2. 新疆农业大学动物科学学院, 乌鲁木齐 830052
2. College of Animal Science, Xinjiang Agricultural University, Urumqi 830052, China
类视黄醇X受体(retinoid X receptors, RXRs)是类固醇受体超家族成员的配体依赖性核受体,由不同基因编码的这类受体有3种亚型,分别为RXRα、RXRβ和RXRγ。RXRs既可以形成同源二聚体(RXRs/RXRs),也可以与其他核受体形成异源二聚体行使功能。有趣的是,作为视黄酸受体(retinoic acid receptors, RARs)、甲状腺激素受体(thyroid hormone receptors, THRs)和维生素D受体(vitamin D receptor, VDR)的异二聚体,RXRs不结合配体,而作为辅助因子存在[1-2],而与法尼酯X受体(farnesoid X receptor, FXR)、肝X受体(liver X receptor, LXR)和过氧化物酶体增殖物激活受体(peroxisome proliferator-activated receptors, PPARs)等结合形成一个有功能的异源二聚体,其中RXRs是一个有活性的配体结合亚单位。而且这2种模式决定了类视黄醇的2种不同反应途径[3]。
动物按发情的季节性特点可分为全年发情动物(如猪)和季节性发情动物(如绵羊、马和仓鼠等)。动物季节性发情的一个关键调控通路是甲状腺激素/甲状腺激素受体-亲吻素-促性腺激素释放激素通路(TH/THRs-KISS-GnRH)[4],其中THRs通常需要结合RXRs发挥作用[5]。季节性发情受动物品种、光照、温度以及营养等多方面的影响,而RXRs参与形成的THRs/RXRs、PPARs/RXRs、LXR/RXRs、RARs/RXRs和VDR/RXRs等二聚体在不同的光照、温度和营养条件下发挥不同作用。本文就RXRs参与形成的多种二聚体在动物季节性发情和发情周期中发挥的生理功能进行了综述。
1 RXRs及其二聚体的基本特征 1.1 RXRs二聚体及其配体简介THRs、PPARs和RARs等这些核受体需要与RXRs进行异二聚化才能产生活化的核受体典型结构。Gampe等[1]报道了RXRs与配体9-cisRA配体结合区的晶体结构,这个结构在分子水平上说明了RXRs在没有配体刺激时,是一个没有功能的四聚体状态,结合配体激活后形成同源二聚体或与其他核受体形成异源二聚体[6],这证明了PPARγ/RXRα等异源二聚体能被9-cisRA激活。Heyman等[7]发现,RXRs靶基因上存在2个或更多个AGGTCA转录激活反应元件。RXRs参与形成的多种异二聚体通过结合各自特定的DNA反应元件激活各种靶基因的转录,其中RXRs结合于元件的3′端,PPARs等核受体则结合于5′端,转录过程中存在一些辅助因子能增强(作为共激活剂,如CBP/p300、ARA70、PGC-1等)或减少(作为共抑制剂,如SMRT、NcoR、mSin3等)它们的活性[8]。
RXRs配体是9-顺式维甲酸(9-cisRA)。维甲酸(retinoic acid, RA)又称视黄酸或维生素A酸,是维生素A的衍生物,它由环己烯环、侧链和极性基团3部分组成,由于极性基团和侧链不同,RA包括多种同分异构体,如9-顺式维甲酸(9-cisRA)、13-顺式维甲酸(13-cisRA)和全反式维甲酸(ATRA)等。研究发现,RA在调控细胞增殖分化、生长发育、形态发生、代谢、内环境的稳定平衡和暗视觉等方面具有广泛的生物学活性。其中,9-cisRA是改变多烯侧链结构合成的第3代维甲酸,与其他RA相比,9-cisRA的生物学作用更强。繁殖上,RXRs被9-cisRA激活与其它核受体形成二聚体后,可以作用于GnRH受体和垂体激素的分泌,并能调节卵泡发育、成熟和排卵过程中增殖分化相关因子表达以及血管生成,实现对季节性发情和发情周期的调控[9-10]。
1.2 PPARs/RXRs的研究进展PPARs属于配体依赖性核受体家族的脂肪酸受体,由PPARα、PPARβ和PPARγ组成。PPARs与RXR进行异二聚化形成核受体的典型结构,异二聚体中当2个受体各自结合配体后,其转录活性大大加强[11]。异二聚体通过结合DNA序列中的过氧化物酶增殖物反应元件(peroxisome proliferator response element, PPRE)激活靶基因的转录。PPARs/RXRs除了能被其天然和合成化合物激活,也可被RXRs的配体9-cisRA激活[12]。天然活化剂包括不饱和脂肪酸、前列腺素(prostaglandin, PG)相关化合物或氧化脂质、胰岛素增敏药(包括噻唑烷二酮类药物(thiazolidinediones, TZDs))等[13]。
PPARγ是连接哺乳动物能量代谢和繁殖生理过程的关键转录因子[8]。其活化剂TZDs可通过诱导多囊卵巢综合征(PCOS)女性排卵来提高其生育能力[14]。垂体细胞通过垂体激素FSH和LH控制卵泡发生,而PPARγ配体具有调节垂体激素分泌的能力[12]。此外,在卵巢中PPARγ激动剂对小卵泡(1~3 mm)和有腔卵泡(>5 mm)两种不同绵羊颗粒细胞群的增殖和类固醇生成有重要作用[12]。通过在绵羊体外颗粒细胞中添加RXR配体(LG1069)极显著增强了PPARγ的表达,并呈RXR配体依赖性,表明内源性PPARγ/RXRα异二聚体在体外颗粒细胞中是有功能的[12]。PPARγ在啮齿动物颗粒细胞中表达,PPARγ激动剂能调节其类固醇生成能力[15-16]。证明PPARγ/RXRs二聚体对卵泡周期性发育有重要作用。
