牦牛是青藏高原重要的动物资源,是牛属动物中唯一对低氧环境具有强适应能力的动物,其繁殖力低下,体外卵母细胞成熟率和胚胎发育能力均较低,严重影响其体外受精、体细胞克隆等繁殖技术的发展[1]。卵母细胞的体外成熟是哺乳动物重要的辅助繁殖技术,其广泛应用于动物的生殖发育[2],同时对体外受精及胚胎发育具有非常重要的作用[3]。肿瘤坏死因子(tumor necrosis factor,TNF-α)是由淋巴细胞、心肌细胞、激活的巨噬细胞、内皮细胞和脂肪细胞等多种细胞产生的一种主要炎性细胞因子[4],可诱导发生各种细胞反应,如细胞存活、细胞增殖和细胞死亡等[5]。有研究表明,TNF-α在不同物种的卵巢中具有活性,大鼠TNF-α主要来源于卵母细胞[6]。Hunt等[7]研究表明,TNF-α及其受体在小鼠胎盘、卵巢、输卵管、子宫以及早期胚胎中均有表达,Naz等[8]研究表明,TNF-α及其受体Ⅱ型(TNFR-Ⅱ)mRNA和蛋白质在人卵母细胞和卵丘细胞均有表达,还有研究表明,低剂量的TNF-α对牛卵母细胞和早期胚胎的发育具有抑制细胞凋亡的作用[9]。有研究表明,TNF-α是排卵的重要介质,可以通过细胞凋亡和自噬重塑卵巢组织,减少卵母细胞释放数量和诱导未破裂卵泡的颗粒细胞死亡[10]。Johnson等[11]研究发现,在促性腺激素释放激素(GnRH)给药前,TNF-α主要局限于卵母细胞,但在排卵中后期主要表达于卵丘细胞中,表明卵母细胞-卵丘细胞复合物是可溶性TNF-α的主要来源。TNF-α还可通过参与颗粒细胞的分化[12]、卵丘细胞的扩张[13]以及对膜间质细胞的聚集[14]等促进卵泡的发育。还有研究证实,卵母细胞也是TNF-α的主要来源及其靶细胞之一,卵母细胞的TNF-α主要表达于其出生前后并持续至整个卵泡成熟及排卵[15]。以上研究表明,TNF-α对卵泡的发育以及卵母细胞的成熟具有重要作用。
在缺氧条件下,低氧诱导因子1α (hypoxia inducible factor-1 alpha, HIF-1α)启动心肌细胞中的外来体介导TNF-α的表达[16],将HIF-1α cRNA注射到非洲爪蟾卵母细胞中证实了通过细胞质多腺苷酸化可以抑制由HIF-1αmRNA的3′-UTR介导的HIF-1α蛋白水平,并且还可影响其卵母细胞的成熟[17]。Van等[18]指出,当TNF-α处理诱导内源性NF-κB时,可以调节缺氧诱导的HIF-1α,还有研究表明,通过抑制NF-κB途径可以抑制TNF-α介导的HIF-1α基因转录的诱导,同时在常氧条件下TNF-α存在时HIF-1α靶基因的转录也会降低[19]。热休克蛋白70(heat shock proteins 70, HSP70)作为重要的保守蛋白之一[20],可以预防TNF-α诱导的致死性炎症休克和肝细胞凋亡[21],还可以与贫血互补组C基因产物协同以防止暴露于TNF-α造血细胞中的细胞凋亡[22]。有研究表明,大多数的HSPA家族成员在正常胚胎中的表达高于退化的胚胎[23]。在轻度应激组中,卵巢反应和卵母细胞发育潜能与对照组相似,而胚胎的HSP70表达显著升高(P < 0.05),在胚胎中观察到的HSP70过表达可能与其慢性不可预测的应激保护作用有关[24]。目前尚未见关于TNF-α对牦牛卵母细胞HIF-1α和HSP70表达及胚胎发育能力影响方面的报道。
