microRNA(miRNA)是一种在转录后水平调节动物多种生物学过程的负调控因子,通过与靶基因的3′-UTR结合转录后抑制或降解靶基因的方式,在调控动物卵巢卵泡的发生、发育、颗粒细胞增殖、凋亡及类固醇激素合成分泌等方面发挥重要作用[1]。多数miRNA具有自己的启动子,但位于内含子区域的一些miRNA基因与宿主基因享有相同的转录调控元件[2]。动物细胞内转录因子可以结合miRNA启动子区域的相应转录因子结合位点,从而调控miRNA的表达。因此,研究miRNA基因的启动子有助于揭示miRNA转录调控过程。类固醇合成因子1(SF-1)可在miR-383的启动子区域结合,增加miR-383的表达水平[3]。TGFβ1作为转录因子在miR-224启动子区结合,从而促进其转录[4]。
生殖激素可调控miRNA的表达,从而调节相关靶基因的表达水平,并影响动物繁殖。与母畜繁殖活动相关的促卵泡素(FSH)和促黄体素(LH)调控卵泡的生长和颗粒细胞的成熟[5-6],同时,FSH和LH也调节miRNA表达。用FSH处理小鼠卵巢颗粒细胞12 h后,发现miR-29a和miR-30b的表达水平显著下降,且表达水平随FSH处理时间延长而逐渐降低[7]。类固醇激素与特异性配体/受体结合可调控卵泡形成和颗粒细胞分化发育[8],这一过程也伴随miRNA表达水平变化。雌二醇(E2)通过激活细胞膜表面的雌激素受体(ER)改变miRNA的表达[9]。
miR-150在哺乳动物组织中广泛表达,可调控多种组织和细胞的分化、发育、凋亡等过程,还可在病理过程中发挥作用[10-12]。除此之外,miR-150也可影响哺乳动物的繁殖性能[13-14]。本课题组前期研究发现,miR-150为吐鲁番黑羊卵泡期和黄体期显著差异表达的miRNA之一,且卵泡期miR-150的表达较黄体期显著降低,推测miR-150差异表达影响绵羊卵巢卵泡发育,从而影响绵羊繁殖活动[15]。此外,本课题组证实绵羊miR-150靶向类固醇激素合成急性调节蛋白(steroidogenic acute regulatory protein gene,STAR)基因且负调控其表达[16],并能通过影响STAR、Cyp11a1和HSD3B1等参与孕酮合成基因的表达而促进卵巢颗粒细胞凋亡[17],为阐明miR-150调控绵羊繁殖活动机制奠定基础。
上述研究主要是从miR-150基因调控的下游出发,而针对其上游的调控机制还未见报道。因此,本研究通过预测绵羊miR-150基因启动子区域及繁殖相关转录因子结合位点,构建miR-150启动子双荧光素酶报告基因重组质粒来确定miR-150的启动子活性区域,并在细胞水平上证实FSH、LH和E2对miR-150表达的调控作用,以期探索miR-150自身的转录调控过程,为miR-150调控卵泡发育的机制研究提供新思路。
1 材料与方法 1.1 材料限制性内切酶购自大连宝生物工程有限公司;T4连接酶购自北京普洛麦格生物科技有限公司;EasyPure PCR Purification Kit、Trans5α感受态细胞、TransScript One-Step gDNA Removal and cDNA购自北京全式金生物技术有限公司;X-tremeGENE HP DNA Transfection Reagent购自瑞士Roche公司;双荧光素酶报告基因检测试剂盒购自美国Promega公司。绵羊(小尾寒羊)卵巢采自保定振宏食品加工有限公司。
1.2 绵羊卵巢颗粒细胞的分离培养将采集的新鲜卵巢组织置于预热的生理盐水中(37 ℃,含1%双抗),2 h内运回实验室。先用75%酒精润洗消毒10 s,预热生理盐水清洗后,去除多余脂肪及系膜。再用PBS(含1%双抗)清洗,然后用注射器抽取卵泡液,并置于无菌EP管内,静置5 min。取上清,1 500 r·min-1离心10 min,弃上清;PBS洗涤后,加入适量预热的DMEM/F12完全培养基(含10%胎牛血清、1%双抗及50 μg·mL-1丙酮酸钠),吹打混匀后接种于细胞培养瓶,37 ℃培养箱培养(5% CO2)。细胞培养24 h后,清洗换液,继续培养至细胞汇合度达80%时可传代。