1.3 RAR/RXR及其他二聚体的研究进展维甲酸受体有两类,均有3种亚型:视黄酸受体(RARα、RARβ和RARγ)和视黄醇X受体(RXRα、RXRβ和RXRγ)[17]。通常两者在细胞内以异二聚体形式存在,异二聚体本身不具有生物学功能,真正发挥生物学效应的是异二聚体与配体的复合体[18]。即9-cisRA先与RXR形成的四聚体结合,使其空间结构发生变化与RAR形成异二聚体,异二聚体被激活后RXR成为转录调节因子,并与目标基因结合,调节目标基因的转录和表达来发挥生物学效应[1]。RAR/RXR与卵巢癌分化程度及恶性转移相关,同时还是抑制肿瘤的关键受体,可以介导维甲酸抑制肿瘤细胞的生长[19]。
人全外显子测序结果显示,FXR/RXR和LXR/RXR是连接脂蛋白、脂质和葡萄糖代谢典型途径的重要部分,能够影响卵巢组织重塑和卵泡周围营养分配[20]。
2 RXRs在动物发情周期中的作用卵巢上的卵泡发育、成熟,雌性激素的产生是发情的本质,卵巢中血管生成是卵泡发育和排卵所必需的生长过程[21]。RXR形成的二聚体可以通过调控参与卵巢血管生成、炎症反应和细胞凋亡的相关基因介导卵泡生长、颗粒细胞的增殖和优势卵泡的选择进而影响发情周期[12],见图 1。其中,已经鉴定的血管生成诱导剂包括:碱性成纤维细胞生长因子(basic fibroblast growth factor, FGF)、表皮生长因子(epidermal growth factor, EGF)、血管内皮生长因子A(vascular endothelial growth factor A, VEGFA)家族成员、转化生长因子(transforming growth factor, TGF)、血管生成素(angiopoietin, ANGPT)和血小板衍生生长因子(PDGF)等[22]。
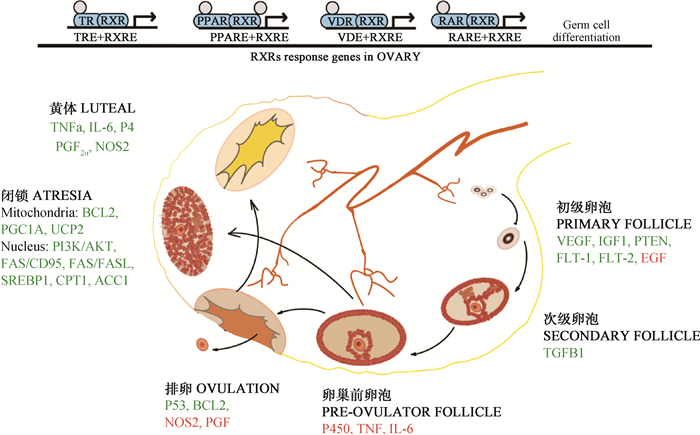
|
图中卵泡发育周期中每个时期下面标注的是对卵泡发育有重要调控作用的因子,其中绿色代表RXR二聚体对其有促进作用,红色代表对其存在抑制作用 In this figure, factors that have important regulatory effects on follicular development are noted below each period of the follicular development cycle. Among them, the factors in green and in red are promoted and inhibited by RXR dimer, respectively 图 1 RXRs二聚体在卵泡发育周期中的间接调控作用 Fig. 1 The indirect regulation role of RXRs dimers in the follicular development cycle |
PPARγ和RXRs在不同类别的卵泡中检测到表达(初级/次级至排卵前卵泡),其表达量随着卵泡的发育而增加,暗示其在卵泡发育过程中具有一定调控作用[12]。在绵羊卵巢的颗粒细胞中,天然配体(罗格列酮)激活PPARγ能够参与调节类固醇激素的合成[12]。因此,PPARγ/RXRα能通过颗粒细胞类固醇激素的分泌间接参与颗粒细胞的增殖和卵母细胞的成熟。在绵羊体外颗粒细胞中的研究也证实了这个观点,通过添加RXR配体(LG1069)极显著增强了罗格列酮刺激PPARγ表达的作用,并呈RXR配体依赖性,证明内源性PPARγ/RXRα异二聚体在体外颗粒细胞中是有功能的[12]。在啮齿动物颗粒细胞中,PPAR/RXR激动剂同样具有调节其类固醇生成的能力[15-16]。
2.1.1 RXRs在原始卵泡发育成初级卵泡过程中的作用原始卵泡发育成为初级卵泡的过程主要受多种生长因子影响(如胰岛素样生长因子(insulin-like growth factors, IGFs)、EGF、FGF等)[22],这些生长因子可以促进颗粒细胞的有丝分裂。染色质免疫沉淀表明,FGF-1能增强大鼠LXR/RXR与目的基因启动子的结合,激活丝裂原活化蛋白激酶/细胞外信号调节激酶(MEK/ERK)通路,增加胆固醇生物合成和磷脂酰肌醇-3-激酶(phosphatidylinositol-3-kinase, PI3K)/蛋白激酶B(Akt)的合成[23]。
PPAR/RXR的靶基因之一是IGFs,可以通过后者实现对卵巢颗粒细胞增殖和凋亡的调控[24]。在卵泡发育的调节系统中起作用的IGFs包括IGF-1、IGF-2及它们的受体IGF结合蛋白(IGF-binding proteins, IGFBps),其中,IGF-1是维持卵巢正常功能所必需的因子[25]。IGF-1与卵泡内膜细胞和颗粒细胞上的受体结合后通过提高FSH诱导的芳香化酶活性,使颗粒细胞合成雌二醇水平增加,IGFs与生殖激素有协同作用,可促进颗粒细胞的有丝分裂,使颗粒细胞数量增多,促进卵泡发育[25]。IGF-1还能通过增强LH与内膜细胞的结合能力,增强内膜细胞对LH的反应性,促进内膜细胞的分化,使雄激素分泌增加,IGF-1可促进LH发挥作用,能增加卵泡对促性腺激素的反应性[26]。
原始卵泡的状态对于维持雌性哺乳动物的生殖寿命至关重要,迄今为止,对于原始卵泡的激活研究是辅助生殖的一大热点。诱导抑癌基因同源性磷酸酶-张力蛋白基因(phosphatase and tensin homolog deleted on chromosome 10,PTEN)的表达可激活储备原始卵泡,治疗卵巢早衰(POF)患者的不孕症[27]。PTEN特异性控制卵泡激活,尤其作用于原始卵泡激活[28]。有研究表明,PPAR γ/RARα可促进PTEN的表达[29-30]。
2.1.2 RXRs在次级卵泡和三级卵泡中的作用在初级卵泡发育成次级卵泡期间,卵泡的发育主要受来自内膜细胞分泌的TGFα的影响[22]。有研究表明,TGFα的过表达可导致视网膜神经元前端RXRA基因表达下调[31]。但是暂无RXR二聚体或者其配体对TGFα调控的报道。