本试验通过在牦牛卵母细胞体外培养时加入不同浓度的TNF-α,检测牦牛卵母细胞的成熟率以及后续胚胎发育能力,采用RT-qPCR和Western blotting分别在mRNA和蛋白水平检测HIF-1α和HSP70的表达,采用免疫荧光染色法检测加入不同浓度TNF-α后卵母细胞和囊胚的表达,以期阐明TNF-α与HIF-1α、HSP70对卵母细胞成熟以及胚胎发育的影响,为进一步探讨TNF-α在牦牛生殖过程中对卵母细胞以及胚胎发育所发挥的作用提供理论依据。
1 材料与方法 1.1 材料和试剂肿瘤坏死因子(TNF-α)购自Kingfisher Biotech.Inc公司,促卵泡素(FSH)、促黄体素(LH)、雌二醇(E2)、透明质酸酶以及M199培养液均购自Sigma公司;胎牛血清FBS为Hyclone公司产品;微量样品总RNA提取试剂盒购自Omega公司;两步法反转录试剂盒购自Promega公司;SYBR Premix DimerEraserTM(2×)购自宝生物(TaKaRa)公司;其他试剂均为国产分析纯。HSP70抗体(ab79852, Munich, Germany)为Abcam公司产品;HIF-1α抗体(bs-0737R)和所有二抗为北京博奥森公司产品。倒置荧光显微镜(olympus,日本),荧光定量PCR仪(ABI ViiA7,Life technologies公司,美国)。
1.2 牦牛卵巢的采集及体外成熟培养牦牛的卵巢样品采自青海省西宁市乐家湾屠宰场。将采取的牦牛卵巢放置于含36 ℃生理盐水的保温壶中,6 h内带回实验室,用生理盐水洗3遍,用带18G针头的注射器采集卵巢表面直径约3~10 mm的卵泡,在显微镜下挑选胞质均匀、形态完好、周围带有3层以上致密卵丘细胞的卵丘-卵母细胞复合体(cumulus-oocyte complexes,COCs),用洗卵液(DPBS + 5% FBS)清洗3遍,将COCs随机分为4组,分别放入已加不同浓度的TNF-α(使其最终浓度为0、10、25、50 ng·mL-1)的成熟液(M199+ 10%FBS + 50 μg·mL-1LH + 100 μg·mL-1 FSH +100 μg·mL-1 E2+ 100 μg·mL-1链霉素+ 100 μg·mL-1青霉素)中,再将其放入38 ℃、5% CO2及饱和湿度的条件下进行成熟培养,培养24 h后,将COCs取出,清洗后收集部分成熟COCs,为提取RNA做准备,部分成熟COCs进行免疫固定,其余成熟的COCs进行体外受精,每个试验组重复5次。
1.3 牦牛卵母细胞成熟率统计将成熟的COCs放入0.1%的透明质酸酶中,用移液枪轻微吹打,除去周围的卵丘细胞而得到裸卵,根据第一极体的排出情况统计成熟的卵母细胞(M Ⅱ期),每个试验组重复5次。
1.4 牦牛体外受精及卵母细胞发育率统计取出冻精管放置在37 ℃的水浴中快速解冻冻精,再将解冻后的精液小心缓慢地移入到含有3 mL预热受精液的15 mL的离心管中,以1 000 r·min-1离心15 min,弃去上清,重复2次。再将沉淀小心移入含1 mL精子获能液的离心管中,放入38 ℃、5% CO2的培养箱中进行上游孵育1 h,取其上清液,将其精子密度调节至(2~4)×106个·mL-1。
将卵母细胞在体外成熟24 h后,用BO液洗涤1次,再用受精液将其洗涤2次,将其移入事先准备好的50 μL受精液滴中,每滴为10~15枚,同时在每个受精滴中加入已调制好的精子50 μL,放置到38 ℃、5% CO2、湿度饱和的培养箱中进行受精12 h。采用吸卵针去除卵母细胞周围的卵丘细胞,将其受精的卵母细胞移入事先平衡好的覆盖有矿物油的SoFaa液中,每100 μL的微滴约有15个卵母细胞,再将其放置在38 ℃、饱和湿度及5% CO2的培养箱中进行培养,于试验所需阶段统计其发育率,并分别收集免疫荧光染色和实时荧光定量的样品。