1.3 绵羊卵巢基因组DNA提取与纯化采用酚氯仿核酸提取法提取绵羊卵巢基因组DNA。DNA纯化体系为20 μL:10×NEB buffer 1 μL,RNase 0.1 μL,DNA 8.9 μL,37 ℃金属浴中消化1 h,纯化产物再次经过酚氯仿核酸提取法进行提取,纯化DNA,Nanodrop 2000测定样品DNA浓度后,-20 ℃保存。
1.4 绵羊miR-150启动子区域预测与克隆下载绵羊miR-150基因组参考序列(Gene ID:102465811),利用Promoter 2.0(http://www.cbs.dtu.dk/services/Promoter/)预测绵羊miR-150基因启动子区域,并设计特异引物,正向引物5′端添加Kpn Ⅰ酶切位点,反向引物5′端添加Bgl Ⅱ酶切位点,正向引物序列为5′-GGGGTACCCTCCTTGGATTTTGTGCACTGA-3′,反向引物序列为5′-GAAGATCTGACCGAGACACACACTGGTA-3′(下划线为保护碱基和酶切位点),预期扩增片段长度为582 bp。PCR反应体系为50 μL:10×TransStart buffer 5 μL,dNTP(2 mmol·L-1) 4 μL,正引物(10 μmol·L-1) 4 μL,反引物(10 μmol·L-1)4 μL,基因组DNA 200 ng,TransStart Taq Polymerase(2.5 U·μL-1) 1 μL,ddH2O补足至50 μL。PCR反应条件:95 ℃ 5 min;95 ℃ 30 s,68 ℃ 30 s,72 ℃ 1 min,共35个循环;72 ℃10 min。采用1%琼脂糖凝胶对PCR产物进行电泳检测。回收PCR产物并克隆至pMD-19T载体上。经菌液PCR产物检测,将阳性克隆送至华大基因公司测序。
1.5 miR-150启动子重组质粒构建提取经菌液PCR和测序验证的阳性克隆质粒,利用限制性内切酶Kpn Ⅰ和Bgl Ⅱ对miR-150启动子片段和LUC荧光素酶报告基因载体PGL3-basic进行双酶切(37 ℃酶切4 h),反应体系为50 μL:10×T Buffer 5 μL,Bgl Ⅱ 1.5 μL,Kpn Ⅰ 1.5 μL,DNA 2 μg,无菌水补足至50 μL。用T4连接酶进行连接,连接体系为10 μL:PGL3-basic与目的基因片段的摩尔比为1:3,10×T4 Buffer 1 μL,T4 DNA Ligase(Promega)0.5 μL,无菌水补齐至10 μL,4 ℃孵育过夜。连接产物转化至感受态细胞,用菌液PCR进行鉴定,送至北京华大生物科技有限公司测序并进行验证。对阳性菌液无内毒素质粒提取,用NanoDrop 2000测定质粒浓度,-80 ℃保存备用。
1.6 绵羊miR-150启动子区域鉴定 1.6.1 绵羊颗粒细胞转染条件的优化在24孔板的每孔中接种1.5×105个颗粒细胞24 h后,将脂质体和eGFP-N1载体按1:1、2:1、3:1、4:1的比例转染细胞,每组3个重复;将PGL3-control载体和内参载体PRL-TK按50:1、100:1、200:1、500:1的比例转染细胞,对照组只转染PGL3-control,每组3个重复。
1.6.2 绵羊miR-150启动子区域鉴定接种绵羊颗粒细胞24 h后,按照“1.6.1”中摸索的最佳转染条件,分别转染PGL3-Basic、PGL3-Control或PGL3-miR-150pm与内参载体PRL-TK至绵羊颗粒细胞,每组3个重复。转染48 h后,收集细胞,并利用双荧光素酶检测试验盒(Promega)进行双荧光素酶活性检测,记录各组的萤火虫荧光素酶值和海肾荧光素酶值。计算相对荧光素酶活性,每次试验至少重复3次,采用t检验比较差异水平,P < 0.05具有统计学意义。
