2. 江苏高校动物重要疫病与人兽共患病防控协同创新中心, 扬州 225009;
3. 江苏省人兽共患病学重点实验室, 扬州 225009
2. Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou 225009, China;
3. Jiangsu Key Laboratory of Zoonosis, Yangzhou 225009, China
1α, 25-(OH)2D3是维生素D的活性形式,在多种生物或生理学过程中发挥作用。维生素D一个主要的功能是通过增加肠道对钙的吸收来维持钙的稳态[1],肠内钙吸收和肾内钙再吸收的增加可以促进骨骼矿化。来源于骨髓间充质干细胞(bone marrow mesenchymal stem cells, BMSCs)的成骨细胞(osteoblast,OB)发挥骨合成代谢作用[2],来源于造血干细胞的破骨细胞(osteoclast,OC)发挥骨分解代谢作用[3],使机体骨骼和钙磷维持动态平衡。BMSCs具有易分离、自我更新和分化成OB、软骨细胞、脂肪细胞等多种细胞的能力[4],因此它成为各种细胞的种子细胞。研究发现,人BMSCs表达维生素D受体(vitamin D receptor,VDR)和维生素D羟化酶(CYP27B1、CYP27A1、CYP24A1),1α, 25-(OH)2D3能够提升BMSCs向OB分化的能力[5]。一定浓度的1α, 25-(OH)2D3还可促进OB或BMSCs中核因子κB受体活化因子配体(receptor activator for nuclear factor-κB ligand,RANKL)的表达,降低骨保护素(osteoprotegerin,OPG)的表达来间接调控OC的形成[6-7],也可以直接促进RANKL诱导RAW264.7分化成成熟OC的作用[8]。
蛋鸡骨骼系统与哺乳动物存在差异,尤其产蛋期骨骼及钙磷代谢非常特殊[9],尽管体外RANKL和巨噬细胞集落刺激因子(macrophage colony stimulating factor,M-CSF)可促进小鼠或人骨髓单核巨噬细胞(bone marrow mononuclear macrophage,BMMs)生成具有骨吸收活性的成熟OC[10-12],但目前由于种属差异,仍无家禽来源的RANKL和M-CSF可用于诱导鸡BMMs分化成OC。鉴于报道认为1α, 25-(OH)2D3可诱导动物BMSCs及OB等分泌RANKL,从而调控BMSCs与BMMs共培养或OB与BMMs共培养体系中BMMs向成熟的OC分化[13-14]。因此,本研究在鸡BMSCs与BMMs体外共培养的基础上,添加不同浓度1α, 25-(OH)2D3,观察维生素D对体外鸡胚BMSCs和BMMs共培养体系中OC形成的影响,以期建立体外家禽OC培养体系,为家禽骨骼及钙磷代谢紊乱性疾病研究奠定基础。
1 材料与方法 1.1 实验动物SPF鸡胚,购自山东益吉达科技有限公司。
1.2 试剂和仪器胎牛血清(fetal bovine serum, FBS)(Gemini, USA);α-MEM(Gbico, USA);胰蛋白酶(Amresco, USA);Trizol(Invitrogen, USA);SYBER Premix TaqTM Ⅱ kit、PrimeScript RT reagent kit with gDNA eraser(TaKaRa, Japan);Ⅱ型碳酸酐酶(carbonic anhydrase Ⅱ, CAⅡ)兔源多克隆抗体,组织蛋白酶K(cathepsin K, CtsK)兔源多克隆抗体,基质金属蛋白酶9(matrix metalloproteinase 9, MMP-9)兔源多克隆抗体,甘油醛-3-磷酸脱氢酶(glyceraldehyde-3-phosphatedehydrogenase, GAPDH)兔源多克隆抗体(cell signal technology, USA),活化T细胞核因子c1(nuclear factor of activated T-cells, cytoplasmic 1, NFATc1)兔单克隆抗体(Abcam, UK);1α, 25-(OH)2D3,骨吸收培养板,抗酒石酸酸性磷酸酶(tartrate-resistant acid phosphatase,TRAP)(Sigma, USA)。