1.5 RNA提取及反转录用微量样品总RNA提取试剂盒提取加不同浓度TNF-α培养后COCs的总RNA,并用反转录试剂盒将其RNA反转录为cDNA,-80 ℃保存备用。
1.6 引物设计及HIF-1α和HSP70基因的扩增根据GenBank数据库中检索的牛HIF-1α(AB018398)、HSP70(U09861.1)和β-actin(JF830811)的mRNA序列,用Primer Premier 5.0软件设计引物(表 1)。以反转录的cDNA为模板,用HIF-1α、HSP70和β-actin的引物扩增牦牛HIF-1α、HSP70和β-actin基因。PCR的反应体系:1 μL cDNA,上下游引物各0.5 μL,10 μL Taq PCR Master Mix,8 μL ddH2O, 总反应体系为20 μL。具体的PCR反应条件:95 ℃预变性4 min;95 ℃变性30 s,(HSP70 60 ℃,HIF-1α 59 ℃,β-actin 60 ℃)退火30 s,72 ℃延伸15 s,35个循环;72 ℃保存10 min。将扩增产物用10 g·L-1的琼脂糖凝胶电泳检测。
|
|
表 1 Real-time PCR引物信息 Table 1 The information of primers used in real-time PCR |
用实时荧光定量PCR仪检测HIF-1α和HSP70基因mRNA水平的表达,其反应体系为20 μL:1 μL cDNA,10 μL FastStart Master SYBR Green MixTM(2×),上下游引物各0.5 μL,ROX Reference Dye Ⅱ(50×)0.5 μL,ddH2O 7.5 μL。反应条件:95 ℃预变性10 s;95 ℃变性4 s,60 ℃(HSP70,β-actin)、59 ℃(HIF-1α)退火20 s,72 ℃延伸10 s,35个循环;每个样品进行3次重复。
1.8 Western blotting检测牦牛HIF-1α和HSP70蛋白的表达提取TNF-α浓度为0、10、25、50 ng·mL-1组的总蛋白,与上样缓冲液(4×)按3:1混合,用100 ℃煮10 min,冰上5 min,然后将其进行SDS-PAGE电泳;再用半干法将其转移至聚偏二氟乙烯(PVDF)膜上,用5%的脱脂奶粉溶液在室温下封闭1.5 h(摇床振动,70 r·min-1),分别用HIF-1α和HSP70的抗体在4 ℃环境下孵育过夜,再用二抗在室温下孵育1.5 h,每步结束后都用TBST洗膜,每遍5 min洗3次,用ECL避光显色,X光片显影、定影,扫描蛋白印迹条带。试验重复做3次,对其扫描的蛋白印迹条带用Image J软件进行图像分析。
1.9 免疫荧光染色检测牦牛HIF-1α和HSP70蛋白的定位根据Western blotting检测HIF-1α和HSP70蛋白表达的结果,将检测出蛋白表达量最高组的COCs用免疫染色固定液在室温下固定1 h,再将其移入含2%BSA的PBS稀释成5 μg·mL-1的一抗HIF-1α和HSP70中,4 ℃过夜孵育,用含2%BSA的PBS洗3遍,再移入二抗中孵育2 h,清洗3遍后封片,用荧光显微镜观察并照相。使用含有2%BSA的PBS替代一抗作为阴性对照组,其他步骤与试验组保持一致。