1.7 绵羊miR-150启动子区域转录因子结合位点预测利用在线软件PROMO(http://alggen.lsi.upc.es.)、Alibaba (http://gene-regulation.com/pub/programs/alibaba2/index.html)预测绵羊miR-150启动子序列转录因子结合位点,并筛选与繁殖相关的转录因子结合位点。
1.8 FSH、LH和E2对绵羊miR-150启动子活性的影响在24孔板内用含血清的DMEM/F12培养基预培养绵羊颗粒细胞24 h,待细胞汇合度达70%~90%时,更换为无血清培养基和单独添加10 IU FSH、4 IU LH、1 μg·mL-1 E2的无血清培养基后进行miR-150启动子重组质粒转染,检测FSH、LH和E2处理后miR-150启动子的相对荧光素酶活性。
2 结果 2.1 绵羊miR-150启动子片段克隆及序列鉴定以绵羊基因组DNA为模板,扩增预测的绵羊miR-150启动子片段(miR-150 promoter)长度为582 bp。在miR-150 promoter正反引物的5′端分别添加酶切位点和保护碱基,PCR扩增的片段大小与预期相符(图 1)。
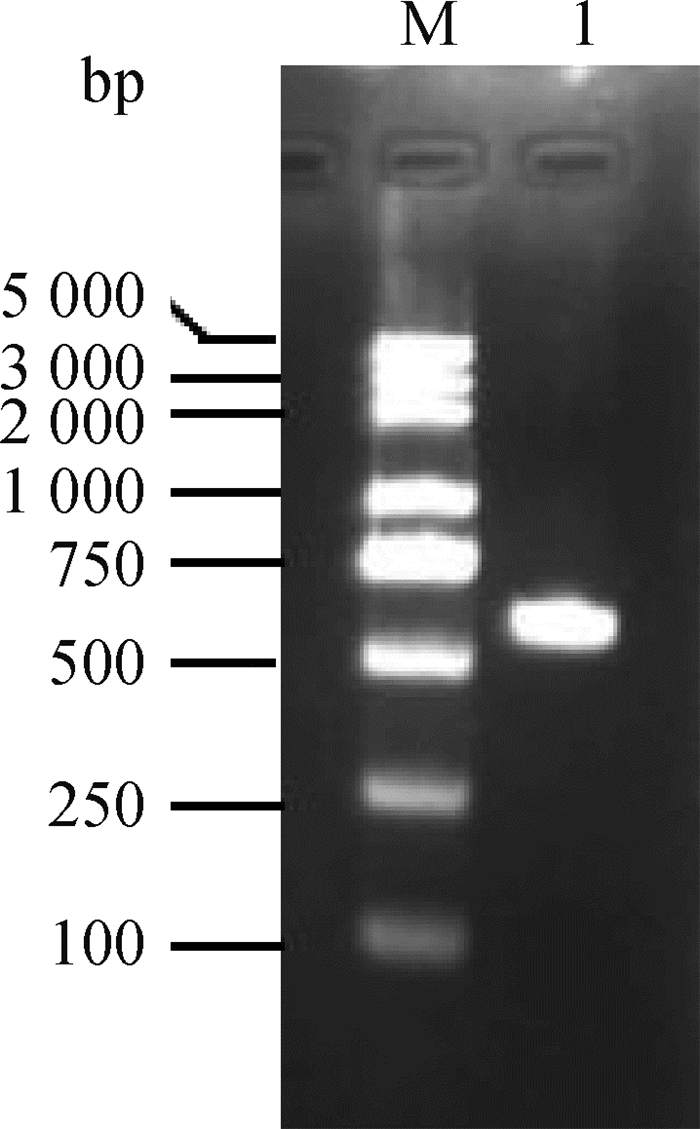
|
M. DNA相对分子质量标准;1. miR-150启动子片段扩增产物 M. DNA marker; 1. miR-150 promoter fragment amplification products 图 1 miR-150启动子片段扩增产物琼脂糖凝胶电泳图 Fig. 1 The agarose gel electrophoresis of amplified products of miR-150 promoter |
用限制内切酶Kpn Ⅰ和Bgl Ⅱ对纯化后的miR-150 promoter与PGL3-basic进行双酶切,用于构建miR-150启动子重组质粒(PGL3-miR-150pm)。取阳性克隆菌液进行PCR扩增初步鉴定,选取扩增良好的菌液提取质粒进行酶切验证(图 2)和测序鉴定(图 3),结果表明,PGL3-miR-150pm构建成功,且插入的miR-150 promoter序列准确无误。

|
M. DNA相对分子质量标准;1.已酶切质粒;2.未酶切质粒 M. DNA marker; 1. Cutted plasmid; 2. No cutted plasmid 图 2 miR-150启动子重组质粒双酶切电泳图 Fig. 2 Electrophoresis of cutted miR-150 promoter recombinant plasmid with double restriction enzymes |
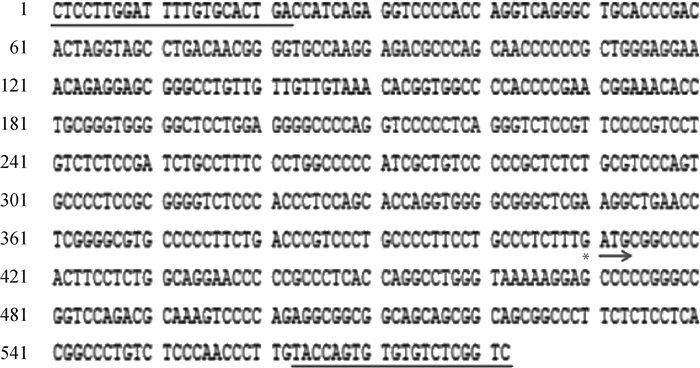
|
下划线表示miR-150基因启动子的正向引物序列和反向引物反向互补序列,*表示预测的转录起始位点,→表示起始密码子ATG The underlines represent the forward primer sequence and reverse primer reverse complementary sequence of the miR-150 gene promoter, * represents the predicted transcriptional starting site, → represents the starting codon ATG 图 3 绵羊miR-150基因启动子序列 Fig. 3 The sequence of miR-150 gene promoter in sheep |
在24孔板内绵羊颗粒细胞汇合度达70%~90%时,将脂质体和eGFP-N1载体按照不同比例转染细胞,筛选最佳转染效率及最适脂质体和DNA的剂量,结果显示,脂质体和绿色荧光蛋白载体eGFP-N1转染比例为1:1时转染效率最高,可达70%,此时细胞的生长状况良好(图 4)。将PGL3-control载体和内参载体PRL-TK按50:1、100:1、200:1、500:1比例转染颗粒细胞,结果表明,50:1为质粒的最佳转染比例(图 5)。
