在模拟动物体内消化过程的方法研究中,消化酶活性的衰变可能会显著地影响体外模拟消化的程度及与体内消化的相关性。因此,探讨仿生消化过程中消化酶活性随消化时间的动力学变化非常关键。目前,在猪饲料的体外模拟消化中,以胃蛋白酶溶液模拟胃液,以胰液素或复合淀粉酶、胰蛋白酶、糜蛋白酶模拟体内小肠液已被普遍使用[1-6]。然而,在模拟消化过程中,模拟消化液都为一次性加入,消化酶活性是平稳还是衰减未见详细报道。Clunies和Leeson[7]、Johnston和Coon[8]、Huang等[9]的研究结果均表明,在猪、禽的模拟消化中胃蛋白酶、胰液素的消化酶活性初始浓度会影响饲料养分的消化程度。由此表明,体外消化过程中若消化酶呈现活性衰减可能会影响模拟消化的效果。由于猪胰液的分泌及消化道内消化酶活性均随采食呈周期性变化[10-11],因此,在体内虽然存在消化酶活性衰变的现象,但因有胰液的分泌而使消化酶的活性呈脉冲式变化。目前,在体外模拟消化中,研究者多集中在饲料养分消化率的动力学变化[12-15],而对模拟猪体内消化过程中消化酶活性的变化鲜见报道。
本试验探讨猪仿生消化过程中模拟消化液注入后消化酶活性的衰变规律以及补注消化酶后的变化情况。为完善模拟猪的体内消化过程,评定饲料养分生物学效价的方法提供参考。
1 材料与方法 1.1 试验材料以玉米、大豆粕、小麦麸、玉米-大豆粕型饲粮为研究对象,用万能粉碎机粉碎后过60目方形筛孔。采用抽真空充氮的方法密封于样本袋后,-20 ℃保存备用,其组成及营养水平见表 1。
|
|
表 1 原料与饲粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of feedstuffs or diet (air-dry basis) |
本研究分为2个试验。试验一:考察以玉米、大豆粕、小麦麸及玉米-大豆粕型饲粮为底物在猪胃-小肠两阶段仿生消化中一次性注入模拟胃液和小肠液后,检测消化液中消化酶活性随消化时间的变化规律。采用单因素完全随机设计,胃消化阶段在消化的0、1、2、3、4 h后取消化液样品,分析胃蛋白酶活性(单位体积的比活性及总活性。比活性为每根透析管中消化液单位体积下的消化酶活性,单位为U·mL-1;总活性为每根透析管中总体积下消化液的消化酶总活性,单位为kU)。小肠消化阶段在胃消化4 h后,注入模拟小肠液,分别在消化0、2、4、6、8、12、16 h后取消化液样品,分析胰蛋白酶、糜蛋白酶和淀粉酶的活性。每个时间点为一个处理,每个处理5个重复,每个重复1根仿生消化管。试验二:根据试验一中消化酶活性变化的规律,设置小肠阶段消化4 h时补注消化酶。考察以玉米、大豆粕、小麦麸、玉米-大豆粕型饲粮为底物在小肠仿生消化4、6、8 h消化液中消化酶活性随消化时间的变化规律。试验设计同试验一。
1.3 仿生消化及消化酶活的测定单胃动物仿生消化系统的原理与仿生消化方法参考Zhao等[3]、王钰明[16]的描述及《单胃动物仿生消化系统操作手册》第二版。试验一模拟胃液中胃蛋白酶活性为890 U·mL-1,每根仿生消化管中消化液体积为20 mL;浓缩小肠液淀粉酶活性2 436 U·mL-1,胰蛋白酶活性760 U·mL-1,糜蛋白酶活性95 U·mL-1,溶于22 mL去离子水中,每根仿生消化管注入2 mL浓缩小肠液。试验二中4 h补酶,浓缩小肠液中淀粉酶活性2 037 U·mL-1,胰蛋白酶活性1 430 U·mL-1,糜蛋白酶活性50 U·mL-1,溶于11 mL去离子水中,每根仿生消化管中注入1 mL浓缩小肠液。消化酶:胃蛋白酶(Sigma P7000)、淀粉酶(Sigma A3306, Sigma-Aldrich Co., St.Louis, MO)、胰蛋白酶(Amersco 0785, Amersco Inc., Solon, OH)、糜蛋白酶(Amersco 0164, Amersco Inc., Solon, OH)。消化结束后,将透析管内的未消化残渣无损失地转移到50 mL量筒中,读取体积。然后,转入50 mL离心管中,在4 ℃ 1 250 g下离心10 min,获得上清液。
胃蛋白酶活性(U·mL-1)的测定参考Anson[17]的方法进行。测定原理:以牛血红蛋白为底物生成可溶于三氯乙酸溶液的氨基酸含量在280 nm处的吸光度变化与胃蛋白酶活性具有一定相关性。淀粉酶活性(U·mL-1)的测定参考Dahlqvist[18]的方法进行。测定原理:可溶性淀粉经淀粉酶作用后的还原物质与3,5-二硝基水杨酸可形成有颜色的络合物,在530 nm下,络合物颜色的深浅与还原物质的量呈线性关系。胰蛋白酶活性(U·mL-1)的测定参考Wirnt[19]的方法进行。测定原理:对-甲苯磺酸-L-精氨酸乙酯经胰蛋白酶水解成的对-甲苯磺酸-L-精氨酸在247 nm下吸光度的变化速度。糜蛋白酶活性(U·mL-1)的测定参考Wirnt[20]的方法进行。测定原理:苯甲酰-L-酪氨酸乙酯经糜蛋白酶水解成苯甲酰-L-酪氨酸在256 nm下吸光度的变化速度。