二氧化碳培养箱,SW-CJ-ZFD型生物安全柜,NanoDrop 2000(Thermo fisher, USA);DMI3000型倒置显微镜(Leica, Germany);Milli-Q Biocel型超纯水系统(Millipore, USA);DK-600型恒温水槽(上海精宏试验设备有限公司);ABI 7500 qRT-PCR仪(ABI, USA);PROTEAH电泳仪(BIO-RAD, USA)。
1.3 鸡胚BMSCs和BMMs的分离及共培养无菌条件下,分离若干18日龄鸡胚股骨和胫骨。剃净股骨和胫骨表面的肌肉和结缔组织,用5 mL注射器吸取无菌PBS冲洗骨髓腔至颜色发白。移液器轻轻吹打含有骨髓细胞的磷酸盐缓冲液(phosphate buffer,PBS)使细胞团块分散,再依次经过100和200目的无菌筛网过滤。过滤的细胞悬液,1 200 r·min-1离心5 min,弃上清。加入PBS再次重悬细胞,1 200 r·min-1离心5 min,弃上清。用含10% FBS的α-MEM培养基重悬细胞,接种于100 mm培养皿中,放入5%CO2、37 ℃培养48 h。48 h后,贴壁细胞即为BMSCs,未贴壁细胞即为BMMs。BMSCs分别传至96、6孔板、骨吸收培养板中,加入BMMs共同培养。培养液为含不同浓度(10-10、10-9、10-8 mol·L-1)1α, 25-(OH)2D3的α-MEM培养基,不添加1α, 25-(OH)2D3组为对照组。上述细胞培养5 d后,弃去96孔内的培养基,用PBS洗两遍,加入4%多聚甲醛室温固定15 min后,按照TRAP试剂盒说明书进行染色。倒置相差显微镜观察TRAP阳性多核(3个及3个以上核)OC并拍照。随机选取10个视野,对OC进行计数。
1.4 RT-PCR检测相关基因mRNA的表达用Trizol法提取6孔板共培养5 d或BMSCs单独培养5 d的细胞总RNA。通过痕量分光光度计NanoDrop2000对样品进行检测,确定提取总RNA的浓度及纯度(A260 nm/A280 nm比值)。以PrimeScript RT reagent kit with gDNA eraser反转录获取的cDNA为模板,检测CtsK、NFATc1、TRAP (Acp5)、MMP-9、CAⅡ、RANKL、OPG mRNA表达水平。查阅NCBI中的相应序列并设计引物(表 1)。反应体系:Power SYBR Green PCR Master Mix 10 μL、上、下游引物(10 μmol·L-1)各0.8 μL、ROX 0.4 μL,模板(cDNA)2 μL,加入无RNA酶水,使反应体系总体积为20 μL。RT-PCR扩增反应条件:95 ℃ 30 s,60 ℃ 34 s,40个循环。2-ΔΔCt法对实时荧光定量PCR试验结果进行数据分析,GAPDH为内参。
|
|
表 1 目的基因引物序列 Table 1 The primer sequence for target genes |
RIPA裂解液提取共培养5 d的各组蛋白样本。BCA法测定蛋白浓度,并调整一致。加入5×SDS loading buffer,100 ℃变性10 min。蛋白样品110 V凝胶电泳,90 min。湿转法110 V蛋白转印,90 min。转印完成后,配5%的脱脂乳室温封闭2 h,加入CAⅡ多克隆抗体,CtsK多克隆抗体,MMP-9多克隆抗体,NFATc1单克隆抗体及GAPDH抗体4 ℃孵育过夜,用TBST进行洗膜,共洗3次,每次洗10 min,再加入羊抗兔二抗,室温孵育2 h。移去二抗,TBST洗膜3次,每次10 min。ECL化学发光试剂显色,显影仪读片。