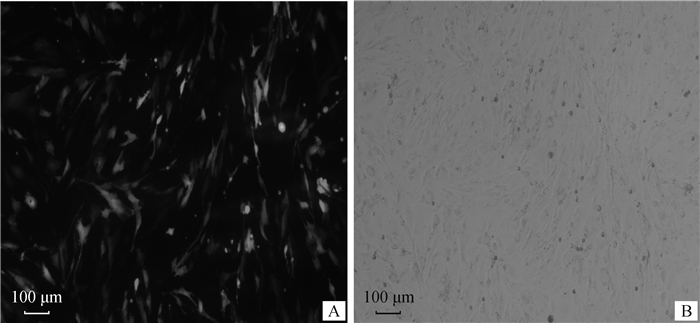
|
A.荧光下观察细胞绿色荧光蛋白表达;B.白光下观察细胞的状态 A. Observation of the expression of green fluorescent protein in cells under fluorescence; B. Observation of the state of cells under white light 图 4 显微镜下观察最佳转染效率时绵羊颗粒细胞荧光表达(100×) Fig. 4 The fluorescence expression in sheep granular cells at the optimal transfection efficiency under the microscope (100×) |
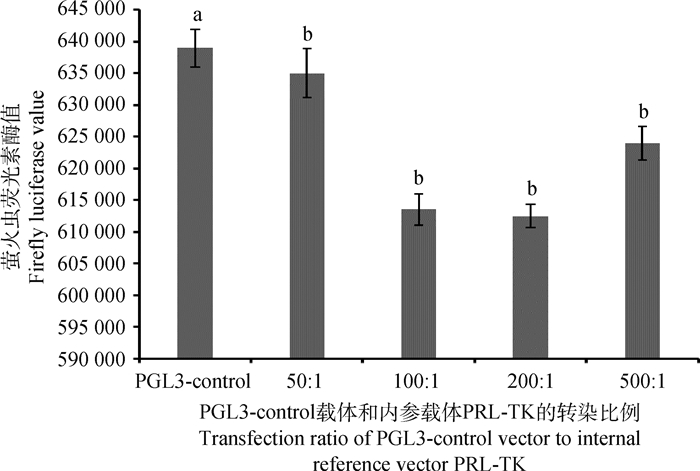
|
数据使用“平均数±标准差”表示。相同字母表示差异不显著(P>0.05),不同字母表示差异显著(P < 0.05)。图 7同 Data is expressed as "mean±SD". The same letters indicate no significant difference (P>0.05), the different letters indicate significant difference (P < 0.05). The same as figure 7 图 5 不同比例PGL3-control与PRL-TK转染的Luc值 Fig. 5 Luc values of different ratio of PGL3-control and PRL-TK transfection |
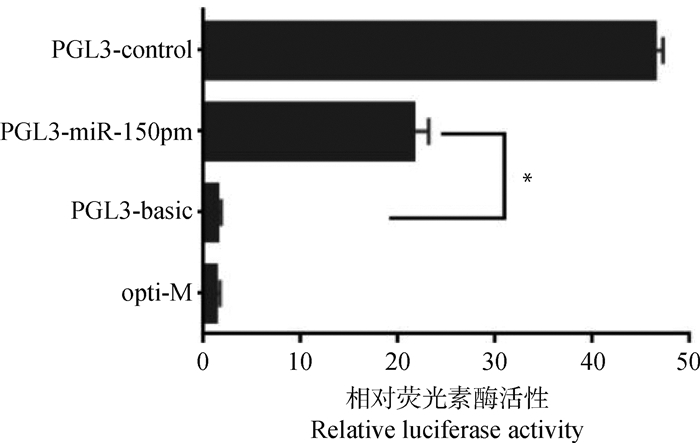
培养绵羊颗粒细胞24 h后,更换培养基为无血清培养基,按优化的绵羊颗粒细胞转染条件,分别转染PGL3-control、PGL3-basic或PGL3-miR-150pm和PRL-TK,48 h后检测各组的荧光素酶活性。PGL3-miR-150pm组与PGL3-basic相比,相对荧光素酶活性值显著提高(P < 0.05),表明miR-150 promoter片段具有显著的启动子活性(图 6)。
|
分别将PGL3-control、PGL3-miR-150pm或PGL3-basic和内参载体PRL-TK共转染于绵羊卵泡颗粒细胞,opti-M组只添加转染试剂,48 h后检测相对荧光素酶活性。数据使用“平均数±标准差”表示(n=3)。*. P < 0.05 PGL3-control, PGL3-miR-150pm or PGL3-basic and PRL-TK were transfected into granular cells of ovine follicles, respectively, only transfection reagents were added in opti-M group. After 48 h, the relative luciferase activity was detected. Data is expressed as "mean±SD" (n=3). *. P < 0.05 图 6 miR-150启动子重组质粒转染后细胞相对荧光素酶活性值 Fig. 6 Relative luciferase activity after transfection of recombinant plasmid of miR-150 promoter |