2 结果 2.1 TNF-α对牦牛卵母细胞成熟及后续胚胎发育能力的影响图 1为牦牛未成熟卵母细胞、成熟卵母细胞、消化成熟卵母细胞后所排出第一极体以及体外受精胚胎不同阶段的发育情况。根据消化成熟卵母细胞后所排出第一极体的情况来统计其卵母细胞的成熟率,同时在受精后48、72、96、120和192 h分别统计卵母细胞的成熟率、卵裂率、囊胚率。统计发现(表 2),在0、10和25 ng·mL-1 TNF-α组中,随着TNF-α浓度的增加,卵母细胞的成熟率、卵裂率和囊胚率逐渐增加,25 ng·mL-1 TNF-α组卵母细胞的成熟率和卵裂率达到最高,分别为84.98%和66.85%,而囊胚率也达到了21.48%,均显著高于对照组的成熟率79.89%、卵裂率61.59%和囊胚率16.12%(P<0.01)。但当TNF-α浓度增加到50 ng·mL-1时,其成熟率、卵裂率和囊胚率均显著下降,分别为74.72%、55.14%和10.66%,均显著低于对照组和25 ng·mL-1 TNF-α组(P<0.01)。
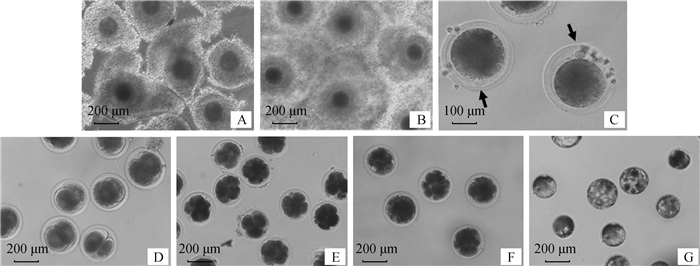
|
A.未成熟卵母细胞;B.成熟卵母细胞;C.成熟卵母细胞排出的第一极体;D. 2-细胞;E. 4-~8-细胞;F.桑椹胚;G.囊胚 A.Immature oocytes; B. Mature oocyte; C. The first polar body exhausted by mature oocyte; D. 2-cell embryos; E. 4--8-cell embryos; F. Morula; G. Blastocysts 图 1 牦牛未成熟的卵母细胞、成熟卵母细胞、体外受精胚胎发育的不同阶段 Fig. 1 Immature oocytes, mature oocytes, the different stages of development of in vitro fertilization embryos of yak |
|
|
表 2 不同浓度TNF-α对牦牛卵母细胞成熟和后续胚胎发育能力的影响 Table 2 The effects of TNF-α with different concentrations on oocyte maturation and subsequent development of embryos of yak |
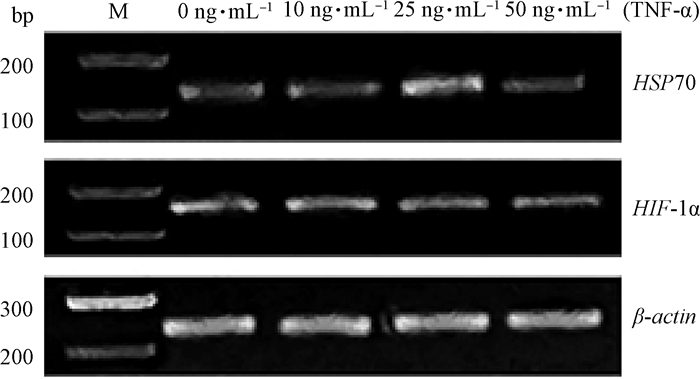
琼脂糖凝胶电泳检测结果见图 2,分别为基因HIF-1α、HSP70和β-actin的扩增产物,说明反转录后的cDNA可以用于后续试验。
|
图 2 HIF-1α、HSP70和β-actin基因RT-PCR产物的电泳分析 Fig. 2 RT-PCR products of electrophoretic analysis of HIF-1α, HSP70 and β-actin genes |
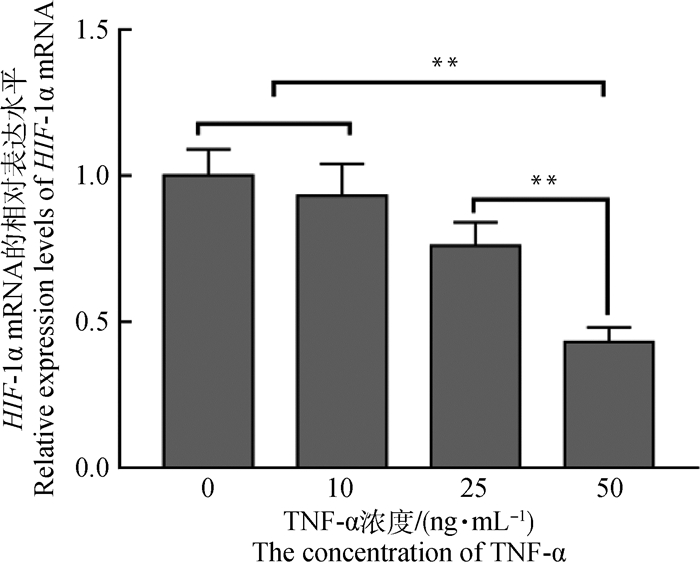
通过实时荧光定量对HIF-1α的相对表达量分析发现(图 3),随着TNF-α浓度的升高,HIF-1α的表达量逐渐降低,在0 ng·mL-1 TNF-α组中,HIF-1α的表达量最高,其极显著高于25和50 ng·mL-1 TNF-α组(P<0.01),在50 ng·mL-1 TNF-α组中HIF-1α的表达量最低,其极显著低于0、10和25 ng·mL-1 TNF-α组(P<0.01)。