1.6 鸡胚OC骨吸收活性鉴定将BMSCs和BMMs接种于用于骨吸收分析的96孔培养板中培养5 d,弃去培养液,用PBS反复清洗,洗去贴壁细胞。用倒置相差显微镜观察骨吸收陷窝并拍照,Image-Pro Plus软件分析骨吸收陷窝的面积。
1.7 数据分析所有试验数值均用x±s表示。SPSS v.17.0软件t-test分析组间差异。P<0.05和P<0.01分别表示差异显著和差异极显著。
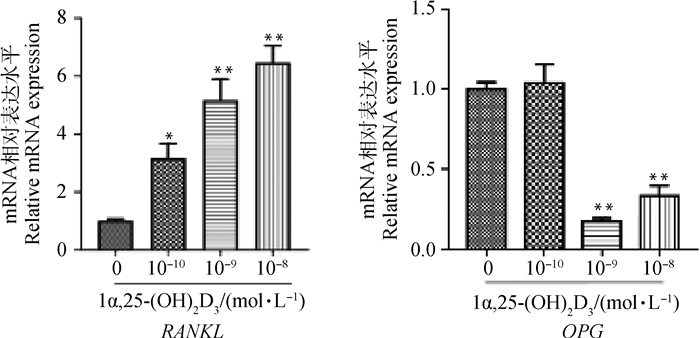
2 结果 2.1 不同浓度1α, 25-(OH)2D3对BMSCs中RANKL、OPG mRNA表达的影响qRT-PCR结果(图 1)表明,不同浓度1α, 25-(OH)2D3处理BMSCs 5 d,RANKL mRNA表达随浓度增加而增强,与对照组相比差异显著(P<0.05)或极显著(P<0.01)。而OPG mRNA表达随浓度增加而减弱。与对照组相比,10-9、10-8 mol·L-1 1α, 25-(OH)2D3均极显著抑制OPG mRNA表达(P<0.01)。
|
与对照组相比,*. P<0.05;**. P<0.01 *. P < 0.05; **. P < 0.01 vs control 图 1 不同浓度1α, 25-(OH)2D3对BMSCs RANKL、OPG mRNA表达的影响 Fig. 1 Effects of different concentrations of 1α, 25-(OH)2D3 on the expression of RANKL and OPG mRNA in BMSCs |
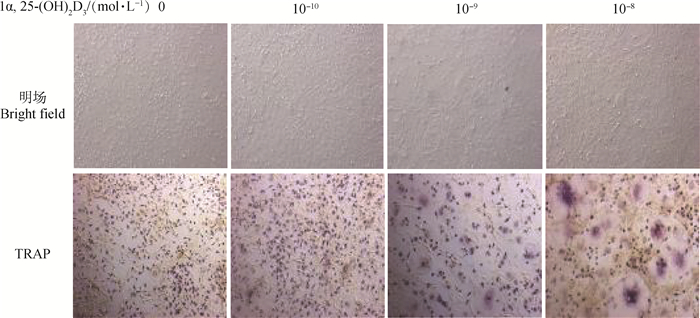
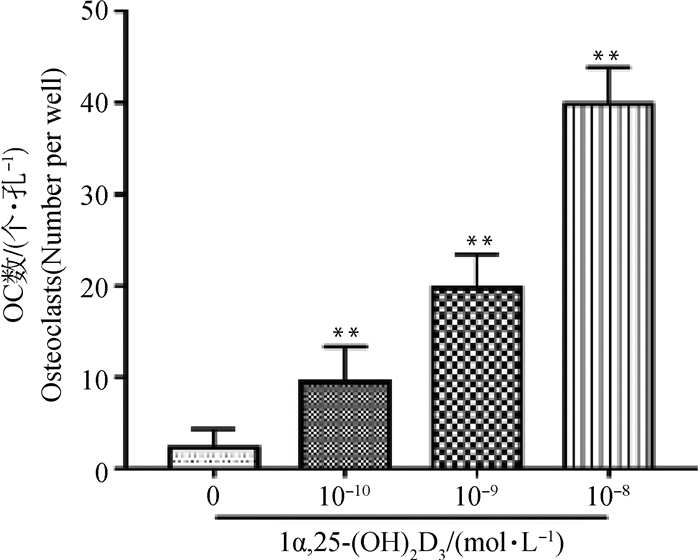
TRAP染色、计数结果可见,对照组[不添加1α, 25-(OH)2D3]细胞呈梭形,极少见细胞核多于3个及3个以上的OC。随着1α, 25-(OH)2D3浓度的升高,圆形、体积较大、细胞质呈酒红色的TRAP阳性的多核OC形成增多(图 2、3)。计数结果显示,与对照组相比,不同浓度1α, 25-(OH)2D3组形成的OC数量极显著增多(P<0.01),且10-8mol·L-1 1α, 25-(OH)2D3组形成的OC最多。
|
图 2 共培养OC在明场和TRAP染色后形态(200×) Fig. 2 The morphology of OC was observed at bright field and after TRAP staining in co-culture system(200×) |
|
与对照组相比,**. P<0.01 **. P < 0.01 vs control 图 3 OC数目统计 Fig. 3 The statistical chart of OC numbers |
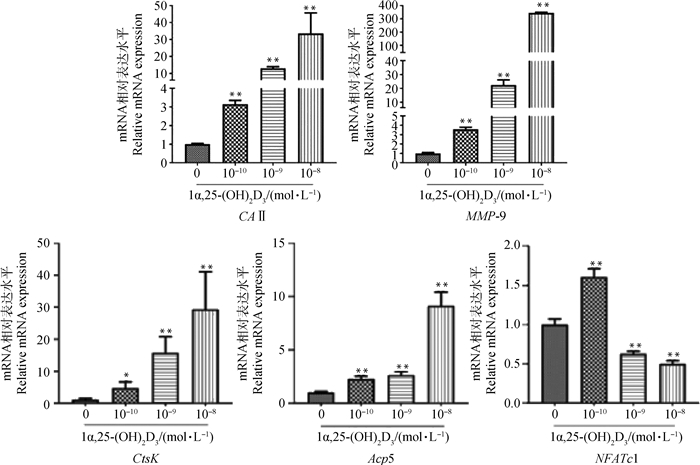
qRT-PCR结果(图 4)表明,不同浓度1α, 25-(OH)2D3培养5 d,OC标志性基因Acp5、CAⅡ、MMP-9、CtsK mRNA表达随浓度增加而增强,与对照组相比差异显著(P<0.05)或极显著(P<0.01)。而NFATc1 mRNA表达在各1α, 25-(OH)2D3处理组随浓度增加(10-10、10-9、10-8 mol·L-1)而下降,与对照组相比差异均极显著(P<0.01)。
|
与对照组相比,*. P<0.05;**. P<0.01 *. P < 0.05; **. P < 0.01 vs control 图 4 1α, 25-(OH)2D3对OC标志基因mRNA表达的影响 Fig. 4 Effect of 1α, 25-(OH)2D3on mRNA expression of OC marker genes |