将绵羊miR-150基因启动子核苷酸序列与NCBI数据库中的miR-150基因(Gene ID:102465811)序列进行比对,确定miR-150基因序列的转录起始位点(图 3)。利用在线软件PROMO预测绵羊miR-150启动子区域转录因子结合位点,设置参数差异(Dissimilar)小于3%,筛选该区域繁殖相关转录因子结合位点(表 1),结果发现了1个ER-alpha结合位点,1个RAR-beta结合位点,10个SP1结合位点,7个Msx-1结合位点,3个p300结合位点,5个PAX-6结合位点,1个Pax-2a结合位点,1个Cdx-1结合位点。
|
|
表 1 绵羊miR-150基因启动子区域预测的繁殖相关转录因子结合位点 Table 1 The predicted transcription factors binding sites related to reproduction in the promoter region of ovine miR-150 gene |
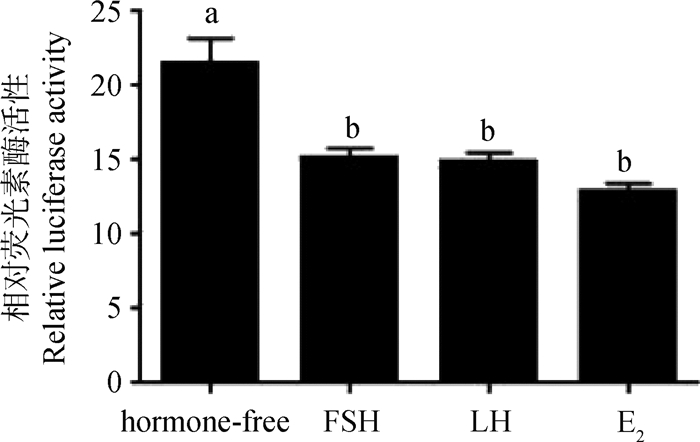
使用含血清培养基预培养绵羊卵泡颗粒细胞24 h后,在无血清培养基内分别添加10 IU FSH、4 IU LH和1 μg·mL-1 E2,并转染miR-150启动子重组质粒,检测各组miR-150启动子活性,无激素组作为对照。结果显示,FSH、LH和E2组与对照组(hormone-free group)相比miR-150启动子的活性显著下调(P < 0.05),且E2组miR-150启动子活性下调最多(图 7)。
|
图 7 FSH、LH和E2对miR-150启动子活性的影响 Fig. 7 The effect of FSH, LH and E2 on miR-150 promoter activity |
基因启动子的鉴定是根据算法预测miRNA的核心启动子,然后利用生物学试验技术验证启动子区域活性以确定预测的片段是否准确。本研究利用在线软件Promoter 2.0预测miR-150的转录起始位点并确定其碱基序列,利用PROMO在线软件预测miR-150的繁殖相关转录因子结合位点。其中,ER-alpha[18]、RAR-beta[19]、SP1[20]与类固醇激素作用相关,RAR-beta[21]、Msx-1[22]、p300[23-24]、PAX-6[25]、Pax-2a[26]、Cdx-1[27]与胚胎发育相关。通过构建重组质粒和双荧光素酶报告基因对绵羊miR-150启动子区域进行鉴定,为研究miR-150在繁殖周期中的表达调控提供理论依据。
3.2 FSH、LH和E2对miR-150的表达调控生殖激素调控miR-150表达的试验中排除了血清中其他因素的干扰,FSH、LH和E2的添加量参考绵羊卵泡体外培养的条件[28]。研究表明,动物卵巢颗粒细胞体外培养时添加FSH[7]、LH[29]和E2[30]均利于卵泡的成熟和颗粒细胞存活。单独添加FSH和E2的剂量越高对卵泡的存活越有利[31-32],而高剂量(5~100 IU)的LH反而不利于卵泡的生长[33]。因此,本试验选择添加10 IU的FSH、4 IU的LH和1 μg·mL-1的E2,以初步判断它们对miR-150的表达调控。而激素处理后miR-150启动子活性降低,表明miR-150的转录受到抑制,间接证明了miR-150不利于颗粒细胞生长的观点。推测细胞在增殖的过程中,某些转录因子可结合在miR-150的转录因子结合位点来上调或下调miR-150的表达。ER-alpha为预测的miR-150繁殖相关转录因子,它在成熟卵泡的颗粒细胞中含量丰富,在生长卵泡的颗粒细胞中含量较少[34]。ER-alpha是否与miR-150启动子区域结合调控miR-150的表达仍需进一步研究。miR-150在绵羊黄体期卵巢皮质表达水平显著高于卵泡期,而激素FSH、LH、E2的总体水平卵泡期高于黄体期[35],表明这些激素可能抑制miR-150的表达。此外,对miR-150进行了研究,发现miR-150通过影响STAR、Cyp11a1和HSD3B1等参与孕酮合成基因的表达进而促进卵巢颗粒细胞凋亡[23],从而直接证明了miR-150不利于颗粒细胞生长。