1.4 数据处理与统计方法采用SAS 9.2的MEANS模块计算基本统计量。对试验一、二猪仿生消化中胃、小肠阶段内不同消化时间点间消化酶活性的差异采用GLM模块进行方差分析,并以Duncan’s法对各处理均值进行多重比较。采用Contrast选项进行多项式正交比较,分析试验一消化酶活性随消化时间的线性和二次效应,同时进行线性失拟检验。以P < 0.05定义为差异显著。
2 结果 2.1 仿生消化过程中胃蛋白酶活性随消化时间的变化当模拟胃液与玉米、大豆粕、小麦麸或玉米-大豆粕型饲粮混合后,消化液中胃蛋白酶的比活性分别为模拟胃液设定值活性的59.1%、28.8%、30.3%、32.7%(表 2)。在模拟消化1、2、3、4 h,消化液中胃蛋白酶的比活性和总活性均显著地高于样品与模拟胃液刚混合时(0 h)消化液中胃蛋白酶的相应活性(P < 0.05)。以玉米为底物时,模拟消化1、2、4 h,胃蛋白酶的比活性和总活性呈二次曲线增加(P < 0.05),在3~4 h胃蛋白酶活性达到稳定。4 h消化液中胃蛋白酶的比活性及总活性达到模拟胃液设定值活性的95.1%和101.4%。在大豆粕模拟消化1、2 h,胃蛋白酶的比活性与总活性依次显著增加(P < 0.05),在3~4 h胃蛋白酶的比活性与总活性达到稳定,但相对于2 h比活性显著下降(P < 0.05),而总活性无显著差异。总体上,胃蛋白酶的比活性与总活性随模拟消化时间呈二次曲线变化(P < 0.05)。4 h消化液中胃蛋白酶的比活性及总活性达到模拟胃液设定值活性的89.5%和102.0%。以小麦麸为底物时,模拟消化1、2 h,胃蛋白酶的比活性差异不显著,在随后3、4 h胃蛋白酶的比活性呈下降变化。总活性在模拟消化1、2、3、4 h无显著差异。总体上,胃蛋白酶的比活性与总活性随模拟消化时间呈二次曲线增加(P < 0.05)。4 h消化液中胃蛋白酶的比活性及总活性达到模拟胃液设定值活性的96.1%和102.7%。以玉米-大豆粕型饲粮为底物时,模拟消化1、2、3 h,胃蛋白酶的比活性和总活性依次显著增加(P < 0.05),在3~4 h胃蛋白酶活性达到稳定。胃蛋白酶的比活性与总活性均随模拟消化时间呈二次曲线变化(P < 0.05)。4 h消化液中胃蛋白酶的比活性及总活性达到模拟胃液设定值活性的78.0%和87.2%。
|
|
表 2 胃仿生消化阶段胃蛋白酶活性的变化 Table 2 Variation of pepsin activity in in vitro gastric digestion |
以玉米为底物时,模拟小肠消化阶段(图 1)消化液中淀粉酶比活性和总活性随消化时间呈二次曲线降低(P < 0.05),其中比活性和总活性在0~4 h依次显著降低(P < 0.05)。胰蛋白酶的比活性和总活性呈现0~8 h急速下降(P < 0.05),8~16 h保持较低值的二次曲线性变化。糜蛋白酶的比活性和总活性呈非线性变化,比活性在2 h达到最高,随后依次显著下降(P < 0.05)。
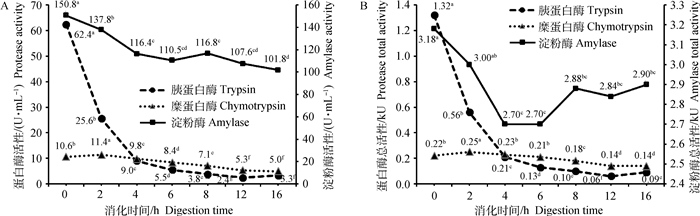
|
淀粉酶活性浓度:SEM=3.23 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;胰蛋白酶活性浓度:SEM=0.14 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶活性浓度:SEM=0.24 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P=0.13。淀粉酶总活性:SEM=0.09 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;胰蛋白酶总活性:SEM=0.003 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶总活性:SEM=0.01 kU,方差P < 0.01,线性失拟P < 0.01,二次P=0.78,不同字母表示差异显著(P < 0.05),下同 Amylase activity concentration:SEM=3.23 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01; Trypsin activity concentration:SEM=0.14 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin activity concentration:SEM=0.24 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P=0.13. Amylase total activity: SEM=0.09 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01; Trypsin