2. 山西农业大学动物科技学院, 太谷 030801
2. College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu 030801, China
牛在一个发情周期通常会出现2~3个卵泡波,其中仅最后一个卵泡波的优势卵泡(dominant follicle, DF)最终排卵[1-3]。低排卵率严重制约着胚胎工程技术的广泛应用和优良种畜的扩繁。课题组和美国密西根州立大学合作研究发现,在牛卵泡发育波中可卡因-苯丙胺调节转录肽(cocaine-amphetamine regulates transcriptional, CART)是一个重要的卵泡发育调节因子[4]。在卵泡发育出现优势化之前和优势早期,通过比较雌激素(estrogen, E2)不活跃(将要闭锁卵泡)和E2活跃卵泡(健康卵泡)表明,CART mRNA在E2不活跃卵泡高表达[5]。体外CART处理颗粒细胞(granulesa cells, GCs)可显著降低FSH[6]和IGF-I[4]诱导的卵泡GCs的E2分泌。为了深入研究CART调控卵泡发育的功能及其作用机理,在免疫共沉淀基础上,经同源建模和分子对接技术筛选发现,跨膜附睾蛋白1(transmembrane epididymal protein 1, TEDDM1)可能是CART候选受体之一[7]。目前,对TEDDM1功能研究相关报道甚少,Zimin等[8]通过全基因组测序对TEDDM1基因全长及其编码氨基酸进行了预测。Yamazaki等[9]通过基因芯片技术研究附睾中高表达基因,结果表明,TEDDM1在小鼠附睾中高表达;阉割后的小鼠附睾中该基因表达量降低,注射睾酮也不能恢复其高表达,雌激素的分泌也不可恢复。本课题组前期对牛DF和从属卵泡(subordinate follicle, SF)转录组测序研究发现,TEDDM1属于高差异表达基因,表明TEDDM1可能对牛卵泡发育具有重要调控作用[10]。
本研究选取牛卵泡作为研究对象,经RT-PCR扩增、克隆牛TEDDM1基因序列并进行序列测定和结构分析,利用qRT-PCR和免疫组织化学定位技术探讨TEDDM1在牛卵泡发育阶段的表达情况,为后期进一步证实TEDDM1是否为CART的受体及深入研究TEDDM1与牛卵泡发育的关系奠定基础。
1 材料与方法 1.1 试验材料选择健康的正常发情中国荷斯坦奶牛,PGF2α同期发情处理后,B超声波监测(每12 h一次)卵泡生长情况,卵泡出现优势化后屠宰母牛,采集双侧卵巢,投入4 ℃ DPBS中保存,实验室修剪卵泡并分离GCs,-80 ℃保存。
1.2 试验方法 1.2.1 牛卵泡GCs分离选择DF和SF,在盛有DPBS的平皿中,将卵泡一分为二剪开,刮刀刮取卵泡内细胞,丢弃卵泡膜性组织,将细胞悬浮液转移至离心管,4 ℃ 2 000 r·min-1离心3 min,弃上清即为GCs。
1.2.2 引物设计与合成参考GenBank提供的牛TEDDM1 mRNA(NW_005396127.1)核酸序列,Primer 5.0设计特异性引物,送大连宝生物公司合成,RT-PCR引物序列见表 1。
|
|
表 1 本研究所使用的引物序列 Table 1 Primer sequences used in this study |