Western blot结果(图 5)显示,1α, 25-(OH)2D3处理共培养细胞可表达NFATc1、CtsK、MMP-9及CA Ⅱ蛋白。灰度值分析显示,不同浓度1α, 25-(OH)2D3培养 5 d,OC标志性蛋白CtsK、MMP-9、CAⅡ的表达随1α, 25-(OH)2D3浓度的增加而上升,与对照组相比差异显著(P<0.05)或极显著(P<0.01);各处理组NFATc1的蛋白表达水平随1α, 25-(OH)2D3浓度的增加而下降,与对照组相比差异显著(P<0.05)。
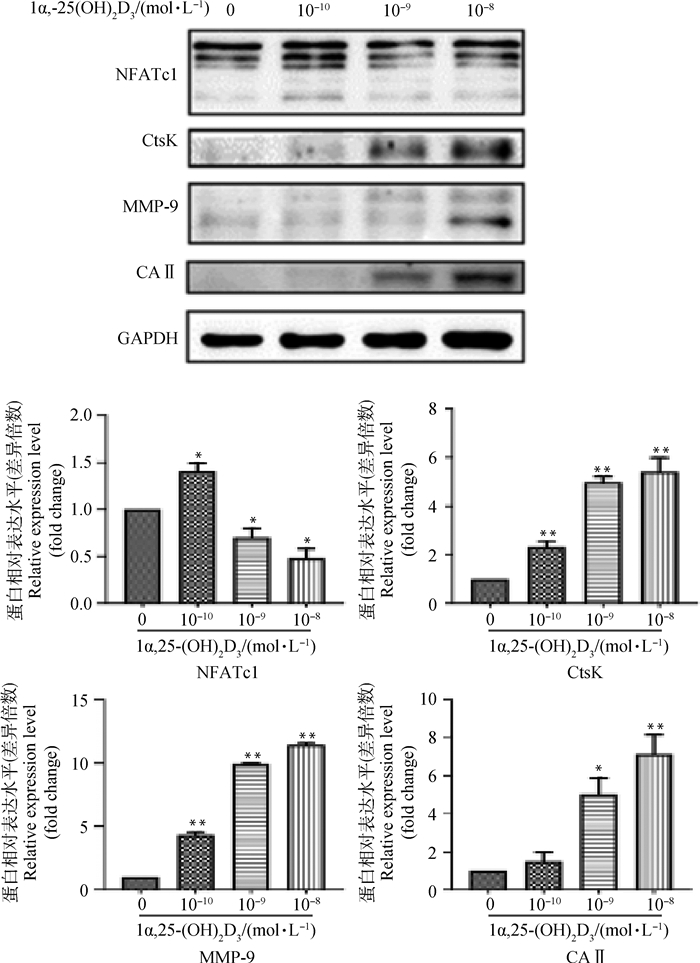
|
与对照组相比,*. P<0.05;**. P<0.01 *. P < 0.05; **. P < 0.01 vs control 图 5 1α, 25-(OH)2D3对OC分化标志性蛋白表达的影响 Fig. 5 Effect of 1α, 25-(OH)2D3on expression of OC marker proteins in differentiation |
骨吸收陷窝结果(图 6)表明,不添加细胞的空白培养板表面平整,无吸收陷窝。单独培养的BMSCs不具有骨吸收活性,培养板表面平整,无1α, 25-(OH)2D3处理的共培养组骨吸收陷窝极少,而添加10-8 mol·L-11α, 25-(OH)2D3共培养形成的OC能产生大量大小不一的骨吸收陷窝。对骨吸收面积进行分析(图 7),10-8 mol·L-1 1α, 25-(OH)2D3组骨吸收陷窝面积极显著大于不添加1α, 25-(OH)2D3组(P<0.01)。
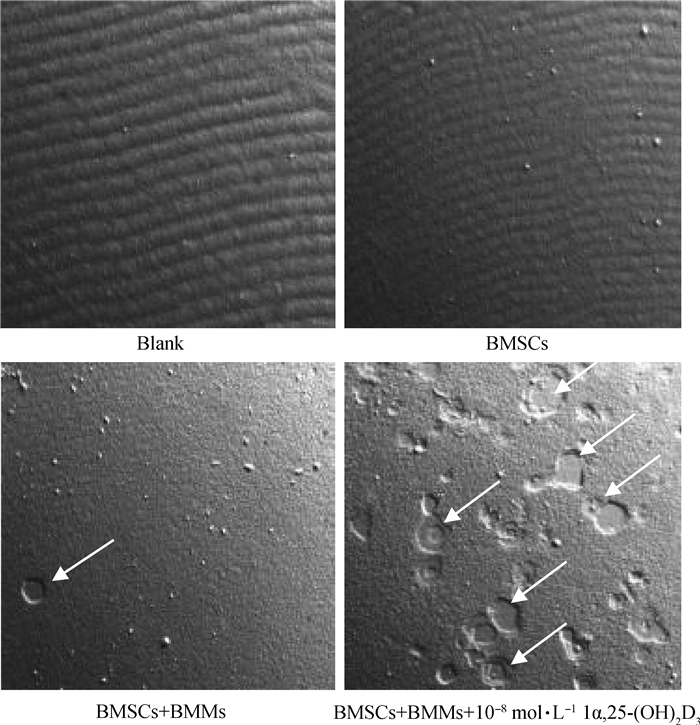
|
白色箭头所指为OC形成的骨吸收陷窝 The white arrow indicates the boneresorption notch formed by OC 图 6 骨吸收陷窝结果(50×) Fig. 6 Bone resorption lacuna results(50×) |
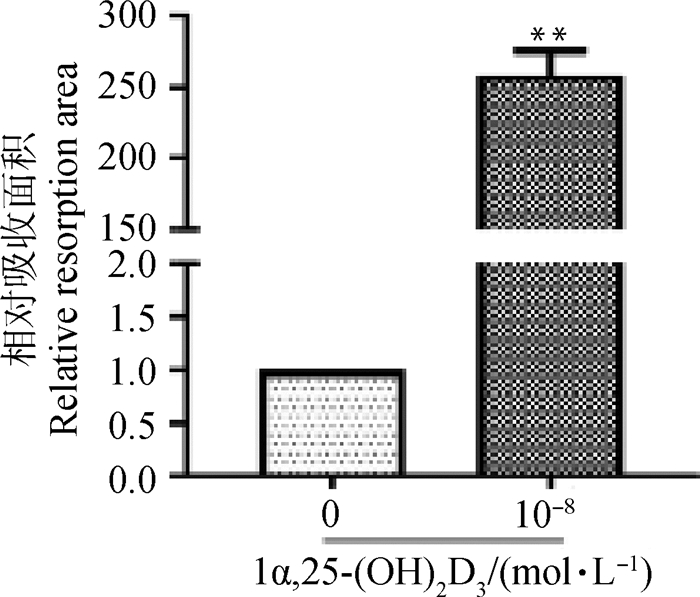
|
与对照组相比,**. P<0.01 **. P < 0.01 vs control 图 7 骨吸收陷窝面积统计 Fig. 7 The statistical chart of bone resorption lacuna area |
家禽骨骼发育异常,如肉仔鸡胫骨软骨发育不良(tibial dyschondroplasia,TD)[15]、佝偻病、股骨头坏死等均给家禽养殖业带来严重的经济损失,直接危及家禽的福利。就蛋鸡而言,目前笼养蛋鸡骨质疏松等疾病发病率仍较高[16],传统鸡笼中的蛋鸡易患骨质疏松症,主要原因是活动受限、缺乏锻炼以及蛋壳生成消耗大量的钙[17]等,这些因素导致笼养蛋鸡在产蛋期骨质疏松的发病率高达6.6%~15.7%,不仅可致骨质疏松性骨折的发生,更可导致产蛋下降或者停产、产软壳蛋等,给蛋鸡生产带来了巨大损失[18-19]。作为与钙磷存在相互调节关系的维生素D,它在家禽机体内也发挥着不可或缺的作用,血清25-(OH)D是维生素D的主要循环形式,较稳定,通常作为检测维生素D水平的指标[20]。根据相关文献的报道,正常人体内的25-(OH)D水平大约为5×10-8 mol·L-1[21]。体外研究中,10-10~10-8 mol·L-1 1α, 25-(OH)2D3是研究最多的生理浓度范围[22],因此,本试验选择接近机体正常生理水平的维生素D浓度范围进行探究。