|
*表示差异显著(P<0.05),**表示差异极显著(P<0.01)。下同 * indicate significant difference (P < 0.05); ** indicate extremely significant difference (P < 0.01). The same as below 图 3 不同浓度TNF-α处理后HIF-1α在成熟COCs中的相对表达量 Fig. 3 Relative abundance of HIF-1α in mature COCs treated with different concentrations of TNF-α |
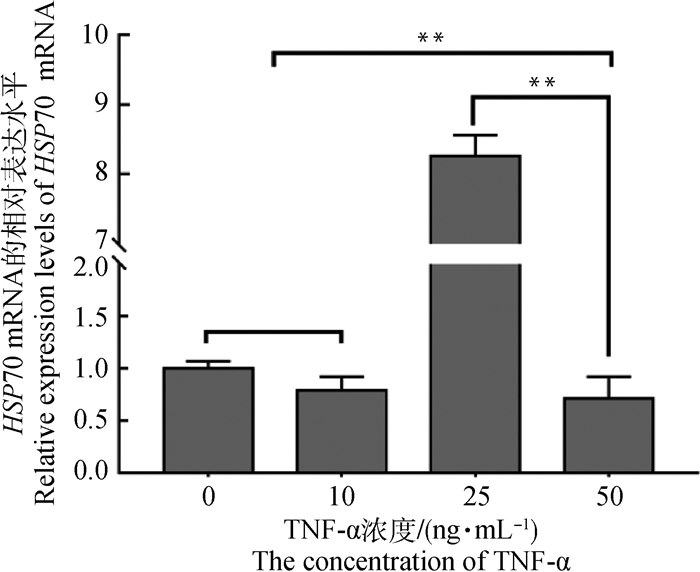
通过实时荧光定量对HSP70的相对表达量分析发现(图 4),在25 ng·mL-1 TNF-α组中,HSP70的表达量达到最高,其极显著高于其他组(P<0.01),而当TNF-α达到50 ng·mL-1时,HSP70的表达量最低。
|
图 4 不同浓度TNF-α处理后HSP70在成熟COCs中的相对表达量 Fig. 4 Relative abundance of HSP70 in mature COCs treated with different concentrations of TNF-α |
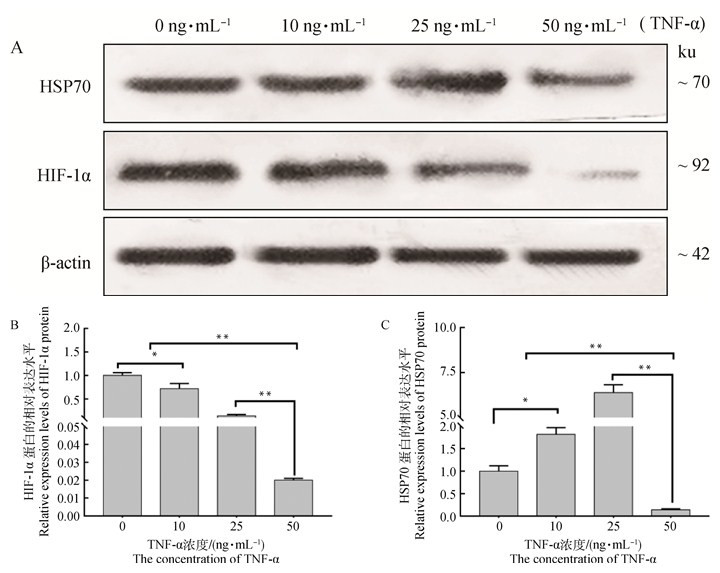
添加TNF-α降低了牦牛COCs中HIF-1α的蛋白表达量,同时也明显增加了牦牛COCs中HSP70的蛋白表达量。在0 ng·mL-1 TNF-α组中HIF-1α表达量最高,而在25 ng·mL-1 TNF-α组中HSP70的蛋白表达量最高(图 5)。
|
图 5 采用Western blotting方法检测不同组HIF-1α和HSP70蛋白水平的表达 Fig. 5 The expression levels of HIF-1α and HSP70 proteins detected by Western blotting in different groups |
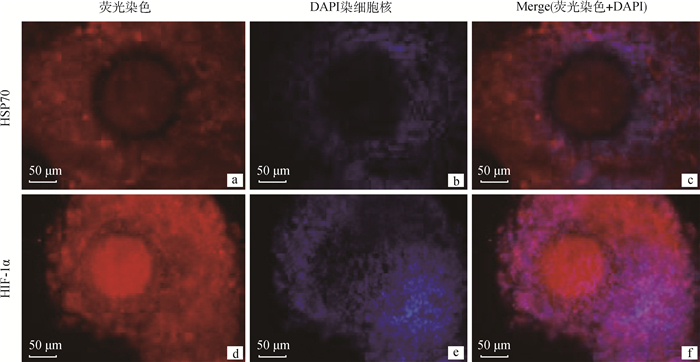
根据Western blotting检测HIF-1α和HSP70蛋白表达的结果发现,在0 ng·mL-1 TNF-α组中HIF-1α表达量最高,其显著高于其他组(P<0.05),而在25 ng·mL-1 TNF-α组中HSP70的蛋白表达量最高,与其他组差异性显著(P<0.05),对0和25 ng·mL-1 TNF-α组HIF-1α和HSP70蛋白分别用荧光进行荧光染色,通过免疫荧光染色检测发现,HSP70主要位于卵丘细胞中,而HIF-1α在卵丘细胞和卵母细胞上均可检测到(图 6)。
|
图 6 TNF-α的成熟液处理后牦牛COCs中HIF-1α、HSP70的蛋白分布(400×) Fig. 6 The distribution of HIF-1α and HSP70 proteins in yak COCs treated with different concentrations of TNF-α(400×) |