4 结论本研究利用生物学软件预测出绵羊miR-150的启动子区域及其转录因子结合位点,通过质粒构建和双荧光素酶活性检测,成功获得了具有活性的miR-150启动子片段,并证明了FSH、LH和E2能够下调miR-150启动子的活性,从而抑制miR-150的表达。本研究结果为miR-150调控卵泡发育的机制研究提供了新思路。
| [1] |
李琴. microRNA对哺乳动物卵泡发育的影响[J]. 畜牧兽医学报, 2018, 49(12): 2558–2566.
LI Q. Roles of microRNA on mammalian follicle development[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(12): 2558–2566. (in Chinese) |
| [2] | BARTEL D P. MicroRNAs:genomics, biogenesis, mechanism, and function[J]. Cell, 2004, 116(2): 281–297. DOI: 10.1016/S0092-8674(04)00045-5 |
| [3] | YIN M M, LV M R, YAO G D, et al. Transactivation of microRNA-383 by steroidogenic factor-1 promotes estradiol release from mouse ovarian granulosa cells by targeting RBMS1[J]. Mol Endocrinol, 2012, 26(7): 1129–1143. DOI: 10.1210/me.2011-1341 |
| [4] | YAO G D, YIN M M, LIAN J, et al. MicroRNA-224 is involved in transforming growth factor-β-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4[J]. Mol Endocrinol, 2010, 24(3): 540–551. DOI: 10.1210/me.2009-0432 |
| [5] | CAYO-COLCA I S, YAMAGAMI Y, PHAN T C, et al. A combination of FSH and dibutyryl cyclic AMP promote growth and acquisition of meiotic competence of oocytes from early porcine antral follicles[J]. Theriogenology, 2011, 75(9): 1602–1612. DOI: 10.1016/j.theriogenology.2010.12.023 |
| [6] | ZAMAH A M, HSIEH M, CHEN J, et al. Human oocyte maturation is dependent on LH-stimulated accumulation of the epidermal growth factor-like growth factor, amphiregulin[J]. Hum Reprod, 2010, 25(10): 2569–2578. DOI: 10.1093/humrep/deq212 |
| [7] | YAO N, YANG B Q, LIU Y, et al. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells[J]. Endocrine, 2010, 38(2): 158–166. DOI: 10.1007/s12020-010-9345-1 |
| [8] |
郭文艳, 丛晶, 吴效科. 类固醇激素在卵泡生长发育过程中的作用[J]. 世界中西医结合杂志, 2008, 3(2): 120–122.