在三级卵泡发育期间,TGFα水平降低,主要受性腺激素(性腺激素依赖期)和TGFβ的调节,此时有丝分裂作用受到抑制。三级卵泡阶段,雌激素作用增强,而PPAR / RXR异二聚体通过结合雌激素反应元件(ERE)抑制雌激素受体的生物学活性[32]。同时,PPARγ和RXRα通过PTEN介导的p70核糖体S6激酶-1(p70 ribosomal S6 kinase-1, S6K1)抑制下调TGFβ1基因表达[33]。因此,PPAR/RXR异二聚体通过上述2个方面抑制三级卵泡的发育。
2.1.3 RXRs在卵泡成熟和排出中的作用与次级卵泡相比,排卵前卵泡具有高度的血管分布,导致营养和促性腺激素的优先递送[34]。卵泡的排出是通过控制血管生成、炎症反应、细胞凋亡和细胞周期相关基因的调节来介导的。次级卵泡发育接近成熟时,PPAR/RXR通过调控靶基因IGF-1促进颗粒细胞分化而增强其孕激素合成[25]。Baker等[35]将小鼠的IGF-1基因敲除后发现小鼠不排卵。
已经证明,PPARγ参与调节卵泡发育、卵母细胞成熟、排卵和黄体形成或消退[36]。罗格列酮(PPARγ配体)在100 mmol·L-1时可联合PPARγ刺激AMP活化蛋白激酶(AMPK),并增强小鼠卵母细胞的减数分裂恢复[30]。有研究显示,与卵泡期相比,黄体期PPARα和PPARβ的mRNA水平增加,而PPARγ基因表达在黄体溶解期显著增高[37]。小鼠中, 颗粒细胞特异性PPARγ缺失可导致卵泡提前破裂,排卵数显著降低[30]。多囊卵巢综合征(polycystic ovary syndrome, PCOS)患者卵巢排卵存在一定障碍[38],PPARγ配体胰岛素增敏剂(如曲格列酮)被证明可以改善PCOS患者的排卵功能[14, 39]。P450芳香化酶是雄激素转化为雌激素的限速酶,PPARγ和RXR激动剂均能显著抑制人卵巢颗粒细胞芳香化酶活性,两者结合使用其抑制作用进一步增强,并且伴有P450 mRNA丰度的下降,说明两者是通过二聚体发挥作用的[38, 40]。
2.2 RXRs在黄体形成和溶解中的作用研究表明,PPAR/RXR能通过抑制人黄体颗粒细胞的促炎细胞因子(如肿瘤坏死因子a(tumor necrosis factor a, TNFa)、白细胞介素-6(interleukin-6, IL-6)等)和血管生成相关因子的活性分泌而参与排卵和黄体化[41]。在卵巢巨噬细胞中,诱导型一氧化氮合酶(nitric oxide synthase, NOS2)是一种调节排卵的重要促炎酶,PPARγ激动剂可抑制其表达[42]。也有证据表明,PPARγ活化影响绵羊颗粒细胞的增殖,但对凋亡基因没有影响[12]。在啮齿动物的黄体和颗粒细胞中,PPARγ活力受到抗细胞凋亡Bcl2蛋白和肿瘤抑制因子p53表达的调节[43-44]。
黄体为分泌孕酮的主要组织。卵巢中PPARγ的组织特异性敲除显著降低了小鼠的生育能力[41],虽然这些小鼠的卵泡数量正常,并且发育出黄体,但其分泌的黄体酮(P4)水平降低,着床率降低,胚胎较小[41]。激活PPARγ/RXR后, 通过PPARγ信号通路可诱导3β-HSD I mRNA转录,从而促进体内孕酮的合成[45]。同样的研究证明,小鼠服用PPARγ激动剂4周不影响卵泡发生,但血浆P4浓度增加[46]。由黄体侧的子宫角分泌的前列腺素F2α(prostaglandin F2α, PGF2α)为主要的溶黄体因子,对猪的研究表明,PPAR配体在黄体中期可抑制PGF2α释放,提示PPARγ/RXR二聚体具有促黄体化的生物学功能[47]。
2.3 RXRs在卵泡闭锁与退化中的作用PPARγ和RXRα及其配体共同参与调节卵巢各级卵泡葡萄糖平衡、脂质代谢和维持稳态[48],调控优势卵泡和劣势卵泡不同的营养分配,诱导细胞凋亡,并通过PPARs、AMPK、PI3K/Akt和Fas(CD95受体)等这些已知的通路在卵巢发育和卵泡闭锁中起关键作用[49]。卵泡闭锁的基本机制是细胞的程序性死亡,一般是从颗粒细胞的凋亡开始,然后引起内膜细胞凋亡。PPARγ可抑制原代颗粒细胞的增殖并诱导细胞凋亡[50],还能通过与一些蛋白质(Bcl2等)的相互作用从细胞核转移至细胞质以诱导细胞凋亡[51]。已证实的细胞凋亡途径有两条。
一条凋亡途径(Fas/FasL通路)是从细胞表面引起的,由细胞外凋亡信号分子(如Fas/CD95)诱发。PPARγ被激活后可增强FasL(CD95配体)的表达,并通过Fas/FasL途径激发细胞凋亡[52]。在体外,人卵巢颗粒细胞中增加PPARγ蛋白表达可上调甾醇调节元件结合转录因子(sterol regulatory element binding transcription factor 1, SREBF1)、Fas、二酰甘油胆碱磷酸转移酶(diacylglycerol cholinephosphotransferase,CPT1)和乙酰辅酶α (acetyl-CoA carboxylase alpha, ACC1)基因表达[53],这些因子是引起卵巢PCOS的重要因素;另外,PPARγ/RXRs的激活明显促进了PTEN的表达,从而抑制PI3K/Akt信号通路,抑制细胞周期进程,促进细胞凋亡[54]。卵巢内的巨噬细胞有吞噬各级卵泡的作用,在卵巢巨噬细胞中,促炎酶NOS2也受PPARγ激动剂负调控[42]。
另一条凋亡途径从线粒体开始,由细胞内凋亡信号(生长因子缺乏、新陈代谢失衡等)引起,主要受Bcl-2蛋白家族调控[43-44]。PPARγ激动剂吡格列酮可增加PGC1α(线粒体生物发生主调节因子)、UCP2(已知能减少ROS产生的线粒体蛋白)和细胞色素氧化酶亚基COX1的表达[54];同时可以抑制Bcl2和BclX配体的表达[54],促进线粒体释放细胞色素C,启动细胞凋亡。在啮齿动物中,黄体和颗粒细胞的活力受抗细胞凋亡Bcl2蛋白和肿瘤抑制因子p53表达的调节[43-44]。PPARγ激活研究表明,RXR在一种激动剂的诱导下结合THRs作用于线粒体Bcl2,使Bcl2构型转变后诱导细胞色素C释放引起细胞凋亡[55]。哺乳动物(绵羊)中PPARγ活化可影响颗粒细胞的增殖,但对凋亡标志物没有影响[12]。