total activity:SEM=0.003 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin total activity:SEM=0.01 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P=0.78. The different letters indicate significant difference (P < 0.05), the same as below 图 1 以玉米为底物模拟小肠消化阶段消化酶活性随消化时间的变化 Fig. 1 Digestive enzyme activities varied with digestion time in in vitro simulated small intestinal digestion for corn |
以大豆粕为底物时,模拟小肠消化阶段(图 2),消化液中淀粉酶比活性和总活性随消化时间呈现非线性变化。在0和4 h淀粉酶比活性以及总活性的差异不显著,但显著低于2 h的相应值(P < 0.05);4~12 h淀粉酶比活性以及总活性显著降低(P < 0.05),随后趋于稳定。胰蛋白酶的比活性和总活性随消化时间呈二次曲线显著降低(P < 0.05),总体上0~8 h呈现急速下降,8~16 h酶活性降到较低水平。糜蛋白酶的比活性和总活性呈二次曲线降低,其中在0~12 h呈缓慢下降,12~16 h显著下降(P < 0.05)。
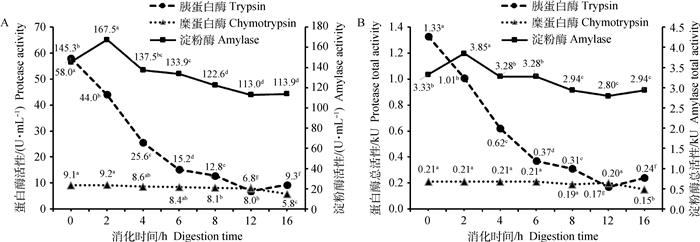
|
淀粉酶活性浓度:SEM=3.27 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P=0.11;胰蛋白酶活性浓度:SEM=0.47 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶活性浓度:SEM=0.30 U·mL-1,方差P < 0.01,线性失拟P < 0.05,二次P < 0.05。淀粉酶总活性:SEM=0.08 kU,方差P < 0.01,线性失拟P < 0.01,二次P=0.15;胰蛋白酶总活性:SEM=0.01 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶总活性:SEM=0.01 kU,方差P < 0.01,线性失拟P < 0.05,二次P < 0.05 Amylase activity concentration:SEM=3.27 U·mL-1, anova P < 0.01, Linear Lof P=0.11, Quadratic P < 0.01;Trypsin activity concentration:SEM=0.47 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin activity concentration:SEM=0.30 U·mL-1, anova P < 0.01, Linear Lof P < 0.05, Quadratic P < 0.05. Amylase total activity: SEM=0.08 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P=0.15; Trypsin total activity:SEM=0.01 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin total activity:SEM=0.01 kU, anova P < 0.01, Linear Lof P < 0.05, Quadratic P < 0.05 图 2 以大豆粕为底物模拟小肠消化阶段消化酶活性随消化时间的变化 Fig. 2 Digestive enzyme activities varied with digestion time in in vitro simulated small intestinal digestion for soybean meal |
以小麦麸为底物时,模拟小肠消化阶段(图 3),消化液中淀粉酶的比活性和总活性随消化时间呈二次曲线依次降低(P < 0.05),0~8 h活性下降较快,8~12 h缓慢下降。胰蛋白酶的比活性和总活性随消化时间呈二次曲线显著降低(P < 0.05),总体上0~8 h呈现急速下降,8~16 h酶活性降到较低水平。糜蛋白酶的比活性和总活性呈二次曲线降低,其中在0~12 h呈快速下降,12~16 h缓慢下降(P < 0.05)。
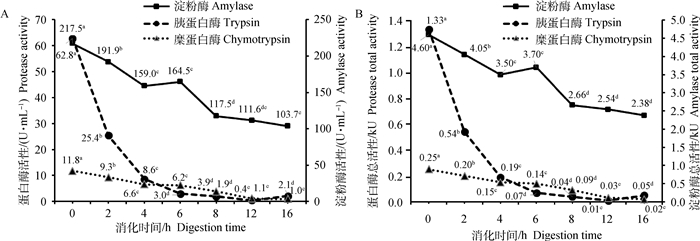
|