按照Trizol RNA提取试剂盒说明书,提取GCs总RNA,经核酸测定仪检测合格后,对总RNA进行反转录合成cDNA,反应体系:5×Prime Script®Buffer 2 4 μL,Prime Script®RT Enzyme Mix1 1 μL,RT Primer Mix 1 μL,总RNA 0.8 μg,RNase Free water 4 μL,定容至20 μL反应。反应条件:37 ℃ 15 min,85 ℃ 5 s。
1.2.4 牛TEDDM1基因克隆与序列测定牛卵泡TEDDM1全CDS区扩增体系:2×Es TaqMasterMix(CWBIO,中国)12.5 μL,反转录cDNA 2 μL,上下游特异性PCR引物各1 μL,ddH2O 8.5 μL。PCR程序:95 ℃ 2 min;95 ℃ 30 s,60 ℃ 30 s,73 ℃ 1 min,30个循环。PCR产物经1%琼脂糖凝胶电泳检测,切胶后经试剂盒胶回收并纯化,克隆到pUC-T载体上(CWBIO,中国),送上海生工测序。
1.2.5 牛TEDDM1序列结构分析测序结果CDS区分析应用ORF finder(https://www.ncbi.nlm.nih.gov/orffinder/);NCBI在线BLAST(https://blast.ncbi.nlm.nih.gov/)对24种动物TEDDM1氨基酸序列比对分析;3D结构和结构域用Conserved Domain Search Services (CD Search) (www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi)分析。
1.2.6 牛TEDDM1基因qRT-PCR反应qRT-PCR反应体系如下(20 μL):SYBR® Green premix Ex TaqTMⅡ 10 μL,ROX Reference Dye Ⅱ 0.4 μL,上下游特异性PCR引物各0.8 μL(引物见表 1),cDNA 2 μL,ddH2O 6 μL。反应在7500型实时荧光定量PCR仪(Thermo Scientific,中国)上进行45个循环,95 ℃ 15 s,64 ℃ 1 min。内源性对照采用RPLP0基因(引物见表 1)。
1.2.7 统计分析TEDDM1在DF和SF中的相对表达量水平=2-△△CT[11],标准曲线的斜率为-3.139(dEff.= 105.6%),RPLP0标准曲线的斜率为-3.111(Eff.= 106.9%)。结果采用“平均值±标准差”表示,表达量结果经内参基因RPLP0表达量校正,以TEDDM1基因在DF的表达量作为对照组,数据用SPSS 18.0统计软件进行t检验分析。
1.2.8 免疫组织化学分析卵泡采集后置于4%多聚甲醛溶液固定,石蜡包埋。TEDDM1蛋白的免疫组化检测采用之前描述的程序[12],采用兔抗TEDDM1多克隆抗体(HA352681,1:100,浙江华安)进行检测。试验中设置阳性对照和阴性对照,其中阳性对照组用PBS代替TEDDM1抗体;阴性对照组用10 μg ·mL-1牛TEDDM1肽(中肽生化,杭州)与TEDDM1抗体预孵育过夜代替TEDDM1抗体。