另外,PPARβ激动剂能通过影响血管生成素-1(angiopoietin-1, ANGPT1)的表达[56]来调节类固醇生成,增加PCNA表达,减少卵巢细胞凋亡,并刺激大卵泡中的细胞增殖[34]。
3 RXRs在季节性发情中的研究进展 3.1 季节性光照变化中THRs/RXR异二聚体的作用THRs和RXRs形成的异二聚体通过调控KISS或者GnRH的释放影响动物季节性发情[4],并且THRs/RXR异二聚体调节季节性发情的功能在黑岩鱼[9]、日本鹌鹑[57]、绵羊[5]以及啮齿动物[58]中得到了证明。对黑岩鱼(Sebastes schlegelii)的研究证明,GnRH基因启动子中含有RXRs和THRs的结合位点[9]。Kaššák等[58]发现,叙利亚黄金仓鼠TH合成被抑制后,GnRH单极神经元数目显著增多,并且在繁殖季节,绵羊下丘脑的GnRH神经元会急剧增加[59],在绵羊下丘脑中TH受体a(THRα)与47%的GnRH神经元共表达于下丘脑基底部,暗示TH通过其受体THRα直接作用于GnRH神经元[60]。TRα2在缺乏配体时有显著负调控作用,对GnRH的分泌产生潜在影响[61]。
3.2 季节性营养代谢变化过程中PPARs/RXRs和LXRs/RXRs的作用PPARs/RXRs和LXR/RXRs信号通路均为脂质代谢调控通路[62]。而PPARγ/RXRα作为繁殖方面的能量传感器,可向性腺轴组织和生殖细胞反映身体的营养状态,在这种情况下,PPARγ/RXRα成为了季节变化过程中能量代谢和繁殖活动的关键因子[63]。季节性繁殖动物发情规律可以随脂肪储存和代谢等营养情况发生变化,这在牧区尤为常见,例如绵羊为适应干旱的草场可推迟发情等。
瘦素(leptin)及其受体(leptin receptor, LEPR)的主要作用是参与摄食活动的调节,调节机体能量代谢的平衡,也是作为调控季节性繁殖的候选基因,当体内脂肪含量上升时,脂肪中分泌进入血液的瘦素量增加,通过LEPR刺激下丘脑GnRH释放[64]。存在PPARγ/RXRα-IL-leptin(LEPR)-GnRH和PPARγ/RXRα-TNF-leptin(LEPR)-GnRH两条通路:细胞介素(IL-a)直接作用于脂肪细胞,刺激瘦素生成,肿瘤坏死因子(TNF-a)系统可直接促进瘦素基因表达,血清瘦素水平升高。PPARγ/RXRα可调控上述这几种因子表达,饲喂类视黄酸可降低小鼠肝TNF、IL-6和IL-1 mRNA的丰度,进而降低LEPR表达[65]。PPARγ配体有调节IL分泌的功能[66],也有研究表明,PPARγ/RXRα可调节leptin表达[67],证明PPARγ/RXRα通过间接调控LEPR的表达影响动物季节性发情以及发情周期[10]。
3.3 季节性温度变化下RXRs发挥的作用气温在一年四季中存在冷热交替的变化规律,按温度一年分为热季(长日照:3~8月份)和冷季(短日照:9月份至次年2月份),相对于热季(低繁殖季节),沼泽水牛(Bubalus bulalis)在冷季(高繁殖季节)发情周期提前,优势卵泡最大尺寸明显增加。水牛发情行为记录主要集中在早晨和傍晚的较冷时间,而白天则停止交配[68]。为了适应环境,一些鸟类随气候迁徙,也有一些鸟类为适应温度变化生成自己独有的抗应激方法,日本鹌鹑在天气冷时肝分泌T3,提高周身甲状腺激素水平,激活THRs/RXRs通路调控鸟类季节性发情[57]。
3.4 PPARs/RXRs在垂体中对促性腺激素分泌的作用在绵羊和人脑垂体[12]和啮齿动物下丘脑[69]中检测到PPARs几种亚型的表达,而且,最近的研究数据表明,PPARγ在垂体功能的调节中起直接作用[70]。PPARγ被激活后可抑制LH和FSH基因表达,PPARγ的条件性敲除导致雌性小鼠LH水平升高和产仔数减少[70]。然而,PPARg被激活后不影响催乳素(PRL)、卵泡刺激素(FSH)或黄体生成素(LH)的分泌[70]。
研究显示,猪垂体中GnRH受体(GnRH receptor, GnRHR)基因-279/-274处存在RXRE结合位点,可与RXRα或RXRβ结合,RXRs被新鉴定为GnRHR启动子活性的调节剂[71],也有研究表明,PPARs能调节GnRHR,并进一步诱导LH转录[63],证明PPARs可与RXRα或RXRβ形成二聚体直接调控GnRHR。另外,PPAR和RXRA能直接结合LH近端启动子区域,也能结合LHB增强子并与LH转录因子相互作用[63],发挥LH的作用。雌二醇在卵泡发育阶段的主要作用是促进垂体中FSH的合成与释放,但GnRHR上也有雌激素受体结合元件[9],而PPAR/RXR可以竞争结合雌激素受体元件,但不发挥作用,可直接影响FSH的合成与释放[32]。
3.5 RXRs其他二聚体对季节性繁殖的影响RARs/RXRs异二聚体参与下丘脑神经元和胎盘细胞中GnRH基因的差异调节[72]。RAR的配体与RXRs的配体调节GnRH的功能不同。例如:RARs配体全反式维甲酸(全反式-RA)通过大鼠GnRH基因的远端启动子元件增强其转录[73]。同时使用RAR和RXR配体全反式视黄酸和9-cisRA体外处理人神经元细胞可减少细胞中的GnRH基因表达,但在人胎盘绒毛膜癌细胞的处理显示增加GnRH基因表达,表明RAR和RXR影响GnRH基因表达存在细胞特异性[72]。在大鼠中,与全反式RA对GnRH转录的刺激作用相反,RXR配体9-顺式-RA以剂量和时间依赖性方式抑制GnRH启动子活性[74]。这些结果也验证了作为RARs的异二聚体,RXRs不是一个结合配体,而是一个辅助因子。更有意思的是,9-cisRA负调控GnRH表达,但是通过RXRs过表达载体的共转染显著增加了GnRH启动子驱动的荧光素酶活性,而用9-cisRA处理,不仅使RXRs过表达的增强作用无效,而且还使基础GnRH启动子驱动的荧光素酶活性降低50%[74]。上述证据表明,在没有(或减少)其同源配体9-cisRA的条件下,RXRs有助于维持GnRH基因转录。