了解炎性细胞因子对卵母细胞成熟的调节作用以及对胚胎发育能力的影响对于研究有效的体外成熟(IVM)体系是至关重要的,本试验主要探讨了TNF-α对牦牛卵母细胞体外成熟的作用及TNF-α与HIF-1α和HSP70对卵母细胞成熟以及胚胎发育的关系。Kong等[25]近期研究发现,凋亡的小鼠COCs在体外衰老过程中可以释放出可溶性的TNF-α(sTNF-α),在TNF-α敲低和敲除的小鼠COCs中,sTNF-α的老化促进作用明显降低,表明小鼠COCs通过分泌sTNF-α来加速卵母细胞衰老。Deb等[26]研究表明,9-顺式维甲酸可以通过降低TNF-α在卵母细胞mRNA的表达提高猪卵母细胞的发育能力和胚胎的质量。Torchinsky等[27]发现,TNF-α可以作为暴露于致畸应激胚胎的保护剂。卵母细胞成熟是决定胚胎发育的重要因素,本研究发现,随着TNF-α浓度的增加,COCs的成熟率、卵裂率和囊胚率逐渐增加,当TNF-α为25 ng·mL-1时,COCs的成熟率和卵裂率达到最高,但当TNF-α浓度的增加到50 ng·mL-1时,其成熟率、卵裂率和囊胚率均显著下降,且均显著低于其他组(P<0.01,表 2)。因此,本研究发现,牦牛卵母细胞体外成熟过程中TNF-α的最适浓度为25 ng·mL-1,当TNF-α的浓度高于最适浓度后,TNF-α的浓度越高,卵母细胞的发育能力和胚胎的质量反而下降。
本研究发现,随着TNF-α浓度的升高,HIF-1α的表达量逐渐降低(图 3、图 5),表明TNF-α在牦牛COCs中抑制了HIF-1α的表达。有研究表明,TNF-α通过NF-κB依赖性途径诱导HIF-1α mRNA合成,但抑制HIF-1α与ARNT和DNA的结合,而缺氧和TNF-α对ASMC炎症具有不同的作用[14],这与本研究相一致。还有研究发现,抑制HIF-1α表达和活性氧生成显著降低了TNF -α诱导的FoxM1过度表达,FoxM1的上调促进了肝癌细胞的增殖,增强了它们对TNF-α诱导的细胞凋亡的抵抗力[28]。Tang等[29]研究发现,HIF-1α作用于TNF-α的下游可抑制VASP表达并可以调节急性肺炎,并且在肺泡-毛细血管屏障的损伤中起着重要作用。
HSP70为热休克蛋白家族中很重要的一族,可抑制凋亡的发生,在细胞分化和胚胎发育过程中具有重要作用[30]。本研究通过实时荧光定量和Western blotting检测发现,在基因和蛋白水平25 ng·mL-1 TNF-α组中HSP70的表达量均最高,显著高于其他组(P<0.05),表明TNF-α诱导了HSP70的表达。有研究表明,单核细胞或巨噬细胞中的HSP70体外诱导抑制细菌脂多糖刺激后TNF-α的产生,并且体内诱导HSP70在受损伤后下调了TNF-α的产生[31]。Mitsuhashi等[32]发现,HSP70、TNF-α的mRNA表达以时间和幅度依赖性方式增加,随着HSP70水平的增加,TNF-α表达在12 h内逐渐下降,表明HSP70可以响应CF调节hPDL细胞中TNF-α的表达。然而TNF-α通过诱导HSP70表达所发挥的生物学作用有待进一步研究。
4 结论在牦牛卵母细胞体外成熟的过程中,低浓度的TNF-α明显提高卵母细胞的发育能力,其最佳浓度为25 ng·mL-1,同时TNF-α还诱导了HSP70的表达、抑制了HIF-1α的表达,推测HIF-1α和HSP70在TNF-α诱导卵母细胞成熟过程中起着重要作用,为探讨TNF-α、HIF-1α和HSP70在牦牛生殖过程中发挥的作用以及对胚胎发育的影响提供了参考资料。
| [1] |
何翃闳, 潘阳阳, 张慧珠, 等. FSH对牦牛卵母细胞EGF、EGFR表达及其细胞凋亡的影响[J]. 畜牧兽医学报, 2018, 49(9): 1899–1907.