淀粉酶活性浓度:SEM=4.28 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;胰蛋白酶活性浓度:SEM=0.46 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶活性浓度:SEM=0.20 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01。淀粉酶总活性:SEM=0.09 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.05;胰蛋白酶总活性:SEM=0.01 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶总活性:SEM=0.005 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01 Amylase activity concentration:SEM=4.28 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Trypsin activity concentration:SEM=0.46 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin activity concentration:SEM=0.20 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01. Amylase total activity: SEM=0.09 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.05; Trypsin total activity:SEM=0.01 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin total activity:SEM=0.005 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01 图 3 以小麦麸为底物模拟小肠消化阶段消化酶活性随消化时间的变化 Fig. 3 Digestive enzyme activities varied with digestion time in in vitro simulated small intestinal digestion for wheat bran |
以玉米-大豆粕型饲粮为底物时,模拟小肠消化阶段(图 4)消化液中淀粉酶的比活性随消化时间变化不显著。总活性随消化时间呈现非线性变化;除4 h外,其余各消化时间的总活性差异不显著。胰蛋白酶的比活性和总活性随消化时间呈二次曲线显著降低(P < 0.05),总体上0~12 h呈现急速下降,12~16 h酶活性缓慢回升。糜蛋白酶比活性在0~4 h最高,6~12 h呈显著降低(P < 0.05),糜蛋白酶总活性前0~4 h显著升高,随后显著性下降(P < 0.05),总体呈现二次曲线变化。
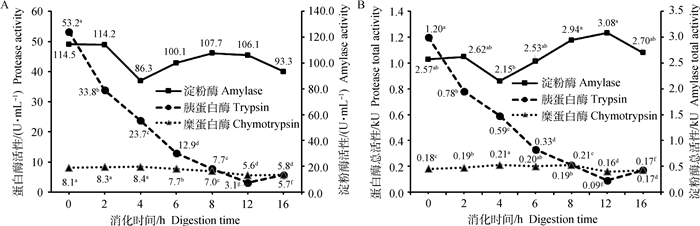
|
淀粉酶活性浓度:SEM=6.50 U·mL-1,方差P=0.05,线性失拟=0.06,二次P=0.79;胰蛋白酶活性浓度:SEM=0.34 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶活性浓度:SEM=0.12 U·mL-1,方差P < 0.01,线性失拟P < 0.01,二次P=0.58。淀粉酶总活性:SEM=0.17 kU,方差P < 0.05,线性失拟P < 0.05,二次P=0.44;胰蛋白酶总活性:SEM=0.01 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01;糜蛋白酶总活性:SEM=0.003 kU,方差P < 0.01,线性失拟P < 0.01,二次P < 0.01 Amylase activity concentration:SEM=6.50 U·mL-1, anova P=0.05, Linear Lof P=0.06, Quadratic P=0.79;Trypsin activity concentration:SEM=0.34 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin activity concentration:SEM=0.12 U·mL-1, anova P < 0.01, Linear Lof P < 0.01, Quadratic P=0.58. Amylase total activity: SEM=0.17 kU, anova P < 0.05, Linear Lof P < 0.05, Quadratic P=0.44; Trypsin total activity:SEM=0.01 