降解生成酚酸和醛类,仅少部分被完整吸收;2)AC进一步随着食糜转移至盲肠,并在微生物的发酵下重新移到肝,未被吸收的部分随粪便排出;3)小肠中的AC吸收进入血液,移至肝及机体各器官;4)肝中的AC通过胆汁盐分泌与小肠形成循环代谢;5)AC通过肝代谢吸收进入各个机体组织(肌肉、乳腺组织等),未吸收的AC经肾随尿液排泄[30](图 2)。Ishida等[31]发现,富含AC的糖蜜残渣对山羊瘤胃微生物活性有抑制作用,降低瘤胃氨态氮浓度。此外,AC可提高泌乳奶山羊瘤胃油酸含量,增加健康脂肪酸的生物加氢[32]。童津津等[33]指出,AC可显著降低奶牛产甲烷菌丰度,抑制甲烷气体产生,对瘤胃发酵参数无明显影响。杨德莲等[34]认为,AC可提高瘤胃液的酸碱度,降低总产气量与甲烷气体总量;亦有抑制原虫、甲烷菌、溶纤维丁酸弧菌和产琥珀酸丝状杆菌含量的趋势。
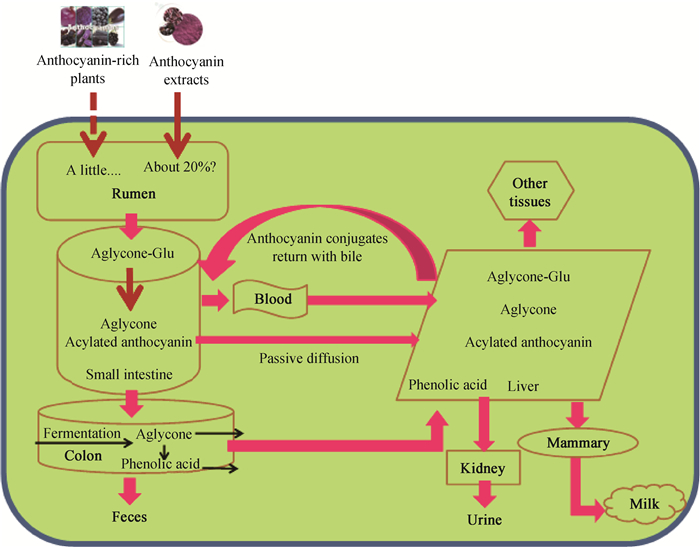
|
Anthocyanin-rich plants.富含花青素的植物性饲料;Anthocyanin extracts.花青素提取物;Aglycone-Glu.糖苷配基(花青素);Aglycone.糖苷元(花色苷);Acylated anthocyanin.酰化花青素;Phenolic acid.酚酸类物质;Passive diffusion.被动扩散;Rumen.瘤胃;Small intestine.小肠;Colon.盲肠;Feces.粪便;Blood.血液;Other tissues.其他组织;Liver.肝;Kidney.肾;Urine.尿液;Mammary.乳腺组织;Milk.乳汁 图 2 花青素在反刍动物体内可能的吸收、代谢和排泄途径 Fig. 2 The possible pathways of anthocyanins absorption, metabolism and excretion in ruminants |
AC具有较高潜在利用价值,但极不稳定的特性与极低的生物利用效率降低了它们在体内的吸收和代谢。影响AC稳定性的因素主要包括pH、温度和金属离子[35]。AC在胃肠道中可同时存在酸碱平衡、水合平衡和环-链异构化平衡;并随pH的改变在蓝色醌式碱、红色黄烊盐阳离子、无色甲醇假碱和淡黄色查尔酮间互换[36]。因此,影响AC在反刍动物体内吸收的主要因素:1)AC是由多个共轭双键构成的高度共轭体系,需穿过细胞壁才可吸收[37];2)分子量的大小是影响AC吸收的重要因素之一[38];3)不同糖基结构可显著影响AC的吸收和代谢[39];4)酰基化作用可增强AC的稳定性,提升转化效率[40];5)AC可与其他营养物质(如蛋白质)结合,影响胃肠道消化酶的活性[41];6)胃肠道酸碱度的差异是影响AC吸收的主要因素。Jordán等[42]试验表明,富含多酚的迷迭香叶提取物可显著提高奶山羊血浆和奶鼠尾草酸的浓度,改善山羊健康,提高羊奶食用价值。Tian等[43]报道,奶山羊可将紫玉米秸秆青贮饲料中矢车菊素-3-葡萄糖苷、飞燕草素、芍药素、锦葵素-3-O-葡萄糖苷(malvidin-3-O-glucoside)、矢车菊色素和天竺葵素转入乳中。胡俊菁[44]指出,饲喂葡萄籽AC可显著提升奶牛乳清GPX活力。诚然,AC在反刍动物体内的吸收率较低,但各组织器官吸收的AC可增强机体抗氧化性能,进入乳中的AC对消费者的健康尤为重要。为此,如何提高AC在体内的吸收代谢效率将成为研究的重点与难点。此外,AC可为过氧自由基提供氢原子,抑制脂肪酸氧化过程,保证乳制品的质量[45]。因此,有必要在将来的饲养试验研究中进一步检测不同花色苷的吸收代谢规律。
4 花青素增强反刍动物机体抗氧化性能的机理 4.1 花青素对反刍动物机体免疫性能的影响AC可与蛋白质结合,被运送至皱胃或小肠进行消化代谢。因此,AC通过与氨基酸作用,调控T淋巴细胞、B淋巴细胞、自然杀伤细胞、巨噬细胞的活化、基因表达和淋巴细胞增殖,以及抗体、细胞因子和其他细胞毒性物质的产生,增强机体免疫反应[46],这在前人研究中得到了验证。羔羊食用富含AC的豆荚显示较高水平的抗体滴度[47]。此外,补饲富含AC的植物性饲料可提高围产期绵羊对环纹背带线虫的免疫力,表现较高水平的白细胞计数和血浆IgE抗感染幼虫的抗体[48]。