OC是体内能够溶解骨有机质和无机质的细胞,在机体骨骼及钙磷内环境稳态中发挥重要作用[23]。因此OC形成及骨吸收功能机制的研究,有助于认识并防控家禽骨骼及钙磷代谢紊乱性疾病。在OC调控机制中,核因子κB受体活化因子(receptor activator of nuclear factor-κB,RANK)/RANKL/OPG轴是研究最深入的[24],该系统对骨骼的调控有着至关重要的作用[25];OB表达的RANKL与OC前体BMMs表面RANK结合[26],促进BMMs进一步融合分化成成熟的OC,并能增强OC的骨吸收功能。OPG属于分泌型蛋白[27],它可以通过阻止OC的分化、成熟而调节骨代谢,当骨吸收大于骨形成时,OB分泌更多OPG,阻止RANKL与RANK的相互作用[28-29],抑制骨吸收功能,抑制OC形成,使骨内环境继续保持相对稳定状态。根据本实验室前期摸索及Song等[30]的研究,BMMs诱导培养5 d即可形成大量OC,用于分子生物学研究。因此,本研究采用BMSCs与BMMs共培养,添加不同浓度1α, 25-(OH)2D3处理5 d,结果显示1α, 25-(OH)2D3能促进RANKL mRNA表达、抑制OPG mRNA,其中10-8 mol·L-1 1α, 25-(OH)2D3效果最明显。与Cong等[7]发现10-8 mol·L-1 1α, 25-(OH)2D3能够促进小鼠OB RANKL的表达,从而促进OC的形成相似。
共培养模型可分为transwell培养、可拆卸型渗透性装置培养、直接培养、选择性培养基培养[31]。研究表明,将MC3T3-E1和RAW264.7在transwell小室中进行共培养,OPG在基因和蛋白水平的表达均低于单一培养组,而RANKL基因和蛋白表达水平高于单一培养组,OC形成增加[32]。本试验利用直接共培养模型探究了1α, 25-(OH)2D3对BMSCs与BMMs共培养过程中OC形成及骨吸收活性的影响。1α, 25-(OH)2D3处理5 d,对OC标志蛋白和mRNA表达均有促进作用,但抑制NFATc1的蛋白和mRNA表达水平,其中10-8 mol·L-1 1α, 25-(OH)2D3效果最明显。对NFATc1呈现的抑制作用跟OC形成过程中的负反馈调节机制相关[33],具体还有待于进一步研究。由于骨吸收活性是OC重要的生物学功能,是检验体外培养的OC是否具有生物学功能的标准[34],因此本研究亦检测了OC的骨吸收活性。10-8 mol·L-1 1α, 25-(OH)2D3处理共培养5 d的BMSCs与BMMs,所形成的OC能在骨吸收培养板上产生大量深浅不一、不规则形状的骨吸收陷窝。由此可见,10-8 mol·L-1 1α, 25-(OH)2D3介导BMSCs与BMMs共培养形成的OC具有较强的生物学功能,为OC在家禽骨骼及钙磷代谢中的作用及机制研究奠定了基础。
4 结论10-10~10-8 mol·L-11α, 25-(OH)2D3均可促进SPF鸡胚来源BMSCs和BMMs共培养体系中OC的形成及相关蛋白的表达,其中10-8 mol·L-1 1α, 25-(OH)2D3效果最明显。所形成的OC具有明显的骨吸收活性,为进一步研究OC在家禽骨骼及钙磷代谢紊乱性疾病中的作用机制奠定了基础。
| [1] | GOLTZMAN D. Functions of vitamin D in bone[J]. Histochem Cell Biol, 2018, 149(4): 305–312. DOI: 10.1007/s00418-018-1648-y |
| [2] | HOU Y C, WU C C, LIAO M T, et al. Role of nutritional vitamin D in osteoporosis treatment[J]. Clin Chim Acta, 2018, 484: 179–191. DOI: 10.1016/j.cca.2018.05.035 |
| [3] | TAKAHASHI N, UDAGAWA N, SUDA T. Vitamin D endocrine system and osteoclasts[J]. Bonekey Rep, 2014, 3: 495. |