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01;Chymotrypsin total activity:SEM=0.003 kU, anova P < 0.01, Linear Lof P < 0.01, Quadratic P < 0.01 图 4 以玉米-大豆粕型饲粮为底物模拟小肠消化阶段消化酶活性随消化时间的变化 Fig. 4 Digestive enzyme activities varied with digestion time in in vitro simulated small intestinal digestion for corn-soybean meal |
在模拟小肠阶段消化4 h时补充消化酶后模拟小肠液中酶活性的变化情况见表 3。以玉米为底物,在小肠消化阶段补加消化酶后,淀粉酶比活性及总活性在4 h显著地高于6~8 h的相应值(P < 0.05),且高于初始值(150.8 U·mL-1),其中4 h的比活性与猪体内值(221 U·mL-1)较接近。胰蛋白酶的比活性和总活性仍呈现急速下降的变化规律(P < 0.05),其中4 h的比活性高于初始值,与体内值(69.1 U·mL-1)较接近。糜蛋白酶比活性及总活性在4 h显著地高于6~8 h的相应值(P < 0.05),总体上与初始值较接近。以大豆粕为底物,在小肠阶段补加消化酶后,淀粉酶比活性及总活性随消化时间的变化差异不显著(P>0.05),且均高于初始值(145.3 U·mL-1,3.33 kU),与猪体内值(221 U·mL-1)较接近。胰蛋白酶的比活性和总活性仍呈现急速下降的变化规律(P < 0.05),其中4 h的比活性与总活性与初始值较接近。糜蛋白酶的比活性和总活性随消化时间呈先升高后降低的变化,总体上与初始值接近。以小麦麸为底物,在小肠阶段补加消化酶后,淀粉酶比活性以及总活性随消化时间的变化差异不显著(P>0.05),但稍低于初始值及体内值。胰蛋白酶的比活性和总活性仍随消化时间呈现急速下降的变化规律(P < 0.05),其中4 h的比活性与初始值较接近。糜蛋白酶的比活性和总活性在4~6 h差异不显著,但显著高于8 h的相应值(P < 0.05),均比初始值低。以玉米-大豆粕型饲粮为底物,在小肠阶段补加消化酶后,淀粉酶比活性在6与8 h间差异不显著,但低于4 h的相应值(P < 0.05),总活性无显著性差异。胰蛋白酶的比活性和总活性仍随消化时间呈现急速下降的变化规律(P < 0.05),其中4 h的比活性与初始值较接近。糜蛋白酶的比活性在4 h显著地高于6~8 h的相应值(P < 0.05),总体上与初始值较接近,而各消化时间段间总活性差异不显著。
|
|
表 3 4 h补加消化酶后小肠仿生消化液中淀粉酶、胰蛋白酶、糜蛋白酶活性的变化 Table 3 Changes of amylase, trypsin and chymotrypsin activity when digestive enzymes were supplemented at 4 h of in vitro small intestine digestion |
在猪的胃消化阶段,饲料主要由猪胃黏膜主细胞所分泌的胃蛋白酶原经修饰后形成的胃蛋白酶消化。胃液的分泌受神经、激素及饲粮物理化学性质的影响,从而导致胃消化期胃液的体积及pH的变化,而胃蛋白酶原的分泌主要受激素、胃及十二指肠食糜养分的影响[21]。胃液与胃蛋白酶原的分泌量直接影响胃消化期胃蛋白酶活性的变异。Lawrence[22]的研究表明,生长猪采食4种不同日粮后,在0.75~5.5 h内胃液中胃蛋白酶的活性总体呈缓慢下降趋势,虽然不同日粮导致胃蛋白酶活性的下降幅度有所不同,但变异幅度不大。本研究中,3种常用饲料原料和1种配合日粮与模拟胃液混合后胃蛋白酶活性迅速下降,这可能是当模拟胃液与饲料底物混合后,由于饲料底物粒度较小(过60目筛)表面积较大从而吸附了部分胃蛋白酶,这导致了消化液中胃蛋白酶活性显著降低。这一现象与饲料原料对溶液中的H+有不同程度的吸附类似[23],与Makkink等[24]报道不同来源的日粮对小肠液中的消化酶活性影响不一致的结果也相似。然而,在消化1 h后胃蛋白酶活性显著回升并随后续消化时间其活性的变异幅度较小。这是由于底物随着模拟胃液的消化总体表面积减少,从而导致被吸附的胃蛋白酶也减少,使检测到的活性呈现回升的现象。不同类别的饲料底物导致消化液中胃蛋白酶的活性变异程度不同,这与生长猪胃液中胃蛋白酶活性随日粮的结构不同而有所变异的结论一致[25]。