4.2 花青素消除反刍动物机体自由基的原理超氧阴离子自由基(superoxide, O2·-)在SOD的作用下转化为过氧化氢(hydrogen peroxide, H2O2),进而在GPX和CAT的作用下转换为H2O。然而,在高代谢负荷、日粮营养失衡等OS状态下可刺激NADPH氧化酶的活性,导致体内过量FR未能及时消除,造成代谢紊乱。AC是一种良好的氧自由基消除剂和脂质过氧化抑制剂,能有效缓解机体OS状态[49]。这是因为AC B环上相邻的二酚羟基具有广泛的电子非定域化特性,可保持其他氧化形式的稳定性[50]。同时,AC可提供多余的酚氢原子,中和体内FR,进行氧化反应,且不易再次还原为游离的FR[51]。Ngo Thi[52]报道,AC可改善生长山羊的抗氧化性能,降低体内寄生虫数量。姜良等[53]试验表明,紫玉米AC有良好的抗牛传染性鼻气管炎病毒的效果。Tian等[2]指出,紫玉米青贮秸秆AC提取液显示较高水平的DPPH自由基清除活性和较低水平的半数抑制浓度,表现出极强的抗氧化性。然而,部分研究显示,反刍动物饲粮添加AC仅提升血浆SOD浓度,不影响机体其他抗氧化指标(GPX、CAT和MDA等)[26, 38]。此外,涂军等[54]发现,奶牛摄入葡萄籽AC有提升血浆H2O2水平的作用。究其原因可能是O2·-作为H2O2线粒体的前体,对机体具有更强的毒害作用。因此,血浆SOD活性的升高间接减轻抗氧化防御系统的负担,从而降低其他抗氧化酶的需求[55]。显然,AC主要从两方面增强机体抗氧化潜力:1)通过酚羟基特有的结构直接清除氧自由基;2)通过改善机体相关抗氧化酶活性间接提高机体抗氧化性能。Schogor等[56]发现,饲予奶牛富含AC的植物性饲粮,显著降低瘤胃液和牛奶的硫代巴比妥酸反应物(thiobarbituric acid-reactive substances, TBARS)水平。宿孝奇[57]报道,AC可提高奶牛血浆TAC、SOD与GPX活性,降低NO浓度。邹须永等[58]指出,饲予产后2周奶牛20 mg·kg-1 BW的AC可显著提高血浆TAC水平。
4.3 花青素增强反刍动物机体抗氧化性能的分子调控机制 4.3.1 NF-κB信号通路当机体受到外界刺激,损伤因子可破坏细胞和组织,致使机体发生炎症反应。核转录因子κB(nuclear factor kappa beta, NF-κB)是一种OS敏感因子,调控炎症反应相关基因的表达[59]。NF-κB以无活性形式存在于胞浆,但蛋白激酶可诱导IKKα/β磷酸化,激活NF-κB信号通路[60]。此外,亲电体可氧化或共价修饰IKKα/β的半胱氨酸残基,抑制酶的活性[61]。因此,AC可直接氧化半胱氨酸巯基或改变细胞氧化还原状态,抑制NF-κB活性[62]。
Jeong等[63]指出,AC通过阻断NF-κB信号途径,显著抑制促炎介质和细胞因子的释放,这也在反刍动物相关研究中得以论证。Elgendy等[64]对饲喂富含AC葡萄渣的奶牛进行RNA转录组分析测序表明,AC不仅可诱导机体的免疫系统及白细胞介素的信号通路,还能抑制核糖体生物合成。De Marchi等[65]报道,饲予多酚类植物性饲料有降低奶牛NF-κB mRNA丰度的趋势。Tian等[66]发现,AC可显著降低乳腺组织肿瘤坏死因子(tumor necrosis factor, TNF)mRNA表达水平。因此,AC主要从两方面保护机体免受炎症的侵害:1)调控相关炎症细胞因子表达;2)限制内皮细胞黏附分子的活性[67]。
4.3.2 Nrf2-ARE信号通路转录因子NF-E2相关因子2 (nuclear factor erythroid 2-related factor 2, Nrf2)在编码氧化还原反应和细胞抗氧化反应元件(antioxidant response element, ARE)协同诱导中起至关重要的作用。Kelch样环氧氯丙烷相关蛋白-1 (kelch-like ECH protein, Keap1)富含半胱氨酸残基,是优良的亲电体和氧化剂的靶标。Nrf2通过ETGE和DLG两个结合位点与两分子的Keap1结合,形成Nrf2-ARE细胞骨架蛋白结构[68]。研究表明,AC主要通过调控Nrf2-ARE信号通路增强机体抗氧化机能[69],这是因为Nrf2是药物代谢酶关键的调节因子[70]。另一方面,Nrf2是碱性亮氨酸拉链(basic-leucine zipper,bZIP)家族成员之一,可调控抗氧化酶和解毒酶基因的表达水平[71]。AC作为一种强亲电体,可吸引带正电荷物质到富含电子的中心,启动细胞保护因子,抑制细胞毒性和抗氧化应激的损伤[72]。Schogor等[56]表明,在奶牛饲粮中添加富含AC的植物性饲料可显著提高机体乳腺组织CAT与Nrf2 mRNA丰度。Côrtes等[5]发现,饲喂多酚类植物性日粮有助于上调奶牛CAT、GPX1及SOD1的基因表达丰度,预防组织发生OS损伤。