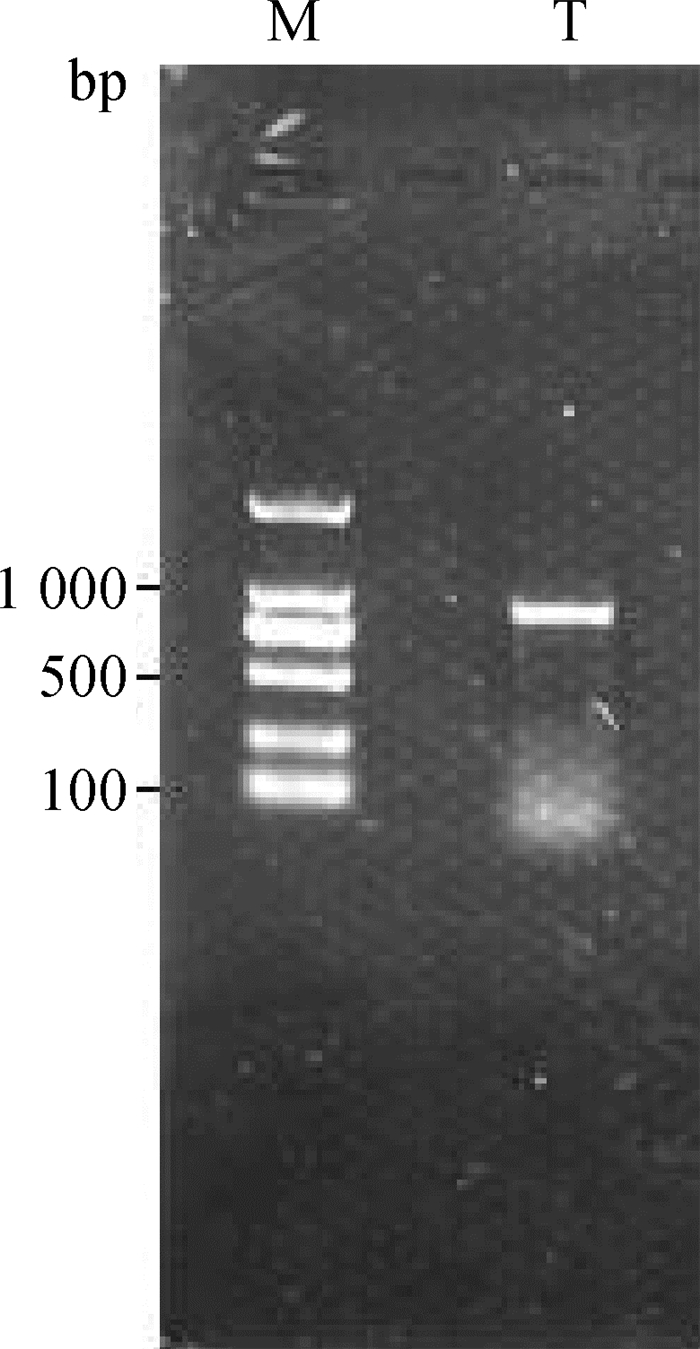
2 结果 2.1 牛TEDDM1基因PCR扩增总RNA提取后,经核酸检测仪检测,RNA浓度和纯度均符合后续试验要求。PCR扩增后,经凝胶电泳检测,可见大小为950 bp左右的条带,与预期结果一致;同时可见电泳条带有拖尾现象,但不影响目的条带的分离纯化(图 1)。
|
M. DNA相对分子质量标准;T.目的产物 M. DNA marker; T. Target product 图 1 TEDDM1的RT-PCR电泳图 Fig. 1 Electrophoresis pattern of RT-PCR product of TEDDM1 |
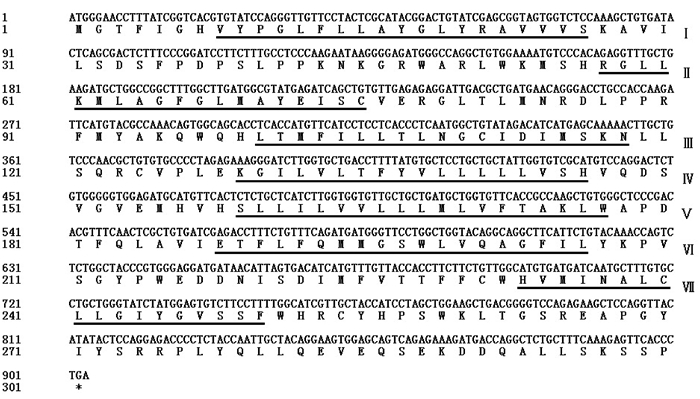
测序结果表明,牛卵泡TEDDM1基因CDS区全长903 bp,编码300个氨基酸;HMMTOP v2.0分析表明,TEDDM1蛋白序列中存在7个跨膜区段(图 2中下划线部分),分别位于8~26、57~75、100~118、129~146、159~177、188~206和233~250区段,符合G蛋白偶联受体(G protein-coupled receptors, GPCRs)的结构特征。
|
*表示终止子;下划线表示跨膜区段 * indicate the terminator; Underscore indicate the transmembrane sections 图 2 TEDDM1序列分析 Fig. 2 TEDDM1 sequence analysis |
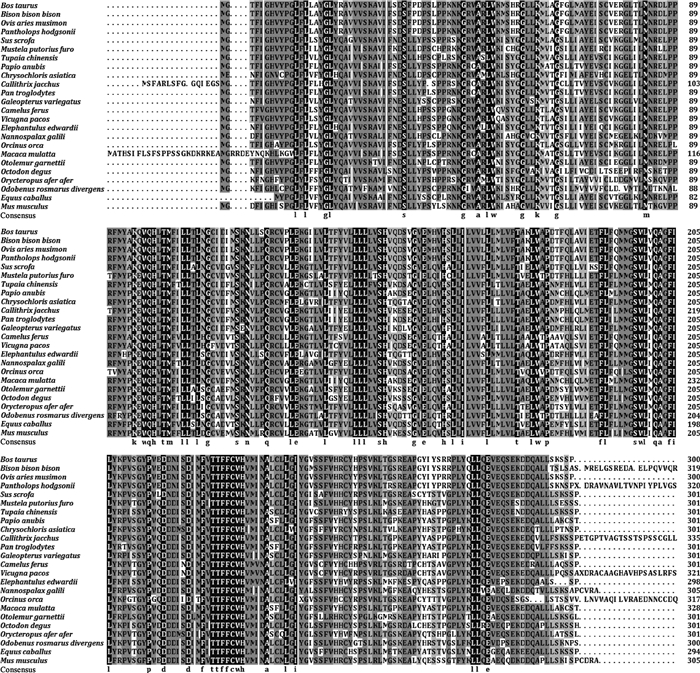
将牛卵泡TEDDM1全CDS区序列翻译为氨基酸序列,经NCBI数据库搜索,获得其它24种动物对应的同源氨基酸序列,利用BLAST在线软件对氨基酸序列进行比对分析。结果表明,牛TEDDM1序列与非洲野牛(Bison bison bison)序列相似性最高,为99.4%;与其它动物序列相似性为71.0%~97.7%,表明TEDDM1在物种进化过程中保守性较高(图 3)。
|
图 3 多物种TEDDM1氨基酸序列比对 Fig. 3 Multi-species amino acid sequence alignment of TEDDM1 |
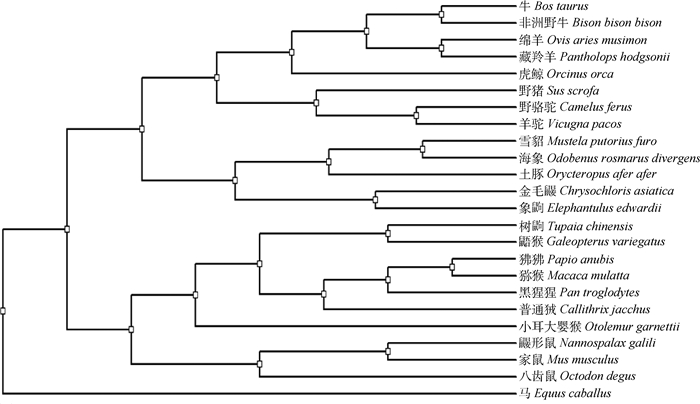
基于不同动物(包括偶蹄类、奇蹄类、鲸类、灵长类和啮齿类)TEDDM1氨基酸序列聚类分析,应用遗传距离邻近法系统发育分析表明,牛与非洲野牛(Bison bison bison)遗传距离最近,与家马(Equus caballus)遗传距离最远(图 4)。
|
图 4 TEDDM1氨基酸序列构建系统发生树 Fig. 4 Phylogenetic tree based on amino acid sequences of TEDDM1 |
由PDB数据库和CDD数据库预测TEDDM1立体结构和功能结构域(图 5),结果显示,氨基酸序列8~250位之间共形成7个螺旋结构(图 5A),并7次横跨胞膜,属于典型的GPCRs;功能域分析表明,TEDDM1蛋白分子中存在未知功能结构域蛋白家族(domains of unknown function protein families, DUFs)716结构域(图 5B)。