3.2 猪小肠内消化酶活性的变化及小肠仿生消化中消化酶活性的变异猪小肠中消化酶主要来源于胰腺分泌的各种消化酶原在十二指肠经过激活后形成具有水解能力的消化酶。其次肠腺及肠黏膜也贡献了少量的肽酶和二糖酶。Partridge等[26]、Botermans和Pierzynowski[27]的研究表明,30~48 kg的生长猪在24 h内胰液的分泌量在1.3~5.0 L间变化,且餐后胰液的分泌量明显地高于餐前,随后逐步降低。胰液中消化酶的活性浓度及总量与胰液的分泌量呈正相关[28]。因此,当猪受到神经、激素的调节,昼夜交替与日粮组成等因素直接影响胰液分泌量,最终会影响进入小肠内相关消化酶活性[10-11, 26, 29-31]。研究发现,生长猪空肠食糜中胰蛋白酶、糜蛋白酶活性的日内变异系数高达34%和55%[32]。与此同时,空肠中消化酶的活性沿消化道纵向长度呈逐渐下降的变化规律[32],这表明,小肠中消化酶一方面由胰液、肠腺分泌补充,另一方面也存在明显的失活衰变。本研究中,当浓缩的模拟小肠液注入消化管后,消化液中3种主要消化酶的活性均随消化时间而衰减,其中胰蛋白酶活性的衰减速度最快。补注浓缩模拟消化液后虽然消化酶活性可完全或部分恢复到小肠消化阶段的初始值但总体上仍呈现前述类似的衰变现象。这表明,消化酶在体外消化的过程中存在明显的失活特性,且胰蛋白酶相对于另外2种消化酶更容易失活。这是由于胰蛋白酶本身很容易自溶,活力也逐步下降而丧失[33]。当补注模拟消化液后,3种消化酶的活性变化可以呈现出类似于猪胰液及肠道中消化酶活性随餐后的阶段性变化[28],但仿生消化中胰蛋白酶下降的变化幅度更大,而猪体内消化酶因能得到及时补充而变异相对小一些。与玉米和玉米-大豆粕型饲粮相比,本试验发现,大豆粕和小麦麸的小肠仿生消化中3种消化酶的活性相对较高。在猪体内, 由于高纤维的饲料底物能刺激胰液的分泌,从而提高肠道中消化酶的活性[31]。然而,本仿生消化系统没有涉及饲料底物的化学成分对模拟消化液注入的反馈控制,因此,是否存在高纤维成分可以降低消化酶的失活速度有待于进一步研究。
4 结论模拟胃液与饲料样品混合后胃蛋白酶的比活性迅速降低,但在模拟消化1~4 h时,胃蛋白酶的活性呈二次曲线回升,且能达到设定值左右。而小肠模拟消化阶段淀粉酶、胰蛋白酶、糜蛋白酶的活性均有不同程度的衰减。不同底物对消化液中酶活性下降程度的影响存在差异。在小肠消化4 h时补加消化酶后消化液中淀粉酶、胰蛋白酶、糜蛋白酶的活性均可恢复到初始值,但仍随消化时间而衰减。因此,在猪仿生消化中胃消化阶段无需补充胃蛋白酶,小肠消化阶段需要补充消化酶。
| [1] | GIUBERTI G, GALLO A, CERIOLI C, et al. In vitro starch digestion and predicted glycemic index of cereal grains commonly utilized in pig nutrition[J]. Anim Feed Sci Technol, 2012, 174(3-4): 163–173. DOI: 10.1016/j.anifeedsci.2012.03.006 |
| [2] | YEGANI M, KORVER D R. Effects of corn source and exogenous enzymes on growth performance and nutrient digestibility in broiler chickens[J]. Poult Sci, 2013, 92(5): 1208–1220. DOI: 10.3382/ps.2012-02390 |
| [3] | ZHAO F, REN L Q, MI B M, et al. Developing a computer-controlled simulated digestion system to predict the concentration of metabolizable energy of feedstuffs for rooster[J]. J Anim Sci, 2014, 92(4): 1537–1547. DOI: 10.2527/jas.2013-6636 |
| [4] | ZHAO F, ZHANG L, MI B M, et al. Using a computer-controlled simulated digestion system to predict the energetic value of corn for ducks[J]. Poult Sci, 2014, 93(6): 1410–1420. DOI: 10.3382/ps.2013-03532 |
| [5] | BOISEN S, FERNÁNDEZ J A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses[J]. Anim Feed Sci Technol, 1997, 68(3-4): 277–286. DOI: 10.1016/S0377-8401(97)00058-8 |