在生理状态下,Keap1可抑制Nrf2信号,以非活性状态存在于胞浆,但在亲电子物质的刺激下,Nrf2从Keap1结构中解离并转移至细胞核与相应蛋白结合,调控下游靶基因的表达[73]。AC可直接作用Keap1的半胱氨酸残基,刺激Nrf2从中解耦连,随后与Maf、JunD等小蛋白形成异源二聚体,结合ARE或亲电响应元件(electrophile response elements, EpRE),上调一系列内源性细胞保护基因的表达[74]。Côrtes等[5]研究表明,富含AC的植物性饲料有助于增加机体相关抗氧化酶基因(CAT、GPX1和SOD1)的表达水平。Tian[75]指出,在奶山羊日粮中补饲1 g·d-1的紫玉米AC提取物可显著提高羊只乳腺组织的Nrf2和GPX1表达量。Sakatani等[76]发现,AC通过减少细胞内OS和增加GSH水平维持热休克牛胚胎细胞内的氧化还原平衡。
综上,AC激活Nrf2-ARE信号通路概括如下:1)Keap1在胞浆中通过BTB和Kelch将Nrf2与E3泛素连接酶结合,抑制Nrf2的活性;2)AC修饰Keap1的半胱氨酸,导致Keap1构象改变并与Nrf2解耦连;3)游离的Nrf2进入细胞核内,与下游靶细胞启动子ARE结合,上调Nrf2相关靶基因的表达[77]。Lima等[78]研究表明,奶牛饲粮中补饲多酚类植物性饲料,可显著增强红细胞GPX活性与乳腺组织GPX1基因的表达量。De Marchi等[65]报道,饲喂富含AC的植物性饲料有助于增强机体组织SOD2丰度,提升机体的抗氧化活性。因此,AC有较强消除机体FR的能力,通过激活Nrf2、抑制NF-κB信号通路增强反刍动物机体的抗氧化性能。
5 小结植物性AC饲料及提取物不仅存在AC复合物,还含有其他天然抗氧化剂(如黄酮类、维生素)。此外,个体AC在反刍动物体内的代谢机制仍不明晰。有必要检测个体AC在各器官和组织的含量分布,明确AC在体内吸收、代谢和排泄的途径及改善机体健康状况的作用机理。显然,AC具有作为天然抗氧化剂增强反刍动物抗氧化性能的潜力。但仍有亟待解决以下问题:1)AC安全性和副作用的深入探索;2)AC在机体中适宜水平添加量的确定;3)提高AC生物利用效率方法的研究;4)个体AC在体内代谢的差异。因此,建议通过饲养试验进一步深入研究AC增强反刍动物抗氧化性能的分子机制,为AC推广应用提供理论支持和参考依据。
| [1] | RAKESH S U, PATIL P R, MANE S R. Use of natural antioxidants to scavenge free radicals: a major cause of diseases[J]. Int J PharmTech Res, 2010, 2(2): 1074–1081. |
| [2] | TIAN X Z, PAENGKOUM P, PAENGKOUM S, et al. Comparison of forage yield, silage fermentative quality, anthocyanin stability, antioxidant activity, and in vitro rumen fermentation of anthocyanin-rich purple corn (Zea mays L.) stover and sticky corn stover[J]. J Integr Agric, 2018, 17(9): 2082–2095. DOI: 10.1016/S2095-3119(18)61970-7 |
| [3] | CHAUDHARI B K, SINGH M, MAURYA P K, et al. Stress markers in the plasma and milk of Murrah buffaloes during summer[J]. Agri Rev, 2013, 34(1): 21–35. |