GUO W Y, CONG J, WU X K. The effect of steroid hormones in the growth and development of follicles[J]. World Journal of Integrated Traditional and Western Medicine, 2008, 3(2): 120–122. DOI: 10.3969/j.issn.1673-6613.2008.02.026 (in Chinese) |
| [9] | KLINGE C M. Estrogen regulation of microRNA expression[J]. Curr Genomics, 2009, 10(3): 169–183. DOI: 10.2174/138920209788185289 |
| [10] | SELIMOGLU-BUET D, RIVIÈRE J, GHAMLOUCH H, et al. A miR-150/TET3 pathway regulates the generation of mouse and human non-classical monocyte subset[J]. Nat Commun, 2018, 9(1): 5455. DOI: 10.1038/s41467-018-07801-x |
| [11] | CHEN X X, XU X N, PAN B, et al. miR-150-5p suppresses tumor progression by targeting VEGFA in colorectal cancer[J]. Aging (Albany NY), 2018, 10(11): 3421–3437. |
| [12] | SRUTOVA K, CURIK N, BURDA P, et al. BCR-ABL1 mediated miR-150 downregulation through MYC contributed to myeloid differentiation block and drug resistance in chronic myeloid leukemia[J]. Haematologica, 2018, 103(12): 2016–2025. DOI: 10.3324/haematol.2018.193086 |
| [13] | KHAN H A, ZHAO Y, WANG L, et al. Identification of miRNAs during mouse postnatal ovarian development and superovulation[J]. J Ovarian Res, 2015, 8: 44. DOI: 10.1186/s13048-015-0170-2 |
| [14] |
郑全辉, 田枫, 李文婷, 等. miR-150基因缺失对小鼠繁殖和血液学指标的影响[J]. 中国免疫学杂志, 2016, 32(10): 1409–1412.
ZHENG Q H, TIAN F, LI W T, et al. miR-150 deletion affects breeding and hematologic parameters of mice[J]. Chinese Journal of Immunology, 2016, 32(10): 1409–1412. DOI: 10.3969/j.issn.1000-484X.2016.10.001 (in Chinese) |
| [15] |
苗艳平, 陈烨, 王涵, 等. 吐鲁番黑羊黄体期和卵泡期卵巢microRNA差异表达分析[J]. 农业生物技术学报, 2018, 26(4): 660–669.
MIAO Y P, CHEN Y, WANG H, et al. Analysis of differentially expressed microRNA in luteal and follicular ovaries of Turpan Black Sheep (Ovis aries)[J]. Journal of Agricultural Biotechnology, 2018, 26(4): 660–669. (in Chinese) |
| [16] |
苗艳平, 刘若男, 魏彦辉, 等. 绵羊卵巢oar-mir-150靶向调节类固醇激素合成急性调节蛋白基因的表达[J]. 农业生物技术学报, 2018, 26(2): 234–245.
MIAO Y P, LIU R N, WEI Y H, et al. Target regulation of the expression of steroidogenicacute regulatory protein gene in sheep (Ovis aries) ovaries by oar-mir-150[J]. Journal of Agricultural Biotechnology, 2018, 26(2): 234–245. (in Chinese) |
| [17] | ZHOU R Y, MIAO Y P, LI Y M, et al. MicroRNA-150 promote apoptosis of ovine ovarian granulosa cells by targeting STAR gene[J]. Theriogenology, 2019, 127: 66–71. DOI: 10.1016/j.theriogenology.2019.01.003 |
| [18] | DE ALMEIDA CHUFFA L G, LUPI-JU'NIOR L A, COSTA A B, et al. The role of sex hormones and steroid receptors on female reproductive cancers[J]. Steroids, 2017, 118: 93–108. DOI: 10.1016/j.steroids.2016.12.011 |
| [19] | UDHANE S S, PANDEY A V, HOFER G, et al. Retinoic acid receptor beta and angiopoietin-like protein 1 are involved in the regulation of human androgen biosynthesis[J]. Sci Rep, 2015, 5: 10132. DOI: 10.1038/srep10132 |
| [20] | KRIKUN G, LOCKWOOD C J. Steroid hormones, endometrial gene regulation and the Sp1 family of proteins[J]. J Soc Gynecol Investig, 2002, 9(6): 329–334. DOI: 10.1177/107155760200900602 |
| [21] | LAURSEN K B, GUDAS L J. Combinatorial knockout of RARα, RARβ, and RARγ completely abrogates transcriptional responses to retinoic acid in murine embryonic stem cells[J]. J Biol Chem, 2018, 293(30): 11891–11900. DOI: 10.1074/jbc.RA118.001951 |
| [22] | BORGES L, IACOVINO M, MAYERHOFER T, et al. A critical role for endoglin in the emergence of blood during embryonic development[J]. Blood, 2012, 119(23): 5417–5428. DOI: 10.1182/blood-2011-11-391896 |
| [23] |
陈国珍, 朱静, 田杰. 组蛋白乙酰化酶p300和CREB结合蛋白在小鼠胚胎心发育中的时序表达[J]. 解剖学杂志, 2008, 31(4): 480–482.