综合上述几方面的研究结果,以短日照季节自然条件为例对RXRs及其二聚体在动物季节性繁殖中的调控作用做了简单总结,见图 2。
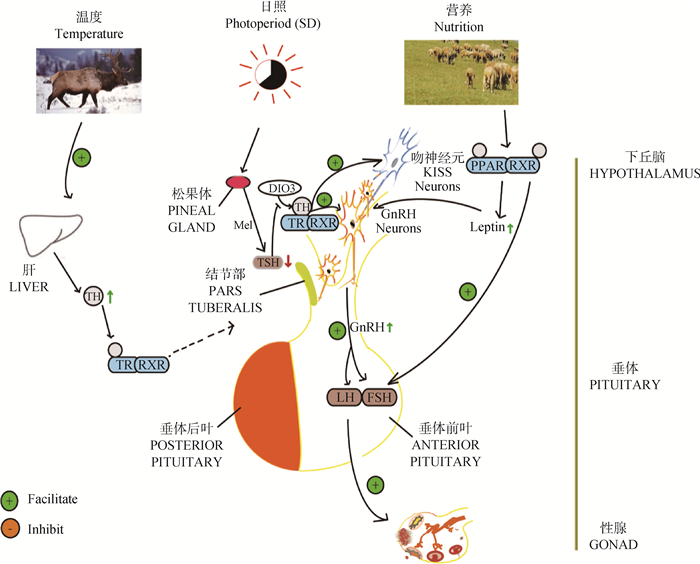
|
图 2 短日照季节下RXRs二聚体在季节性繁殖神经内分泌方面的调控作用 Fig. 2 Regulatory effect of RXRs dimer on seasonal reproduction neuroendocrine in short-day season |
RXRs参与组成的二聚体对动物繁殖发挥着多种重要的调控作用。但是之前的研究多集中于分析二聚体中某个成员在发情周期和季节性发情中的作用,而对于RXRs与THRs、PPARs和RARs等核受体组成的二聚体研究相对较少。更重要的是,不同二聚体又可以发挥不同的作用。因此,目前RXRs组成的二聚体在发情周期和季节性发情中的具体分子机制不够明晰。在对其进一步研究时,应将RXRs与THRs、PPARs和RARs等核受体作为一个整体分析其对动物繁殖性状的调控作用,考虑二聚体的两个成员间的协同作用,以更加深入、完善的认识动物繁殖关键通路和分子机制。
| [1] | GAMPE R J Jr, MONTANA V G, LAMBERT M H, et al. Structural basis for autorepression of retinoid X receptor by tetramer formation and the AF-2 helix[J]. Genes Dev, 2000, 14(17): 2229–2241. DOI: 10.1101/gad.802300 |
| [2] | PINTO V M S, MINAKHINA S, QIU S Q, et al. Naturally occurring amino acids in helix 10 of the thyroid hormone receptor mediate isoform-specific TH gene regulation[J]. Endocrinology, 2017, 158(9): 3067–3078. DOI: 10.1210/en.2017-00314 |
| [3] | WILLY P J, MANGELSDORF D J. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR[J]. Genes Dev, 1997, 11(3): 289–298. DOI: 10.1101/gad.11.3.289 |
| [4] |
李华振, 刘武军, 刘秋月, 等. 甲状腺激素受体基因调控动物繁殖的研究进展[J]. 畜牧兽医学报, 2019, 50(2): 243–252.
LI H Z, LIU W J, LIU Q Y, et al. Research progress on the regulative role of thyroid hormone receptor gene in animal reproduction[J]. Acta Veterinaria et Zootechnica Sinica, 2019, 50(2): 243–252. (in Chinese) |
| [5] | SINGH B K, SINHA R A, OHBA K, et al. Role of thyroid hormone in hepatic gene regulation, chromatin remodeling, and autophagy[J]. Mol Cell Endocrinol, 2017, 458: 160–168. DOI: 10.1016/j.mce.2017.02.018 |
| [6] | KERSTEN S, PAN L, CHAMBON P, et al. Role of ligand in retinoid signaling.9-cis-retinoic acid modulates the oligomeric state of the retinoid X receptor[J]. Biochemistry, 1995, 34(42): 13717–13721. DOI: 10.1021/bi00042a001 |
| [7] | HEYMAN R A, MANGELSDORF D J, DYCK J A, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor[J]. Cell, 1992, 68(2): 397–406. DOI: 10.1016/0092-8674(92)90479-V |
| [8] | BOGACKA I, KURZYNSKA A, BOGACKI M, et al. Peroxisome proliferator-activated receptors in the regulation of female reproductive functions[J]. Folia Histochem Cytobiol, 2015, 53(3): 189–200. DOI: 10.5603/fhc.a2015.0023 |
| [9] |
周晓苏, 王旭波, 宋华玉, 等. 许氏平鲉三种促性腺激素释放激素基因的启动子克隆及生物信息学分析[J]. 中国海洋大学学报, 2012, 42(10): 58–64.