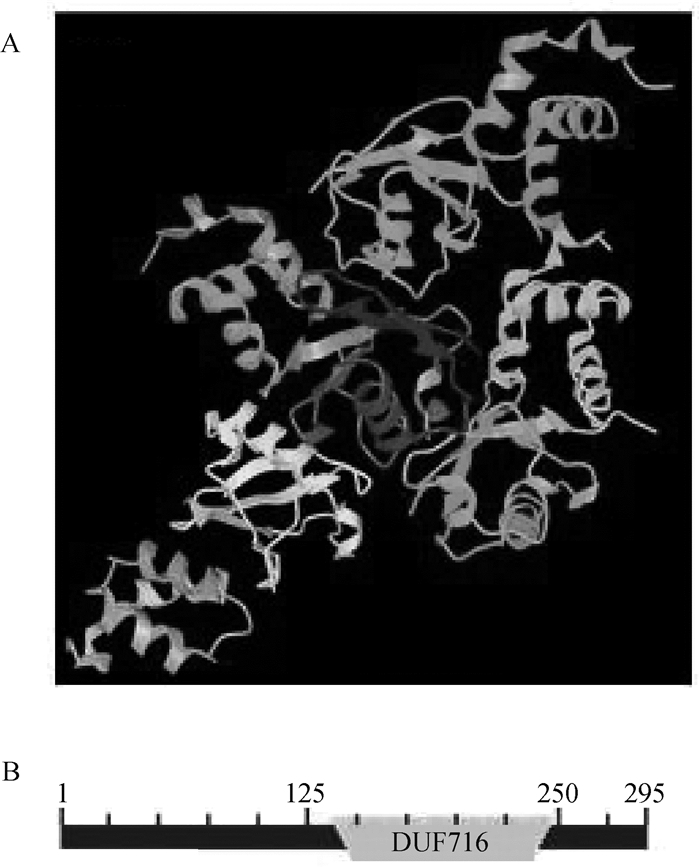
|
A.TEDDM1三维结构;B.TEDDM1功能域 A. Three-dimensional structure of TEDDM1;B. Functional domains of TEDDM1 图 5 牛TEDDM1立体结构和功能域预测 Fig. 5 Predicted three-dimensional structure and functional domains of bovine TEDDM1 |
经样本重复校正、物理校正和RPLP0内参基因校正,利用△△CT算法对TEDDM1在牛发情周期第一卵泡波DF和SF的表达量进行分析(图 6)。结果显示,TEDDM1在SF中的表达量显著高于DF(P<0.05)。
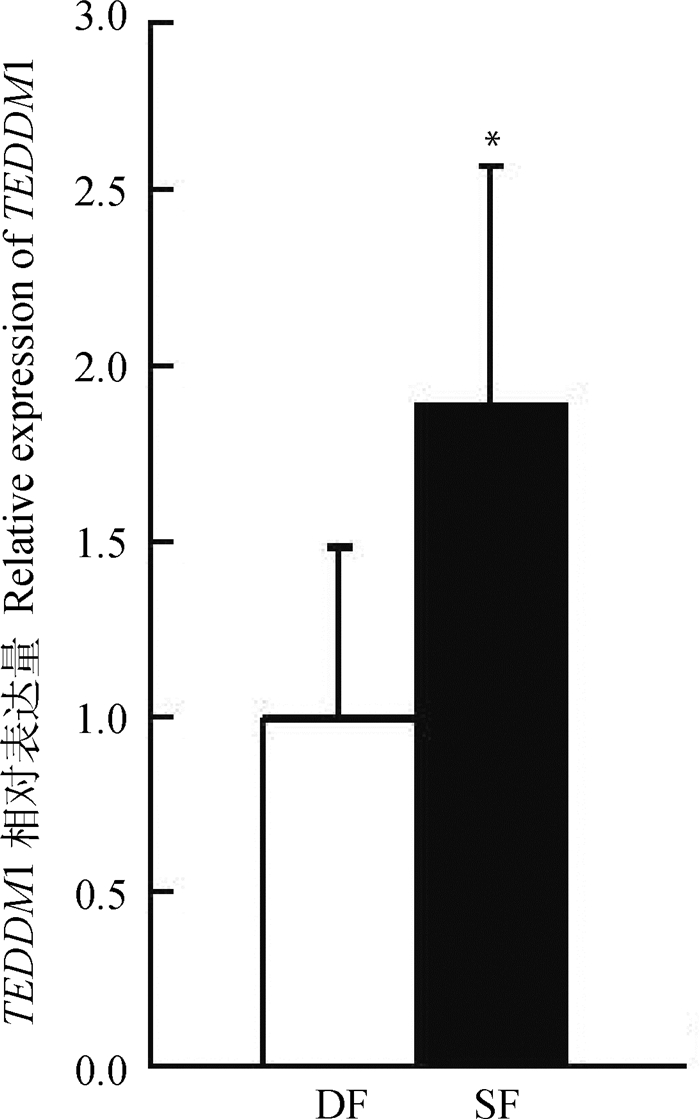
|
*表示在显著水平0.05的结果 * indicate significant difference at the level of 0.05 图 6 牛TEDDM1在DF和SF中的表达量 Fig. 6 Real-time PCR analysis of TEDDM1 expression in ovine DF and SF |
免疫组化分析表明,TEDDM1在牛DF和SF颗粒层、膜层细胞均有表达(图 7B、E),阳性对照组和阴性对照组均无特异性显色(图 7A、D、C、F)。显色反应表明,TEDDM1在SF颗粒层、膜层细胞显色强度明显高于DF,这与qRT-PCR分析结果相一致。
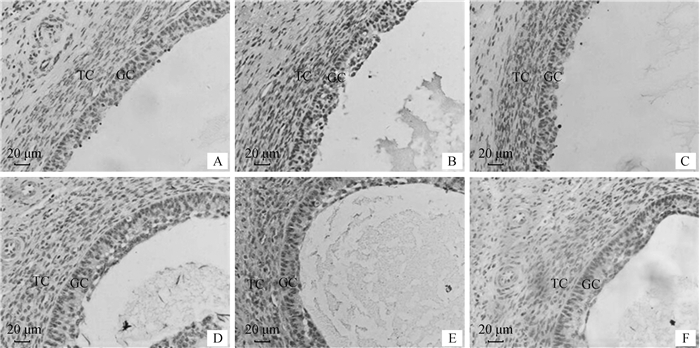
|
A、B、C分别为优势卵泡阳性对照组、试验组和阴性对照组;D、E、F分别为从属卵泡阳性对照组、试验组和阴性对照组。GC.颗粒细胞;TC.膜细胞 A, B, C. Control group, experiment group and negative control group of DF, respectively; D, E, F. Control group, experiment group and negative control group of SF, respectively. GC. Granulosa cells; TC. Theca cells 图 7 牛卵泡组织TEDDM1的表达(免疫组化400×) Fig. 7 Expression of TEDDM1 in ovine follicular tissue(immunohistochemical 400×) |
动物卵泡生长发育的整个过程受到各种内分泌激素和卵泡内生长因子的调控,为了进一步厘清有腔卵泡生长发育的内分泌调控机制,近年来,本课题组一直致力于筛选和识别参与卵泡生长发育的局部调控分子。该研究经RT-PCR技术获得牛卵泡TEDDM1全CDS区,经生物信息学分析表明,牛卵泡TEDDM1属于GPCRs,蛋白分子结构中存在DUF716结构域。