| [4] | TSAI T L, LI W J. Identification of bone marrow-derived soluble factors regulating human mesenchymal stem cells for bone regeneration[J]. Stem Cell Rep, 2017, 8(2): 387–400. DOI: 10.1016/j.stemcr.2017.01.004 |
| [5] | GENG S, ZHOU S H, BI Z G, et al. Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells[J]. Metabolism, 2013, 62(6): 768–777. DOI: 10.1016/j.metabol.2013.01.003 |
| [6] | WANG D, GU J H, FENG L L, et al. 1α, 25-dihydroxyvitamin D3 potentiates avian osteoclast activation by increasing the formation of zipper-like structure via Src/Rac1 signaling[J]. Biochem Biophys Res Commun, 2018, 501(2): 576–583. DOI: 10.1016/j.bbrc.2018.05.048 |
| [7] | CONG L, ZHANG C Y, TU G J. Osteoblastic NF-κB pathway is involved in 1α, 25(OH)2D3-induced osteoclast-like cells formation in vitro[J]. Int J Clin Exp Pathol, 2015, 8(5): 5988–5996. |
| [8] | GU J H, TONG X S, CHEN G H, et al. Effects of 1α, 25-(OH)2D3 on the formation and activity of osteoclasts in RAW264. 7 cells[J]. J Steroid Biochem Mol Bio, 2015, 152: 25–33. DOI: 10.1016/j.jsbmb.2015.04.003 |
| [9] | KÜÇÜKYILMAZ K, ERKEK R, BOZKURT M. The effects of boron supplementation of layer diets varying in calcium and phosphorus concentrations on performance, egg quality, bone strength and mineral constituents of serum, bone and faeces[J]. Br Poult Sci, 2014, 55(6): 804–816. DOI: 10.1080/00071668.2014.975782 |
| [10] |
耿欢, 累鸣, 刘水涛, 等. 体外冲击波对骨髓源巨噬细胞向破骨细胞分化及骨吸收活性的影响[J]. 中国医学前沿杂志, 2017, 9(2): 20–24.
GENG H, LEI M, LIU S T, et al. The effect of extracorporeal shock wave on osteoclast formation of bone marrow-derived macrophage and bone resorption activity[J]. Chinese Journal of the Frontiers of Medical Science (Electronic Version), 2017, 9(2): 20–24. (in Chinese) |