HE H H, PAN Y Y, ZHANG H Z, et al. The effects of follicle-stimulating hormone (FSH) on the expression of EGF and EGFR in yak oocytes and apoptosis[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(9): 1899–1907. (in Chinese) |
| [2] | XU X M, DUAN X, LU C F, et al. Dynamic distribution of NuMA and microtubules in human fetal fibroblasts, developing oocytes and somatic cell nuclear transferred embryos[J]. Hum Reprod, 2011, 26(5): 1052–1060. DOI: 10.1093/humrep/der067 |
| [3] | BINELLI M, MURPHY B D. Coordinated regulation of follicle development by germ and somatic cells[J]. Reprod Fertil Dev, 2009, 22(1): 1–12. |
| [4] | KAPADIA S, LEE J, TORRE-AMIONE G, et al. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration[J]. J Clin Invest, 1995, 96(2): 1042–1052. DOI: 10.1172/JCI118090 |
| [5] | VARFOLOMEEV E E, ASHKENAZI A. Tumor necrosis factor:an apoptosis JuNKie?[J]. Cell, 2004, 116(4): 491–497. DOI: 10.1016/S0092-8674(04)00166-7 |
| [6] | MARCINKIEWICZ J L, BALCHAK S K, MORRISON L J. The involvement of tumor necrosis factor-alpha (TNF) as an intraovarian regulator of oocyte apoptosis in the neonatalrat[J]. Front Biosci, 2002, 7: d1997–2005. |
| [7] | HUNT J S, CHEN H L, HU X L, et al. Normal distribution of tumor necrosis factor-α messenger ribonucleic acid and protein in the uteri, placentas, and embryos of osteopetrotic (op/op) mice lacking colony-stimulating factor-1[J]. Biol Reprod, 1993, 49(3): 441–452. DOI: 10.1095/biolreprod49.3.441 |
| [8] | NAZ R K, ZHU X L, MENGE A C. Expression of tumor necrosis factor-α and its receptors type Ⅰ and type Ⅱ in human oocytes[J]. Mol Reprod Dev, 1997, 47(2): 127–133. DOI: 10.1002/(ISSN)1098-2795 |
| [9] | SOTO P, NATZKE R P, HANSEN P J. Actions of tumor necrosis factor-α on oocyte maturation and embryonic development in cattle[J]. Am J Reprod Immunol, 2003, 50(5): 380–388. DOI: 10.1034/j.1600-0897.2003.00101.x |
| [10] | YAMAMOTO Y, KUWAHARA A, TANIGUCHI Y, et al. Tumor necrosis factor alpha inhibits ovulation and induces granulosa cell death in rat ovaries[J]. Reprod Med Biol, 2015, 14(3): 107–115. DOI: 10.1007/s12522-014-0201-5 |
| [11] | JOHNSON M L, MURDOCH J, VAN KIRK E A, et al. Tumor necrosis factor α regulates collagenolytic activity in preovulatory ovine follicles:relationship to cytokine secretion by the oocyte-cumulus cell complex[J]. Biol Reprod, 1999, 61(6): 1581–1585. DOI: 10.1095/biolreprod61.6.1581 |
| [12] |
刘汝宏. TNF系统与自身免疫性疾病[J]. 医学综述, 2003, 9(4): 200–202.
LIU R H. TNF system and autoimmune diseases[J]. Medical Recapitulate, 2003, 9(4): 200–202. DOI: 10.3969/j.issn.1006-2084.2003.04.004 (in Chinese) |
| [13] | ROBY K F, WEED J, LYLES R, et al. Immunological evidence for a human ovarian tumor necrosis factor-α[J]. J Clin Endocrinol Metab, 1990, 71(5): 1096–1102. DOI: 10.1210/jcem-71-5-1096 |
| [14] | SANCHO-TELLO M, PÉREZ-ROGER I, IMAKAWA K, et al. Expression of tumor necrosis factor-α in the rat ovary[J]. Endocrinology, 1992, 130(3): 1359–1364. |
| [15] | MARCINKIEWICZ J L, KRISHNA A, CHEUNG C M Y, et al. Oocytic tumor necrosis factor alpha:localization in the neonatal ovary and throughout follicular development in the adult rat[J]. Biol Reprod, 1994, 50(6): 1251–1260. DOI: 10.1095/biolreprod50.6.1251 |
| [16] | YU X, DENG L Y, WANG D, et al. Mechanism of TNF-α autocrine effects in hypoxic cardiomyocytes:initiated by hypoxia inducible factor 1α, presented by exosomes[J]. J Mol Cell Cardiol, 2012, 53(6): 848–857. DOI: 10.1016/j.yjmcc.2012.10.002 |
| [17] | HÄGELE S, KÜHN U, BÖNING M, et al. Cytoplasmic polyadenylation-element-binding protein (CPEB)1 and 2 bind to the HIF-1α mRNA 3'-UTR and modulate HIF-1α protein expression[J]. Biochem J, 2009, 417(1): 235–246. DOI: 10.1042/BJ20081353 |