DUFs属于一类生物数据库中没有功能注释的蛋白家族[13],在生物体中广泛分布,其中有20%左右在真核生物有表达[14]。研究表明,一些DUFs在动物、真菌和植物中均有表达,如DUF221[15]、DUF547[16]和DUF647[17]等;而有些DUFs仅在植物中有表达,如DUF579[18]、DUF640[19]和DUF860[20]等。通过动植物转录组和蛋白组表达谱分析表明,DUFs在动植物生命活动中发挥重要生物学作用,因此,探究牛卵泡TEDDM1蛋白中DUF716结构域的功能具有重要意义。Okada等[21]研究发现,MtPiI4预测蛋白中包含有DUF716结构域,在真核生物非典型膜蛋白中,该结构域具有显著的家族蛋白特征,参与对细菌入侵植物的免疫调节,可能与信号通路的抑制有关[22]。人TMEM45A编码区包含有DUF716结构域,在人体中参与机体抗病毒应答[23-24],TMEM45A表达量降低会导致导管癌发展为浸润性乳腺癌[25];哺乳动物相关研究发现,TMEM45A可以刺激宿主的免疫,这说明TMEM45A可以有效防御病毒性感染。TEDDM1虽包含有DUF716结构域,然而,对其功能仍未有明确注释[9]。TEDDM1基因表达局限于动物附睾初始段上皮细胞,在精子成熟过程中起关键作用。多瘤病毒增强激活因子3(polyomavirus enhancer activator 3, PEA3)转录因子主要在附睾起始段表达,在该区域表达的基因启动子中发现二者具有一致位点AGGAAA/G,表明TEDDM1可能与早期精子成熟过程中起始信号传导途径相关[26-27]。
TEDDM1属于7次跨膜螺旋的GPCRs。从结构上看,由胞外N端和胞内C端组成,参与细胞识别和信号转导。胞外区域结合细胞外信号分子,胞内区域招募并结合下游信号分子,如G蛋白和第二信使等,下游信号分子主要通过cAMP信号通路和磷脂酰肌醇信号通路参与调节体内多项生理活动[28]。研究表明,CART可能是牛卵泡TEDDM1胞外区域的结合因子[7],CART是一种内源性神经肽,对牛卵泡发育有着负调控作用,是引起卵泡闭锁的原因之一[29]。Sen等[30]对CART信号传导通路进行了研究,发现CART加速FSH诱导的牛卵泡颗粒细胞Erk1/2和Akt信号的终止,单独刺激诱导Erk1/2激活过程中Go/i-PKC-MEK依赖的通路。提示,TEDDM1可能在CART调控牛卵泡发育过程中发挥特异性信号调控作用。
牛卵泡GCs的凋亡是引起卵泡闭锁的重要原因,研究表明,正常发育卵泡E2分泌量显著高于闭锁卵泡[5, 30]。在卵泡选择过程中,随着卵泡优势化出现,DF的抑制素和E2分泌量增加,抑制促卵泡激素的分泌,同时,DF分泌的抑制素使其它SF发育受阻[31]。本研究经qRT-PCR和免疫组化分析表明,TEDDM1在SF颗粒层和膜层表达量均显著高于DF,推测TEDDM1可能通过调控信号传导途径从而引起牛卵泡闭锁。
4 结论牛卵泡TEDDM1作为GPCRs可能通过Erk1/2和Akt信号传导途径调控GCs增殖和E2分泌,从而参与牛卵泡发育调节。本研究为进一步探讨TEDDM1在牛卵泡发育过程中的调控作用及其在信号转导和激素调节中的功能提供了依据,为后期深入研究牛卵泡发育机理奠定基础。