| [11] | DAI Q G, XIE F R, HAN Y J, et al. Inactivation of Regulatory-associated protein of mTOR (Raptor)/mammalian target of rapamycin complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice[J]. J Biol Chem, 2017, 292(1): 196–204. DOI: 10.1074/jbc.M116.764761 |
| [12] | ZHANG S, WANG X, LI G, et al. Osteoclast regulation of osteoblasts via RANK-RANKL reverse signal transduction in vitro[J]. Mol Med Rep, 2017, 16(4): 3994–4000. DOI: 10.3892/mmr.2017.7039 |
| [13] | SIMON D, DERER A, ANDES F T, et al. Galectin-3 as a novel regulator of osteoblast-osteoclast interaction and bone homeostasis[J]. Bone, 2017, 105: 35–41. DOI: 10.1016/j.bone.2017.08.013 |
| [14] | MCSHEEHY P M, CHAMBERS T J. 1, 25-Dihydroxyvitamin D3 stimulates rat osteoblastic cells to release a soluble factor that increases osteoclastic bone resorption[J]. J Clin Invest, 1987, 80(2): 425–429. DOI: 10.1172/JCI113089 |
| [15] | FIKADU W, TEGEGNE D, ABDELA N, et al. Milk fever and its economic consequences in dairy cows: a review[J]. Global Vet, 2016, 16(5): 441–452. |
| [16] | CLARK W D, COX W R, SILVERSIDES F G. Bone fracture incidence in end-of-lay high-producing, noncommercial laying hens identified using radiographs[J]. Poult Sci, 2008, 87(10): 1964–1970. DOI: 10.3382/ps.2008-00115 |
| [17] | REGMI P, SMITH N, NELSON N, et al. Housing conditions alter properties of the tibia and humerus during the laying phase in Lohmann white Leghorn hens[J]. Poult Sci, 2016, 95(1): 198–206. DOI: 10.3382/ps/pev209 |
| [18] |
夏令. 笼养蛋鸡产软壳蛋的原因及对策[J]. 现代畜牧科技, 2018(8): 34.
XIA L. Causes and countermeasures of soft-shell eggs produced by caged layer hens[J]. Modern Animal Husbandry Science & Technology, 2018(8): 34. (in Chinese) |
| [19] |
王静, 刘贵巧. 蛋鸡产薄壳、软壳蛋的原因分析[J]. 中国畜牧兽医文摘, 2017, 33(4): 96.