| [4] | SHARMA N, SINGH N K, SINGH O P, et al. Oxidative stress and antioxidant status during transition period in dairy cows[J]. Asian-Austral J Anim Sci, 2011, 24(4): 479–484. DOI: 10.5713/ajas.2011.10220 |
| [5] | CÔRTES C, PALIN M F, GAGNON N, et al. Mammary gene expression and activity of antioxidant enzymes and concentration of the mammalian lignan enterolactone in milk and plasma of dairy cows fed flax lignans and infused with flax oil in the abomasum[J]. Br J Nutr, 2012, 108(8): 1390–1398. DOI: 10.1017/S0007114511006829 |
| [6] | GUO H H, LI D, LING W H, et al. Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ[J]. J Lipid Res, 2011, 52(5): 908–922. DOI: 10.1194/jlr.M013375 |
| [7] | LIN L Z, SUN J, CHEN P, et al. UHPLC-PDA-ESI/HRMS/MS(n) analysis of anthocyanins, flavonol glycosides, and hydroxycinnamic acid derivatives in red mustard greens (Brassica juncea Coss variety)[J]. J Agric Food Chem, 2011, 59(22): 12059–12072. DOI: 10.1021/jf202556p |
| [8] | HAN K H, SEKIKAWA M, SHIMADA K I, et al. Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats[J]. Br J Nutr, 2006, 96(6): 1125–1134. DOI: 10.1017/BJN20061928 |
| [9] | WU X L, PITTMAN H E, PRIOR R L. Fate of anthocyanins and antioxidant capacity in contents of the gastrointestinal tract of weanling pigs following black raspberry consumption[J]. J Agric Food Chem, 2006, 54(2): 583–589. DOI: 10.1021/jf052108+ |
| [10] | YOU J Y, KIM J, LIM J, et al. Anthocyanin stimulates in vitro development of cloned pig embryos by increasing the intracellular glutathione level and inhibiting reactive oxygen species[J]. Theriogenology, 2010, 74(5): 777–785. DOI: 10.1016/j.theriogenology.2010.04.002 |
| [11] |
汪水平, 彭希豪, 牛有军, 等. 葡萄籽提取物原花青素对肉兔屠宰性能与肉质的影响[J]. 畜牧兽医学报, 2014, 45(7): 1135–1141.