| [6] | PAN L, MA H, PIAO X S, et al. A computer-controlled simulated digestion system is a promising in vitro digestibility technique to predict digestible energy of corn grain for growing pigs[J]. Anim Feed Sci Technol, 2018, 235: 43–49. DOI: 10.1016/j.anifeedsci.2017.10.005 |
| [7] | CLUNIES M, LEESON S. In vitro estimation of dry matter and crude protein digestibility[J]. Poult Sci, 1984, 63(1): 89–96. DOI: 10.3382/ps.0630089 |
| [8] | JOHNSTON J, COON C N. The use of varying levels of pepsin for pepsin digestion studies with animal proteins[J]. Poult Sci, 1979, 58(5): 1271–1273. DOI: 10.3382/ps.0581271 |
| [9] | HUANG R L, TAN Z L, XING T X, et al. An in vitro method for the estimation of ileal crude protein and amino acids digestibility using the dialysis tubing for pig feedstuffs[J]. Anim Feed Sci Technol, 2000, 88(1-2): 79–89. DOI: 10.1016/S0377-8401(00)00209-1 |
| [10] | PIERZYNOWSKI S G, WESTRÖM B R, KARLSSON B W, et al. Pancreatic cannulation of young pigs for long-term study of exocrine pancreatic function[J]. Can J Anim Sci, 1988, 68(3): 953–959. DOI: 10.4141/cjas88-105 |
| [11] | THAELA M J, JENSEN M S, CORNÉLISSEN G, et al. Circadian and ultradian variation in pancreatic secretion of meal-fed pigs after weaning[J]. J Anim Sci, 1998, 76(4): 1131–1139. DOI: 10.2527/1998.7641131x |
| [12] | WILFART A, JAGUELIN-PEYRAUD Y, SIMMINS H, et al. A step-wise in vitro method to estimate kinetics of hydrolysis of feeds[J]. Livest Sci, 2007, 109(1-3): 179–181. DOI: 10.1016/j.livsci.2007.01.139 |
| [13] | WILFART A, JAGUELIN-PEYRAUD Y, SIMMINS H, et al. Kinetics of enzymatic digestion of feeds as estimated by a stepwise in vitro method[J]. Anim Feed Sci Technol, 2008, 141(1-2): 171–183. DOI: 10.1016/j.anifeedsci.2007.05.021 |
| [14] | SOPADE P A, GIDLEY M J. A rapid in-vitro digestibility assay based on glucometry for investigating kinetics of starch digestion[J]. Starch-Stärke, 2009, 61(5): 245–255. DOI: 10.1002/star.v61:5 |
| [15] | PÉREZ-VENDRELL A M, TORRALLARDONA D. In vitro digestibility kinetics of diets containing different cereal sources[J]. Livest Sci, 2010, 134(1-3): 47–49. DOI: 10.1016/j.livsci.2010.06.093 |
| [16] |
王钰明.猪模拟小肠液的制备及仿生消化法测定饲料可消化养分含量的研究[D].北京: 中国农业科学院, 2015.