CHEN G Z, ZHU J, TIAN J. Spatiotemporal expressions of histone acetyltransferases p300 and CREB binding protein during mouse embryonic heart development[J]. Chinese Journal of Anatomy, 2008, 31(4): 480–482. DOI: 10.3969/j.issn.1001-1633.2008.04.009 (in Chinese) |
| [24] |
蒋静. 影响小鼠胚胎时期心脏发育的相关蛋白[J]. 实验与检验医学, 2014, 32(2): 153–156, 171.
JIANG J. Related proteins of affecting mouse embryonic heart development[J]. Experimental and Laboratory Medicine, 2014, 32(2): 153–156, 171. DOI: 10.3969/j.issn.1674-1129.2014.02.017 (in Chinese) |
| [25] | EDQVIST P H, MYERS S M, HALLBÖÖK F. Early identification of retinal subtypes in the developing, pre-laminated chick retina using the transcription factors Prox1, Lim1, Ap2α, Pax6, Isl1, Isl2, Lim3 and Chx10[J]. Eur J Histochem, 2006, 50(2): 147–154. |
| [26] | PHELPS D E, DRESSLER G R. Identification of novel Pax-2 binding sites by chromatin precipitation[J]. J Biol Chem, 1996, 271(14): 7978–7985. DOI: 10.1074/jbc.271.14.7978 |
| [27] | DUPREY P, CHOWDHURY K, DRESSLER G R, et al. A mouse gene homologous to the Drosophila gene caudal is expressed in epithelial cells from the embryonic intestine[J]. Genes Dev, 1988, 2(12A): 1647–1654. DOI: 10.1101/gad.2.12a.1647 |
| [28] |
常迪, 董晓晨, 郭勇, 等. 不同培养方法及培养密度对绵羊腔前卵泡发育的影响[J]. 中国农学通报, 2017, 33(8): 130–134.
CHANG D, DONG X C, GUO Y, et al. Effect of different culture methods and densities on sheep preantral follicles development[J]. Chinese Agricultural Science Bulletin, 2017, 33(8): 130–134. (in Chinese) |
| [29] | WU J, XU B, WANG W. Effects of luteinizing hormone and follicle stimulating hormone on the developmental competence of porcine preantral follicle oocytes grown in vitro[J]. J Assist Reprod Genet, 2007, 24(9): 419–424. DOI: 10.1007/s10815-007-9154-5 |
| [30] | QUIRK S M, COWAN R G, HARMAN R M. The susceptibility of granulosa cells to apoptosis is influenced by oestradiol and the cell cycle[J]. J Endocrinol, 2006, 189(3): 441–453. DOI: 10.1677/joe.1.06549 |
| [31] | YAVAS Y, JOHNSON W H, WALTON J S. Modification of follicular dynamics by exogenous FSH and progesterone, and the induction of ovulation using hCG in postpartum beef cows[J]. Theriogenology, 1999, 52(6): 949–963. DOI: 10.1016/S0093-691X(99)00185-5 |
| [32] |
杨世华.几种生殖激素对体外培养的牦牛卵泡颗粒细胞凋亡的影响[D].兰州: 甘肃农业大学, 2002.
YANG S H.Effects of several reproductive hormones on apoptosis of ovarian granulose cells of Yaks in vitro[J]. Lanzhou: Gansu Agricultural University, 2002.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10733-2002073331.htm |
| [33] | SARAIVA M V A, CELESTINO J J H, CHAVES R N, et al. Influence of different concentrations of LH and FSH on in vitro caprine primordial ovarian follicle development[J]. Small Ruminant Res, 2008, 78(1-3): 87–95. DOI: 10.1016/j.smallrumres.2008.05.008 |
| [34] |
孙健红, 张涌, 郑月茂, 等. ERα免疫反应产物在山羊卵巢中的分布[J]. 畜牧兽医学报, 2006, 37(9): 920–923.
SUN J H, ZHANG Y, ZHENG Y M, et al. Distribution of estrogen receptor α immunoreactivity in goats ovary[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(9): 920–923. DOI: 10.3321/j.issn:0366-6964.2006.09.016 (in Chinese) |
| [35] | ORTEGA I, GARCÍA-VELASCO J A, PELLICER A. Ovarian manipulation in ART:going beyond physiological standards to provide best clinical outcomes[J]. J Assist Reprod Genet, 2018, 35(10): 1751–1762. DOI: 10.1007/s10815-018-1258-6 |