WANG S P, PENG X H, NIU Y J, et al. Effects of grape seed proanthocyanidin extracts on slaughter performance and meat quality of meat rabbit[J]. Acta Veterinaria et Zootechnica Sinica, 2014, 45(7): 1135–1141. (in Chinese) |
| [12] | FELGINES C, TALAVÉRA S, TEXIER O, et al. Blackberry anthocyanins are mainly recovered from urine as methylated and glucuronidated conjugates in humans[J]. J Agric Food Chem, 2005, 53(20): 7721–7727. DOI: 10.1021/jf051092k |
| [13] | CHANG H, YU B, YU X P, et al. Anticancer activities of an anthocyanin-rich extract from black rice against breast cancer cells in vitro and in vivo[J]. Nutr Cancer, 2010, 62(8): 1128–1136. DOI: 10.1080/01635581.2010.494821 |
| [14] | FORMICA J V, REGELSON W. Review of the biology of quercetin and related bioflavonoids[J]. Food Chem Toxicol, 1995, 33(12): 1061–1080. DOI: 10.1016/0278-6915(95)00077-1 |
| [15] | BLOOR S J, ABRAHAMS S. The structure of the major anthocyanin in Arabidopsis thaliana[J]. Phytochemistry, 2002, 59(3): 343–346. DOI: 10.1016/S0031-9422(01)00460-5 |
| [16] | KONCZAK I, ZHANG W. Anthocyanins-more than nature's colours[J]. J Biomed Biotechnol, 2004, 2004(5): 239–240. DOI: 10.1155/S1110724304407013 |
| [17] | CASTAÑEDA-OVANDO A, DE LOURDES PACHECO-HERNÁNDEZ M, PÁEZ-HERNÁNDEZ M E, et al. Chemical studies of anthocyanins: a review[J]. Food Chem, 2009, 113(4): 859–871. DOI: 10.1016/j.foodchem.2008.09.001 |
| [18] | ADEDOKUN O, TITILOPE K, AWODUGBA A O. Review on natural dye-sensitized solar cells (DSSCs)[J]. Int J Eng Technol, 2016, 2(2): 34–41. |
| [19] | REIN M J.Copigmentation reactions and color stability of berry anthocyanins[D]. Finland, Helsinki: University of Helsinki, 2005. |
| [20] | CLIFFORD M N. Anthocyanins-nature, occurrence and dietary burden[J]. J Sci Food Agric, 2000, 80(7): 1063–1072. DOI: 10.1002/(SICI)1097-0010(20000515)80:7<1063::AID-JSFA605>3.0.CO;2-Q |
| [21] | AOKI H, KUZE N, KATO Y. Anthocyanins isolated from purple corn (Zea mays L.)[J]. Foods Food Ingredients J Jpn, 2002, 199: 41–45. |
| [22] | HOU Z H, QIN P Y, ZHANG Y, et al. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics[J]. Food Res Int, 2013, 50(2): 691–697. DOI: 10.1016/j.foodres.2011.07.037 |
| [23] | MOJICA L, BERHOW M, DE MEJIA E G. Black bean anthocyanin-rich extracts as food colorants: physicochemical stability and antidiabetes potential[J]. Food Chem, 2017, 229: 628–639. DOI: 10.1016/j.foodchem.2017.02.124 |
| [24] | WHO.Toxicological evaluation of certain food additives and contaminants[R]. Geneva: WHO Food Additives Series, 1996. |
| [25] | HOSODA K, MIYAJI M, MATSUYAMA H, et al. Effect of supplementation of purple pigment from anthocyanin-rich corn (Zea mays L.) on blood antioxidant activity and oxidation resistance in sheep[J]. Livest Sci, 2012, 145(1-3): 266–270. DOI: 10.1016/j.livsci.2011.12.001 |
| [26] | HOSODA K, ERUDEN B, MATSUYAMA H, et al. Effect of anthocyanin-rich corn silage on digestibility, milk production and plasma enzyme activities in lactating dairy cows[J]. Anim Sci J, 2012, 83(6): 453–459. DOI: 10.1111/j.1740-0929.2011.00981.x |
| [27] | SONG T H, HAN O K, PARK T I, et al. Anthocyanin stability and silage fermentation quality of colored barley[J]. J Korean Soc Grassland Forage Sci, 2012, 32(4): 335–342. DOI: 10.5333/KGFS.2012.32.4.335 |
| [28] | LEATHERWOOD W L.The effect of anthocyanins from purple-fleshed sweetpotato on in vitro fermentation by rumen microbial cultures[D]. Raleigh: North Carolina State University, 2013. |
| [29] |
伍树松, 贺喜, 伍小松, 等. 花青素作为饲料添加剂的应用前景[J]. 动物营养学报, 2011, 23(12): 2097–2104.
WU S S, HE X, WU X S, et al. The use of anthocyanins as a feed additive[J]. Chinese Journal of Animal Nutrition, 2011, 23(12): 2097–2104. DOI: 10.3969/j.issn.1006-267x.2011.12.009 (in Chinese) |
| [30] | GONTHIER M P, DONOVAN J L, TEXIER O, et al. Metabolism of dietary procyanidins in rats[J]. Free Radical Bio Med, 2003, 35(8): 837–844. DOI: 10.1016/S0891-5849(03)00394-0 |
| [31] | ISHIDA K, KISHI Y, OISHI K, et al. Effects of feeding polyphenol-rich winery wastes on digestibility, nitrogen utilization, ruminal fermentation, antioxidant status and oxidative stress in wethers[J]. Anim Sci J, 2015, 86(3): 260–269. DOI: 10.1111/asj.12280 |
| [32] | CORREDDUF, NUDDA A, BATTACONE G, et al. Effects of grape seed supplementation, alone or associated with linseed, on ruminal metabolism in Sarda dairy sheep[J]. Anim Feed Sci Technol, 2015, 199: 61–72. DOI: 10.1016/j.anifeedsci.2014.11.002 |
| [33] |
童津津, 张华, 孙铭维, 等. 采用Illumina MiSeq测序技术分析葡萄籽原花青素对奶牛体外瘤胃发酵产甲烷菌区系的影响[J]. 动物营养学报, 2019, 31(1): 314–323.