ZHOU X S, WANG X B, SONG H Y, et al. Cloning and bioinformati analysis of the promoters from three gonadotropin-releasing hormone genes of sebastes schlegelii[J]. Periodical of Ocean University of China, 2012, 42(10): 58–64. (in Chinese) |
| [10] | ROSS R A, LEON S, MADARA J C, et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse[J]. eLife, 2018, 2(7): e35960. |
| [11] | KLIEWER S A, UMESONO K, NOONAN D J, et al. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors[J]. Nature, 1992, 358(6389): 771–774. DOI: 10.1038/358771a0 |
| [12] | FROMENT P, FABRE S, DUPONT J, et al. Expression and functional role of peroxisome proliferator-activated receptor-γ in ovarian folliculogenesis in the sheep[J]. Biol Reprod, 2003, 69(5): 1665–1674. DOI: 10.1095/biolreprod.103.017244 |
| [13] | VISWAKARMA N, JIA Y Z, BAI L, et al. Coactivators in PPAR-regulated gene expression[J]. PPAR Res, 2010, 2010: 250126. |
| [14] | NESTLER J E, STOVALL D, AKHTER N, et al. Strategies for the use of insulin-sensitizing drugs to treat infertility in women with polycystic ovary syndrome[J]. Fertil Steril, 2002, 77(2): 209–215. DOI: 10.1016/S0015-0282(01)02963-6 |
| [15] | KOMAR C M, BRAISSANT O, WAHLI W, et al. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period[J]. Endocrinology, 2001, 142(11): 4831–4838. DOI: 10.1210/endo.142.11.8429 |
| [16] | KOMAR C M, CURRY T J Jr. Localization and expression of messenger RNAs for the peroxisome proliferator-activated receptors in ovarian tissue from naturally cycling and pseudopregnant rats[J]. Biol Reprod, 2002, 66(5): 1531–1539. DOI: 10.1095/biolreprod66.5.1531 |
| [17] | VIERA-VERA J, GARCIA-ARRARAS J E. Molecular characterization and gene expression patterns of retinoid receptors, in normal and regenerating tissues of the sea cucumber, Holothuria glaberrima[J]. Gene, 2018, 654: 23–35. DOI: 10.1016/j.gene.2018.01.102 |
| [18] | SWIFT C B, HAYS J L, PETTY W J. Distinct functions of retinoic acid receptor beta isoforms: implications for targeted therapy[J]. Endocr Metab Immune Disord Drug Targets, 2008, 8(1): 47–50. DOI: 10.2174/187153008783928389 |
| [19] | XU X C. Tumor-suppressive activity of retinoic acid receptor-β in cancer[J]. Cancer Lett, 2007, 253(1): 14–24. DOI: 10.1016/j.canlet.2006.11.019 |
| [20] | TANG D, FAKIOLA M, SYN G, et al. Arylsulphatase A pseudodeficiency (ARSA-PD), hypertension and chronic renal disease in Aboriginal Australians[J]. Sci Rep, 2018, 8(1): 10912. DOI: 10.1038/s41598-018-29279-9 |
| [21] | MIZUNUMA H, LIU X W, ANDOH K, et al. Activin from secondary follicles causes small preantral follicles to remain dormant at the resting stage[J]. Endocrinology, 1999, 140(1): 37–42. DOI: 10.1210/endo.140.1.6409 |
| [22] | ANTOSIK P, KEMPISTY B, JACKOWSKA M, et al. Differential expression of genes encoding EGF, IGF-I, TGFβ1, TGFβ2 and TGFβ3 in porcine endometrium during estrus cycle at different ages[J]. Med Weter, 2010, 66(9): 618–621. |
| [23] | LU R, ITO J, IWAMOTO N, et al. FGF-1 induces expression of LXRα and production of 25-hydroxycholesterol to upregulate the apoE gene in rat astrocytes[J]. J Lipid Res, 2009, 50(6): 1156–1164. DOI: 10.1194/jlr.M800594-JLR200 |
| [24] | WAN X J, WANG S B, XU J R, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation[J]. PLoS One, 2017, 12(3): e173174. |
| [25] | ARMSTRONG D G, WEBB R. Ovarian follicular dominance: the role of intraovarian growth factors and novel proteins[J]. Rev Reprod, 1997, 2(3): 139–146. DOI: 10.1530/ror.0.0020139 |
| [26] |
白爱红, 付秀虹, 李荣香, 等. 多囊卵巢综合征患者血清miR-206和IGF-1的表达水平及临床意义[J]. 中国妇幼保健, 2019, 34(1): 139–142.
BAI A H, FU X H, LI R X, et al. Expression levels of serum miR-206 and IGF-1 in patients with polycystic ovary syndrome and its clinical significance[J]. Maternal and Child Health Care of China, 2019, 34(1): 139–142. (in Chinese) |
| [27] | NICKKHO-AMIRY M, MCVEY R, HOLLAND C. Peroxisome proliferator-activated receptors modulate proliferation and angiogenesis in human endometrial carcinoma[J]. Mol Cancer Res, 2012, 10(3): 441–453. DOI: 10.1158/1541-7786.MCR-11-0233 |
| [28] | JAGARLAMUDI K, LIU L, ADHIKARI D, et al. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation[J]. PLoS One, 2009, 4(7): e6186. DOI: 10.1371/journal.pone.0006186 |
| [29] | DE VRIES W N, BINNS L T, FANCHER K S, et al. Expression of Cre recombinase in mouse oocytes: a means to study maternal effect genes[J]. Genesis, 2000, 26(2): 110–112. DOI: 10.1002/(SICI)1526-968X(200002)26:2<110::AID-GENE2>3.0.CO;2-8 |
| [30] | KIM J, SATO M, LI Q, et al. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice[J]. Mol Cell Biol, 2008, 28(5): 1770–1782. DOI: 10.1128/MCB.01556-07 |
| [31] | YUAN Y, YEH L K, LIU H S, et al. Targeted overexpression of TGF-α in the corneal epithelium of adult transgenic mice induces changes in anterior segment morphology and activates noncanonical Wnt signaling[J]. Invest Ophthalmol Vis Sci, 2013, 54(3): 1829–1837. DOI: 10.1167/iovs.12-11477 |