| [1] | RAJAKOSKI E. The ovarian follicular system in sexually mature heifers with special reference to seasonal, cyclical, end left-right variations[J]. Acta Endocrinol Suppl (Copenh), 1960, 34(S52): 1–68. |
| [2] | GOODMAN A L, NIXON W E, JOHNSON D K, et al. Regulation of folliculogenesis in the cycling rhesus monkey:selection of the dominant follicle[J]. Endocrinology, 1977, 100(1): 155–161. |
| [3] | IRELAND J J, ROCHE J F.Hypotheses regarding development of dominant follicles during a bovine estrous cycle[M]//ROCHE J F.Follicular growth and ovulation rate in farm animals.The Netherlands: Martinus Nijhoff Publishers, 1987: 1-18. |
| [4] | SMITH G W, SEN A, FOLGER J K, et al. Putative role of cocaine- and amphetamine-regulated transcript (CARTPT) in dominant follicle selection in cattle[J]. Soc Reprod Fertil, 2010, 67(S1): 105–117. |
| [5] | LV L H, JIMENEZ-KRASSEL F, SEN A, et al. Evidence supporting a role for cocaine- and amphetamine- regulated transcript(CARTPT) in control of granulosa cell estradiol production associated with dominant follicle selection in cattle[J]. Biol Reprod, 2009, 81(3): 580–586. |
| [6] | SEN A, BETTEGOWDA A, JIMENEZ-KRASSEL F, et al. Cocaine- and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells[J]. Endocrinology, 2007, 148(9): 4400–4410. DOI: 10.1210/en.2007-0332 |
| [7] |
李鹏飞.牛卵泡可卡因-苯丙胺调节转录肽(CART)受体的筛选[D].太谷: 山西农业大学, 2014.