TONG J J, ZHANG H, SUN M W, et al. Effects of grape seed procyanidine on Methanogens flora of in vitro rumen fermentation of dairy cows using Illumina MiSeq sequencing technology[J]. Chinese Journal of Animal Nutrition, 2019, 31(1): 314–323. DOI: 10.3969/j.issn.1006-267x.2019.01.038 (in Chinese) |
| [34] |
杨德莲, 童津津, 张婕, 等. 葡萄籽原花青素对奶牛瘤胃体外发酵参数及微生物区系的影响[J]. 动物营养学报, 2018, 30(2): 717–725.
YANG D L, TONG J J, ZHANG J, et al. Effects of grape seed procyanidine on rumen fermentation parameters and microflora of dairy cows in vitro[J]. Chinese Journal of Animal Nutrition, 2018, 30(2): 717–725. DOI: 10.3969/j.issn.1006-267x.2018.02.037 (in Chinese) |
| [35] | CEVALLOS-CASALS B A, CISNEROS-ZEVALLOS L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants[J]. Food Chem, 2004, 86(1): 69–77. DOI: 10.1016/j.foodchem.2003.08.011 |
| [36] | KHOO H E, AZLAN A, TANG S T, et al. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits[J]. Food Nutr Res, 2017, 61(1): 1361779. DOI: 10.1080/16546628.2017.1361779 |
| [37] | CAVALCANTI R N, SANTOS D T, MEIRELES M A A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems - An overview[J]. Food Res Int, 2011, 44(2): 499–509. DOI: 10.1016/j.foodres.2010.12.007 |
| [38] | MARTÍNEZ C, JORDÁN M J, MOÑINO M I, et al. Identification of polyphenolic components in Murciano-Granadina goat milk and suckling goat kid plasma[J]. Planta Med, 2007, 73(9): 923. |
| [39] | HE J, MAGNUSON B A, LALA G, et al. Intact anthocyanins and metabolites in rat urine and plasma after 3 months of anthocyanin supplementation[J]. Nutr Cancer, 2006, 54(1): 3–12. |
| [40] | NETZEL M, NETZEL G, KAMMERER D R, et al. Cancer cell antiproliferation activity and metabolism of black carrot anthocyanins[J]. Innov Food Sci Emerg Technol, 2007, 8(3): 365–372. DOI: 10.1016/j.ifset.2007.03.011 |
| [41] | MANGAN J L. Nutritional effects of tannins in animal feeds[J]. Nutr Res Rev, 1988, 1(1): 209–231. |
| [42] | JORDÁN M J, MOÑINO M I, MARTÍNEZ C, et al. Introduction of distillate rosemary leaves into the diet of the Murciano-granadina goat: transfer of polyphenolic compounds to goats' milk and the plasma of suckling goat kids[J]. J Agric Food Chem, 2010, 58(14): 8265–8270. DOI: 10.1021/jf100921z |
| [43] | TIAN X Z, PAENGKOUM P, PAENGKOUM S, et al. Short communication: purple corn (Zea mays L.) stover silage with abundant anthocyanins transferring anthocyanin composition to the milk and increasing antioxidant status of lactating dairy goats[J]. J Dairy Sci, 2019, 102(1): 413–418. DOI: 10.3168/jds.2018-15423 |
| [44] |
胡俊菁.葡萄籽原花青素对奶牛抗氧化状况和酮病等指标的影响[D].南宁: 广西大学, 2017.