| [18] | VAN UDEN P, KENNETH N S, ROCHA S. Regulation of hypoxia-inducible factor-1α by NF-κB[J]. Biochem J, 2008, 412(3): 477–484. DOI: 10.1042/BJ20080476 |
| [19] | TSAPOURNIOTI S, MYLONIS I, HATZIEFTHIMIOU A, et al. TNFα induces expression of HIF-1α mRNA and protein but inhibits hypoxic stimulation of HIF-1 transcriptional activity in airway smooth muscle cells[J]. J Cell Physiol, 2013, 228(8): 1745–1753. DOI: 10.1002/jcp.24331 |
| [20] | VAN MOLLE W, WIELOCKX B, MAHIEU T, et al. HSP70 protects against TNF-induced lethal inflammatory shock[J]. Immunity, 2002, 16(5): 685–695. DOI: 10.1016/S1074-7613(02)00310-2 |
| [21] | YANG R C, JAO H C, HUANG L J, et al. The essential role of PKCα in the protective effect of heat-shock pretreatment on TNFα-induced apoptosis in hepatic epithelial cell line[J]. Exp Cell Res, 2004, 296(2): 276–284. DOI: 10.1016/j.yexcr.2004.01.027 |
| [22] | PANG Q S, KEEBLE W, CHRISTIANSON T A, et al. FANCC interacts with Hsp70 to protect hematopoietic cells from IFN-γ/TNF-α-mediated cytotoxicity[J]. EMBO J, 2014, 20(16): 4478–4489. |
| [23] | HUANG W, YANDELL B S, KHATIB H. Transcriptomic profiling of bovine IVF embryos revealed candidate genes and pathways involved in early embryonic development[J]. BMC Genomics, 2010, 11: 23. DOI: 10.1186/1471-2164-11-23 |
| [24] |
李小红, 罗珊, 樊伟, 等. HSP70在不同应激程度CUMS昆明小鼠胚胎中的表达及作用[J]. 四川大学学报:医学版, 2017, 48(4): 515–519.
LI X H, LUO S, FAN W, et al. Expression of HSP70 in Kunming mouse embryos stimulated by chronic mild anticipatory stress[J]. Journal of Sichuan University:Medical Science Edition, 2017, 48(4): 515–519. (in Chinese) |
| [25] | KONG Q Q, WANG J, XIAO B, et al. Cumulus cell-released tumor necrosis factor (TNF)-α promotes post-ovulatory aging of mouse oocytes[J]. Aging, 2018, 10(7): 1745–1757. DOI: 10.18632/aging.v10i7 |
| [26] | DEB G K, DEY S R, BANG J I, et al. 9-cis retinoic acid improves developmental competence and embryo quality during in vitro maturation of bovine oocytes through the inhibition of oocyte tumor necrosis factor-α gene expression[J]. J Anim Sci, 2011, 89(9): 2759–2767. DOI: 10.2527/jas.2011-3848 |
| [27] | TORCHINSKY A, SHEPSHELOVICH J, ORENSTEIN H, et al. TNF-α protects embryos exposed to developmental toxicants[J]. Am J Reprod Immunol, 2003, 49(3): 159–168. DOI: 10.1034/j.1600-0897.2003.01174.x |
| [28] | XIA L M, MO P, HUANG W J, et al. The TNF-α/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis[J]. Carcinogenesis, 2012, 33(11): 2250–2259. DOI: 10.1093/carcin/bgs249 |
| [29] | TANG M J, TIAN Y H, LI D L, et al. TNF-α mediated increase of HIF-1α inhibits VASP expression, which reduces alveolar-capillary barrier function during acute lung injury (ALI)[J]. PLoS One, 2014, 9(7): e102967. DOI: 10.1371/journal.pone.0102967 |
| [30] | MASSEY A J, WILLIAMSON D S, BROWNE H, et al. A novel, small molecule inhibitor of Hsc70/Hsp70 potentiates Hsp90 inhibitor induced apoptosis in HCT116 colon carcinoma cells[J]. Cancer Chemother Pharmacol, 2010, 66(3): 535–545. DOI: 10.1007/s00280-009-1194-3 |
| [31] | MENG X Z, HARKEN A H. The interaction between Hsp70 and TNF-α expression:a novel mechanism for protection of the myocardium against post-injury depression[J]. Shock, 2002, 17(5): 345–353. DOI: 10.1097/00024382-200205000-00001 |
| [32] | MITSUHASHI M, YAMAGUCHI M, KOJIMA T, et al. Effects of HSP70 on the compression force-induced TNF-α and RANKL expression in human periodontal ligament cells[J]. Inflamm Res, 2011, 60(2): 187–194. DOI: 10.1007/s00011-010-0253-x |