| [32] | KELLER H, GIVEL F, PERROUD M, et al. Signaling cross-talk between peroxisome proliferator-activated receptor/retinoid X receptor and estrogen receptor through estrogen response elements[J]. Mol Endocrinol, 1995, 9(7): 794–804. |
| [33] | LEE S J, YANG E K, KIM S G. Peroxisome proliferator-activated receptor-γ and retinoic acid X receptor α represses the TGFβ1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: role for Zf9 dephosphorylation[J]. Mol Pharmacol, 2006, 70(1): 415–425. |
| [34] | PARBORELL F, ABRAMOVICH D, IRUSTA G, et al. Angiopoietin 1 reduces rat follicular atresia mediated by apoptosis through the PI3K/Akt pathway[J]. Mol Cell Endocrinol, 2011, 343(1-2): 79–87. DOI: 10.1016/j.mce.2011.06.009 |
| [35] | BAKER J, HARDY M P, ZHOU J, et al. Effects of an Igf1 gene null mutation on mouse reproduction[J]. Mol Endocrinol, 1996, 10(7): 903–918. |
| [36] | FROMENT P, GIZARD F, DEFEVER D, et al. Peroxisome proliferator-activated receptors in reproductive tissues: from gametogenesis to parturition[J]. J Endocrinol, 2006, 189(2): 199–209. DOI: 10.1677/joe.1.06667 |
| [37] | BOGACKA I, BOGACKI M. The quantitative expression of peroxisome proliferator activated receptor (PPAR) genes in porcine endometrium through the estrous cycle and early pregnancy[J]. J Physiol Pharmacol, 2011, 62(5): 559–565. |
| [38] | HE Y, WANG C L. Effects of testosterone on PPARγ and P450arom expression in polycystic ovary syndrome patients and related mechanisms[J]. Eur Rev Med Pharmacol Sci, 2018, 22(6): 1549–1553. |
| [39] | IUORNO M J, NESTLER J E. Insulin-lowering drugs in polycystic ovary syndrome[J]. Obstetr Gyneco Clin North Amer, 2001, 28(1): 153–164. |
| [40] | MU Y M, YANASE T, NISHI Y, et al. Combined treatment with specific ligands for PPARγ: RXR nuclear receptor system markedly inhibits the expression of cytochrome P450arom in human granulosa cancer cells[J]. Mol Cell Endocrinol, 2001, 181(1-2): 239–248. DOI: 10.1016/S0303-7207(00)00457-3 |
| [41] | CUI Y Z, MIYOSHI K, CLAUDIO E, et al. Loss of the peroxisome proliferation-activated receptor gamma (PPARγ) does not affect mammary development and propensity for tumor formation but leads to reduced fertility[J]. J Biol Chem, 2002, 277(20): 17830–17835. DOI: 10.1074/jbc.M200186200 |
| [42] | MINGE C E, RYAN N K, VAN DER HOEK K H, et al. Troglitazone regulates peroxisome proliferator-activated receptors and inducible nitric oxide synthase in murine ovarian macrophages[J]. Biol Reprod, 2006, 74(1): 153–160. DOI: 10.1095/biolreprod.105.043729 |
| [43] | LOVEKAMP-SWAN T, CHAFFIN C L. The peroxisome proliferator-activated receptor γ ligand troglitazone induces apoptosis and p53 in rat granulosa cells[J]. Mol Cell Endocrinol, 2005, 233(1-2): 15–24. DOI: 10.1016/j.mce.2005.01.011 |
| [44] | TINFO N, KOMAR C. Potential role for peroxisome proliferator-activated receptor γ in regulating luteal lifespan in the rat[J]. Reproduction, 2007, 133(1): 187–196. DOI: 10.1530/REP-06-0134 |
| [45] | HIROMORI Y, YUI H, NISHIKAWA J I, et al. Organotin compounds cause structure-dependent induction of progesterone in human choriocarcinoma Jar cells[J]. J Steroid Biochem Mol Biol, 2016, 155: 190–198. DOI: 10.1016/j.jsbmb.2014.10.010 |
| [46] | LEBOVIC D I, KIR M, CASEY C L. Peroxisome proliferator-activated receptor-γ induces regression of endometrial explants in a rat model of endometriosis[J]. Fertil Steril, 2004, 82(3): 1008–1013. |
| [47] | KURZYNSKA A, CHOJNOWSKA K, BOGACKI M, et al. PPAR ligand association with prostaglandin F2α and E2 synthesis in the pig corpus luteum-an in vitro study[J]. Anim Reprod Sci, 2016, 172: 157–163. DOI: 10.1016/j.anireprosci.2016.07.014 |
| [48] | DARNERUD P O, RISBERG S. Tissue localisation of tetra- and pentabromodiphenyl ether congeners (BDE-47, -85 and -99) in perinatal and adult C57BL mice[J]. Chemosphere, 2006, 62(3): 485–493. DOI: 10.1016/j.chemosphere.2005.04.004 |
| [49] | DUPONT J, REVERCHON M, CLOIX L, et al. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer[J]. Int J Dev Biol, 2012, 56(10-12): 959–967. |
| [50] | ZHANG H, LI Q L, LIN H Y, et al. Role of PPARγ and its gonadotrophic regulation in rat ovarian granulosa cells in vitro[J]. Neuro Endocrinol Lett, 2007, 28(3): 289–294. |
| [51] | CAO X H, LIU W, LIN F, et al. Retinoid X receptor regulates Nur77/thyroid hormone receptor 3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting[J]. Mol Cell Biol, 2004, 24(22): 9705–9725. DOI: 10.1128/MCB.24.22.9705-9725.2004 |
| [52] | BONOFIGLIO D, GABRIELE S, AQUILA S, et al. Peroxisome proliferator-activated receptor gamma activates fas ligand gene promoter inducing apoptosis in human breast cancer cells[J]. Breast Cancer Res Treat, 2009, 113(3): 423–434. |
| [53] | BELANI M, DEO A, SHAH P, et al. Differential insulin and steroidogenic signaling in insulin resistant and non-insulin resistant human luteinized granulosa cells-A study in PCOS patients[J]. J Steroid Biochem Mol Biol, 2018, 178: 283–292. DOI: 10.1016/j.jsbmb.2018.01.008 |