HU J J.Effects of feeding grape seed procyanidins on antioxidant status and ketosis-related indicators in dairy cows[D]. Nanning: Guangxi University, 2017.(in Chinese) |
| [45] | NARAYAN M S, NAIDU K A, RAVISHANKAR G A, et al. Antioxidant effect of anthocyanin on enzymatic and non-enzymatic lipid peroxidation[J]. Prostag Leukotr ESS, 1999, 60(1): 1–4. DOI: 10.1054/plef.1998.0001 |
| [46] | PROVENZA F D, VILLALBA J J. The role of natural plant products in modulating the immune system: an adaptable approach for combating disease in grazing animals[J]. Small Ruminant Res, 2010, 89(2-3): 131–139. DOI: 10.1016/j.smallrumres.2009.12.035 |
| [47] | NIEZEN J H, CHARLESTON W A G, ROBERTSON HA, et al. The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and development of immunity to gastrointestinal nematodes[J]. Vet Parasitol, 2002, 105(3): 229–245. |
| [48] | HOUDIJK J G M, JACKSON F, COOP R L, et al. Rapid improvement of immunity to Teladorsagia circumcincta is achieved through a reduction in the demand for protein in lactating ewes[J]. Int J Parasitol, 2006, 36(2): 219–227. DOI: 10.1016/j.ijpara.2005.09.014 |
| [49] | MORAIS C A, DE ROSSO V V, ESTADELLA D, et al. Anthocyanins as inflammatory modulators and the role of the gut microbiota[J]. J Nutr Biochem, 2016, 33: 1–7. DOI: 10.1016/j.jnutbio.2015.11.008 |
| [50] | CORREDDU F, GASPA G, PULINA G, et al. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep[J]. J Dairy Sci, 2016, 99(3): 1725–1735. DOI: 10.3168/jds.2015-10108 |
| [51] | JORDĀO A M, CORREIA A C. Relationship between antioxidant capacity, proanthocyanidin and anthocyanin content during grape maturation of Touriga Nacional and Tinta Roriz grape varieties[J]. S Afr J Enol Vitic, 2012, 33(2): 214–224. |
| [52] | NGO THI M S.Utilization of anthocyanin-rich Napier grass silage in growing goat diets[D]. Thailand: Suranaree University of Technology, 2018. |
| [53] |
姜良, 黄秀芬, 刘杉杉, 等. 紫玉米花色苷抑制牛传染性鼻气管炎病毒在牛肾细胞中增殖的研究[J]. 中国畜牧兽医, 2014, 41(8): 127–130.
JIANG L, HUANG X F, LIU S S, et al. Study on purple corn anthocyanin inhibits replication of infectious bovine Rhinotracheitis virus on bovine kidney cells[J]. China Animal Husbandry & Veterinary Medicine, 2014, 41(8): 127–130. (in Chinese) |
| [54] |
涂军, 黄云飞, 胡俊菁, 等. 葡萄籽原花青素对产后奶牛血浆中H2O2和酮体含量的影响[J]. 广西畜牧兽医, 2018, 34(2): 100–102.
TU J, HUANG Y F, HU J J, et al. Effects of grape seed proanthocyanidins on the plasma hydrogen peroxide and ketone bodies of postpartum dairy cows[J]. Guangxi Journal of Animal Husbandry & Veterinary Medicine, 2018, 34(2): 100–102. DOI: 10.3969/j.issn.1002-5235.2018.02.017 (in Chinese) |
| [55] | MITTLER R. Oxidative stress, antioxidants and stress tolerance[J]. Trends Plant Sci, 2002, 7(9): 405–410. DOI: 10.1016/S1360-1385(02)02312-9 |
| [56] | SCHOGOR A L B, PALIN M F, SANTOS G T, et al. Mammary gene expression and activity of antioxidant enzymes and oxidative indicators in the blood, milk, mammary tissue and ruminal fluid of dairy cows fed flax meal[J]. Br J Nutr, 2013, 110(10): 1743–1750. DOI: 10.1017/S0007114513001220 |
| [57] |
宿孝奇.酮病奶牛氧化应激特征及原花青素对奶牛氧化应激的影响[D].南宁: 广西大学, 2015.
SU X Q.Characetristics of oxidative stress in cows with ketosis and the effect of adding procyanidins on oxidative stress in cows[D]. Nanning: Guangxi University, 2015.(in Chinese) |
| [58] |
邹须永, 胡俊菁, 冯馨莹, 等. 喂食原花青素对产后奶牛血浆抗氧化指标的影响[J]. 广西畜牧兽医, 2017, 33(2): 83–85.