WANG J, LIU G Q. Analysis of reasons for laying eggs with thin and soft shells[J]. China Animal Husbandry & Veterinary Medicine Digest, 2017, 33(4): 96. (in Chinese) |
| [20] | TSUPRYKOV O, BUSE C, SKOBLO R, et al. Comparison of free and total 25-hydroxyvitamin D in normal human pregnancy[J]. J Steroid Biochem Mol Biol, 2019, 190: 29–36. DOI: 10.1016/j.jsbmb.2019.03.008 |
| [21] | MCVEY M K, GERAGHTY A A, O'BRIEN E C, et al. An exploratory analysis of associations of diet, sun exposure, and body composition with 25OHD at five years of age:findings from the ROLO Kids Study[J]. J Steroid Biochem Mol Biol, 2019, 188: 111–116. DOI: 10.1016/j.jsbmb.2018.12.014 |
| [22] | GU J H, TONG X S, CHEN G H, et al. Regulation of matrix metalloproteinase-9 protein expression by 1α, 25-(OH)2D3 during osteoclast differentiation[J]. J Vet Sci, 2014, 15(1): 133–140. DOI: 10.4142/jvs.2014.15.1.133 |
| [23] | CAPPARIELLO A, MAURIZI A, VEERIAH V, et al. The Great beauty of the osteoclast[J]. Arch Biochem Biophys, 2014, 558: 70–78. DOI: 10.1016/j.abb.2014.06.017 |
| [24] | BOYCE B F, XING L P. The RANKL/RANK/OPG pathway[J]. Curr Osteoporos Rep, 2007, 5(3): 98–104. DOI: 10.1007/s11914-007-0024-y |
| [25] | LIU W, ZHANG X L. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues[J]. Mol Med Rep, 2015, 11(5): 3212–3218. DOI: 10.3892/mmr.2015.3152 |
| [26] | CORRADO A, MARUOTTI N, CANTATORE F P. Osteoblast role in rheumatic diseases[J]. Int J Mol Sci, 2017, 18(6): 1272. DOI: 10.3390/ijms18061272 |
| [27] | PIRI F, KHOSRAVI A, MOAYERI A, et al. The effects of dietary supplements of calcium, vitamin D and estrogen hormone on serum levels of OPG and RANKL cytokines and their relationship with increased bone density in rats[J]. J Clin Diagn Res, 2016, 10(9): AF01–AF04. |
| [28] | WAN Q L, SCHOENMAKER T, JANSEN I D C, et al. Osteoblasts of calvaria induce higher numbers of osteoclasts than osteoblasts from long bone[J]. Bone, 2016, 86: 10–21. DOI: 10.1016/j.bone.2016.02.010 |
| [29] | XU F, DONG Y H, HUANG X, et al. Pioglitazone affects the OPG/RANKL/RANK system and increase osteoclastogenesis[J]. Mol Med Rep, 2016, 14(3): 2289–2296. DOI: 10.3892/mmr.2016.5515 |
| [30] | SONG C C, YANG X B, LEI Y S, et al. Evaluation of efficacy on RANKL induced osteoclast from RAW264. 7 cells[J]. J Cell Physiol, 2019, 234(7): 11969–11975. |
| [31] | ZHU S, EHNERT S, ROUB M, et al. From the clinical problem to the basic research—co-culture models of osteoblasts and osteoclasts[J]. Int J Mol Sci, 2018, 19(8): 2284. DOI: 10.3390/ijms19082284 |
| [32] |
莫国业, 张顺聪, 李永贤, 等. Transwell小室内建立小鼠成骨-破骨细胞共培养体系[J]. 中国骨伤, 2018, 31(3): 241–247.
MO G Y, ZHANG S C, LI Y X, et al. Establish mouse osteoblast-osteoclast cell co-culture system in a Transwell chamber[J]. China Journal of Orthopaedics and Traumatology, 2018, 31(3): 241–247. DOI: 10.3969/j.issn.1003-0034.2018.03.010 (in Chinese) |
| [33] | KIM J H, KIM N. Signaling pathways in osteoclast differentiation[J]. Chonnam Med J, 2016, 52(1): 12–17. DOI: 10.4068/cmj.2016.52.1.12 |
| [34] | SAMBANDAM Y, SUNDARAM K, SAIGUSA T, et al. NFAM1 signaling enhances osteoclast formation and bone resorption activity in Paget's disease of bone[J]. Bone, 2017, 101: 236–244. DOI: 10.1016/j.bone.2017.05.013 |

 图 1(Fig. 1)
图 1(Fig. 1)