| [54] | HYUN S, KIM M S, SONG Y S, et al. Peroxisome proliferator-activated receptor-gamma agonist 4-O-methylhonokiol induces apoptosis by triggering the intrinsic apoptosis pathway and inhibiting the PI3K/Akt survival pathway in SiHa human cervical cancer cells[J]. J Microbiol Biotechnol, 2015, 25(3): 334–342. |
| [55] | SZANTO A, NARKAR V, SHEN Q, et al. Retinoid X receptors: X-ploring their (patho)physiological functions[J]. Cell Death Differ, 2004, 11(2): S126–S143. |
| [56] | HUANG Y S, CHANG C W, CHEN Y M, et al. Investigating expression profiles of VEGF-Flk, and Angpt1 during development of gas glands in Japanese eel (Anguilla japonica)[J]. Comp Biochem Physiol A: Mol Integr Physiol, 2010, 155(3): 350–360. DOI: 10.1016/j.cbpa.2009.11.021 |
| [57] | IKEGAMI K, YOSHIMURA T. Comparative analysis reveals the underlying mechanism of vertebrate seasonal reproduction[J]. Gen Comp Endocrinol, 2016, 227: 64–68. DOI: 10.1016/j.ygcen.2015.05.009 |
| [58] | KAŠŠÁK F, HÁNA V, SAUDEK V, et al. Novel mutation (T273R) in thyroid hormone receptor β gene provides further insight into cryptic negative regulation by thyroid hormone[J]. Folia Biol (Praha), 2017, 63(2): 60–66. |
| [59] | XIONG J J, KARSCH F J, LEHMAN M N. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in synaptic inputs onto GnRH neurons[J]. Endocrinology, 1997, 138(3): 1240–1250. DOI: 10.1210/endo.138.3.5000 |
| [60] | ANDERSON G M, CONNORS J M, HARDY S L, et al. Thyroid hormones mediate steroid-independent seasonal changes in luteinizing hormone pulsatility in the ewe[J]. Biol Reprod, 2002, 66(3): 701–706. DOI: 10.1095/biolreprod66.3.701 |
| [61] | BURGOS-TRINIDAD M, KOENIG R J. Dominant negative activity of thyroid hormone receptor variant α2 and interaction with nuclear corepressors[J]. Mol Cell Endocrinol, 1999, 149(1-2): 107–114. DOI: 10.1016/S0303-7207(98)00253-6 |
| [62] | DUFFY D J, KRSTIC A, HALASZ M, et al. Retinoic acid and TGF-β signalling cooperate to overcome MYCN-induced retinoid resistance[J]. Genome Med, 2017, 9(1): 15. DOI: 10.1186/s13073-017-0407-3 |
| [63] | SHIUE Y L, CHEN L R, TSAI C J, et al. Emerging roles of peroxisome proliferator-activated receptors in the pituitary gland in female reproduction[J]. Biomark Genom Med, 2013, 5(1-2): 1–11. DOI: 10.1016/j.gmbhs.2013.04.008 |
| [64] |
于嘉瑞, 王翔宇, 郭晓飞, 等. LEPR及LEP基因表达量对FecB不同基因型小尾寒羊产羔数的影响[J]. 畜牧兽医学报, 2018, 49(5): 954–961.
YU J R, WANG X Y, GUO X F, et al. Effects of expression of LEPR and LEP genes on litter size of Small Tail Han sheep with different FecB genotypes[J]. Acta Veterinaria et zootechnica Sinica, 2018, 49(5): 954–961. (in Chinese) |
| [65] | SHIMIZU M, SAKAI H, SHIRAKAMI Y, et al. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J- + Leprdb/+Leprdb mice[J]. Cancer Prev Res (Phila), 2011, 4(1): 128–136. DOI: 10.1158/1940-6207.CAPR-10-0163 |
| [66] | WANICHKUL T, HAN S W, HUANG R P, et al. Cytokine regulation by peroxisome proliferator-activated receptor gamma in human endometrial cells[J]. Fertil Steril, 2003, 79(Suppl 1): 763–769. |
| [67] | RUEBNER M, LANGBEIN M, STRISSEL P L, et al. Regulation of the human endogenous retroviral Syncytin-1 and cell-cell fusion by the nuclear hormone receptors PPARγ/RXRα in placentogenesis[J]. J Cell Biochem, 2012, 113(7): 2383–2396. DOI: 10.1002/jcb.24110 |
| [68] | TAILOR S P, JAIN L S, GUPTA H K, et al. Oestrus and conception rates in buffaloes under village conditions[J]. Indian J Anim Sci, 1990, 60(8): 1020–1021. |
| [69] | SARRUF D A, YU F, NGUYEN H T, et al. Expression of peroxisome proliferator-activated receptor-γ in key neuronal subsets regulating glucose metabolism and energy homeostasis[J]. Endocrinology, 2009, 150(2): 707–712. DOI: 10.1210/en.2008-0899 |
| [70] | SHARMA S, SHARMA P M, MISTRY D S, et al. PPARG regulates gonadotropin-releasing hormone signaling in LbetaT2 cells in vitro and pituitary gonadotroph function in vivo in mice[J]. Biol Reprod, 2011, 84(3): 466–475. DOI: 10.1095/biolreprod.110.088005 |
| [71] | CEDERBERG R A, SMITH J E, MCDONALD E A, et al. Activity of the porcine gonadotropin-releasing hormone receptor gene promoter is partially conferred by a distal gonadotrope specific element (GSE) within an upstream enhancing region, two proximal GSEs and a retinoid X receptor binding site[J]. Reprod Biol Endocrinol, 2015, 13: 45. DOI: 10.1186/s12958-015-0033-0 |
| [72] | HOO R L C, CHAN K Y Y, LEUNG F K Y, et al. Involvement of NF-κB subunit p65 and retinoic acid receptors, RARα and RXRα, in transcriptional regulation of the human GnRH Ⅱ gene[J]. FEBS J, 2007, 274(11): 2695–2706. DOI: 10.1111/j.1742-4658.2007.05804.x |
| [73] | CHO S, CHO H, GEUM D, et al. Retinoic acid regulates gonadotropin-releasing hormone (GnRH) release and gene expression in the rat hypothalamic fragments and GT1-1 neuronal cells in vitro[J]. Mol Brain Res, 1998, 54(1): 74–84. DOI: 10.1016/S0169-328X(97)00325-2 |
| [74] | CHO S, CHUNG J, HAN J, et al. 9-cis-Retinoic acid represses transcription of the gonadotropin-releasing hormone (GnRH) gene via proximal promoter region that is distinct from all-trans-retinoic acid response element[J]. Mol Brain Res, 2001, 87(2): 214–222. DOI: 10.1016/S0169-328X(01)00020-1 |