WANG Y M.Study on the preparation of mimic intestinal fluid and simulated digestion method for determination of digestible nutrient content of feeds for pigs[D]. Beijing: Chinese Academy of Agricultural Sciences, 2015.(in Chinese) |
| [17] | ANSON M L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin[J]. J Gen Physiol, 1938, 22(1): 79–89. DOI: 10.1085/jgp.22.1.79 |
| [18] | DAHLQVIST A. A method for the determination of amylase in intestinal content[J]. Scand J Clin Lab Invest, 1962, 14(2): 145–151. DOI: 10.3109/00365516209079686 |
| [19] | WIRNT R.Trypsin: measurements with Nα-p-toluene-sulphonyl-L-arginine methyl ester as substrate[M]//BERGMEYER H U.Methods of Enzymatic Analysis. New York: Verlag Chemie, 1974: 1021-1024. |
| [20] | WIRNT R.Chymotrypsin measurements with N-benzoyl-L-tyrosin methyl ester as substrate[M]//BERGMEYER H U.Methods of Enzymatic Analysis.Weinheinm: Verlag Chemie, 1974: 1009-1012. |
| [21] | LOW A G. Nutritional regulation of gastric secretion, digestion and emptying[J]. Nutr Res Rev, 1990, 3(1): 229–252. DOI: 10.1079/NRR19900014 |
| [22] | LAWRENCE T L J. The effect of certain dietary factors on in vivo pH changes and pepsin activity in the stomach of the growing pig[J]. Br Vet J, 1972, 128(8): 402–411. DOI: 10.1016/S0007-1935(17)36834-3 |
| [23] | LAWLOR P G, LYNCHP B, CAFFREY P J, et al. Measurements of the acid-binding capacity of ingredients used in pig diets[J]. Ir Vet J, 2005, 58(8): 447–452. DOI: 10.1186/2046-0481-58-8-447 |
| [24] | MAKKINK C A, NEGULESCU G P, QIN G X, et al. Effect of dietary protein source on feed intake, growth, pancreatic enzyme activities and jejunal morphology in newly-weaned piglets[J]. Br J Nutr, 1994, 72(3): 353–368. DOI: 10.1079/BJN19940039 |
| [25] | ZEBROWSKA T, LOW A G, ZEBROWSKA H. Studies on gastric digestion of protein and carbohydrate, gastric secretion and exocrine pancreatic secretion in the growing pig[J]. Br J Nutr, 1983, 49(3): 401–410. DOI: 10.1079/BJN19830049 |
| [26] | PARTRIDGE I G, LOW A G, SAMBROOK I E, et al. The influence of diet on the exocrine pancreatic secretion of growing pigs[J]. Br J Nutr, 1982, 48(1): 137–145. DOI: 10.1079/BJN19820096 |
| [27] | BOTERMANS J A M, PIERZYNOWSKI S G. Relations between body weight, feed intake, daily weight gain, and exocrine pancreatic secretion in chronically catheterized growing pigs[J]. J Anim Sci, 1999, 77(2): 450–456. DOI: 10.2527/1999.772450x |
| [28] | THAELA M J, PIERZYNOWSKI S G, JENSEN M S, et al. The pattern of the circadian rhythm of pancreatic secretion in fed pigs[J]. J Anim Sci, 1995, 73(11): 3402–3408. DOI: 10.2527/1995.73113402x |
| [29] | DAL BORGO G, SALMAN A J, PUBOLS M H, et al. Exocrine function of the chick pancreas as affected by dietary soybean meal and carbohydrate[J]. Proc Soc Exp Biol Med, 1968, 129(3): 877–881. DOI: 10.3181/00379727-129-33448 |
| [30] | JOHNSON L R. Gastrointestinal Physiology[M]. Philadelphia: Mosby Elsevier, 2007. |
| [31] | WENK C. The role of dietary fibre in the digestive physiology of the pig[J]. Anim Feed Sci Technol, 2001, 90(1-2): 21–33. DOI: 10.1016/S0377-8401(01)00194-8 |
| [32] | LOW A G. The activity of pepsin, chymotrypsin and trypsin during 24 h periods in the small intestine of growing pigs[J]. Br J Nutr, 1982, 48(1): 147–159. DOI: 10.1079/BJN19820097 |
| [33] |
茹炳根, 杜锦珠, 曾耀辉, 等. 猪胰蛋白酶自溶后的活性产物[J]. 北京大学学报, 1979(4): 32–47.
RU B G, DU J Z, ZENG Y H, et al. Active products of porcine trypsin after autolysis[J]. Acta Scicentiarum Naturalum Universitis Pekinesis, 1979(4): 32–47. (in Chinese) |