ZOU X Y, HU J J, FENG X Y, et al. Effects of procyanidins on plasma antioxidant parameters in postpartum dairy cows[J]. Guangxi Journal of Animal Husbandry & Veterinary Medicine, 2017, 33(2): 83–85. (in Chinese) |
| [59] | PARK S Y, LEE J H, KIM Y K, et al. Cilostazol prevents remnant lipoprotein particle-induced monocyte adhesion to endothelial cells by suppression of adhesion molecules and monocyte chemoattractant protein-1 expression via lectin-like receptor for oxidized low-density lipoprotein receptor activation[J]. J Pharmacol Exp Ther, 2005, 312(3): 1241–1248. |
| [60] | SURH Y J, NA H K. NF-κB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals[J]. Genes Nutr, 2008, 2(4): 313–317. DOI: 10.1007/s12263-007-0063-0 |
| [61] | HEISS E, HERHAUS C, KLIMO K, et al. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms[J]. J Biol Chem, 2001, 276(34): 32008–32015. DOI: 10.1074/jbc.M104794200 |
| [62] | HOLLANDS W, BRETT G M, RADREAU P, et al. Processing blackcurrants dramatically reduces the content and does not enhance the urinary yield of anthocyanins in human subjects[J]. Food Chem, 2008, 108(3): 869–878. DOI: 10.1016/j.foodchem.2007.11.052 |
| [63] | JEONG J W, LEE W S, SHIN S C, et al. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in BV2 microglial cells by suppressing the NF-κB and Akt/MAPKs signaling pathways[J]. Int J Mol Sci, 2013, 14(1): 1502–1515. DOI: 10.3390/ijms14011502 |
| [64] | ELGENDY R, CASTELLANI F, PALAZZO F, et al. RNA sequencing-based transcriptome profiling of dairy cows fed with a polyphenol-rich grape pomace-supplemented diet[J]. Ital J Anim Sci, 2017, 16(S1): 137–138. |
| [65] | DE MARCHI F E, PALIN M F, SANTOS G T D, et al. Flax meal supplementation on the activity of antioxidant enzymes and the expression of oxidative stress- and lipogenic-related genes in dairy cows infused with sunflower oil in the abomasum[J]. Anim Feed Sci Tech, 2015, 199: 41–50. DOI: 10.1016/j.anifeedsci.2014.10.018 |
| [66] | TIAN X Z, XIN H L, PAENGKOUM P, et al. Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on nutrient utilization, rumen fermentation, plasma antioxidant capacity, and mammary gland gene expression in dairy goats[J]. J Anim Sci, 2019, 97(3): 1384–1397. DOI: 10.1093/jas/sky477 |
| [67] | CHEN C Y, YI L, JIN X, et al. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-κB pathway[J]. Cell Biochem Biophys, 2011, 61(2): 337–348. |
| [68] | NIOI P, NGUYEN T, SHERRATT P J, et al. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation[J]. Mol Cell Biol, 2005, 25(24): 10895–10906. DOI: 10.1128/MCB.25.24.10895-10906.2005 |
| [69] | ZHANG X, YANG Y, WU Z F, et al. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro[J]. J Agric Food Chem, 2016, 64(12): 2582–2590. DOI: 10.1021/acs.jafc.6b00586 |
| [70] | HE J L, ZHOU Z W, YIN J J, et al. Schisandra chinensis regulates drug metabolizing enzymes and drug transporters via activation of Nrf2-mediated signaling pathway[J]. Drug Des Dev Ther, 2014, 9: 127–146. |
| [71] | HUANG W, SHANG Y F, CHEN P L, et al. Basic leucine zipper (bZIP) domain transcription factor MBZ1 regulates cell wall integrity, spore adherence, and virulence in Metarhizium robertsii[J]. J Biol Chem, 2015, 290(13): 8218–8231. DOI: 10.1074/jbc.M114.630939 |
| [72] | DOMITROVIC R. The molecular basis for the pharmacological activity of anthocyans[J]. Curr Med Chem, 2011, 18(29): 4454–4469. |
| [73] | YE J, YAO J P, WANG X, et al. Neuroprotective effects of ginsenosides on neural progenitor cells against oxidative injury[J]. Mol Med Rep, 2016, 13(4): 3083–3091. DOI: 10.3892/mmr.2016.4914 |
| [74] | KRUGER M J, DAVIES N, MYBURGH K H, et al. Proanthocyanidins, anthocyanins and cardiovascular diseases[J]. Food Res Int, 2014, 59: 41–52. DOI: 10.1016/j.foodres.2014.01.046 |
| [75] | TIAN X Z.Effects of anthocyanin-rich purple corn (Zea mays L.) stover silage on antioxidant activities in dairy goats[D]. Thailand: Suranaree University of Technology, 2017. |
| [76] | SAKATANI M, SUDA I, OKI T, et al. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock[J]. J Reprod Dev, 2007, 53(3): 605–614. DOI: 10.1262/jrd.18124 |
| [77] | SPECIALE A, CIMINO F, SAIJA A, et al. Bioavailability and molecular activities of anthocyanins as modulators of endothelial function[J]. Genes Nutr, 2014, 9(4): 404. DOI: 10.1007/s12263-014-0404-8 |
| [78] | LIMA L S, PALIN M F, SANTOS G T, et al. Effects of supplementation of flax meal and flax oil on mammary gene expression and activity of antioxidant enzymes in mammary tissue, plasma and erythrocytes of dairy cows[J]. Livest Sci, 2015, 176: 196–204. DOI: 10.1016/j.livsci.2015.03.015 |