LI P F.Screening of cocaine-and amphetamine-regulated transcript peptide(CART) receptor of cattle follicle[D].Taigu: Shanxi Agricultural University, 2014.(in Chinese) |
| [8] | ZIMIN A V, DELCHER A L, FLOREA L, et al. A whole-genome assembly of the domestic cow, Bos Taurus[J]. Genome Biol, 2009, 10: R42. DOI: 10.1186/gb-2009-10-4-r42 |
| [9] | YAMAZAKI K, ADACHI T, SATO K, et al. Identification and characterization of novel and unknown mouse epididymis-specific genes by complementary DNA microarray technology[J]. Biol Reprod, 2006, 75(3): 462–468. |
| [10] | LI P F, MENG J Z, ZHU Z W, et al. Detection of genes associated with follicle development through tran-scriptome analysis of bovine ovarian follicles GCs[J]. Curr Bioinform, 2018, 13(2): 127–140. |
| [11] | SHI L G, ZHAO H, REN Y S, et al. Effects of different levels of dietary selenium on the proliferation of spermatogonial stem cells and antioxidant status in testis of roosters[J]. Anim Reprod Sci, 2014, 149(3-4): 266–272. DOI: 10.1016/j.anireprosci.2014.07.011 |
| [12] |
李鹏飞, 毕锡麟, 王锴, 等. CART在不同发育阶段牛卵泡颗粒细胞中的表达和定位[J]. 中国农业科学, 2016, 49(12): 2389–2396.
LI P F, BI X L, WANG K, et al. Research on the expression and localization of CART in bovine granulosa cells at different developmental stages[J]. Scientia Agricultura Sinica, 2016, 49(12): 2389–2396. DOI: 10.3864/j.issn.0578-1752.2016.12.014 (in Chinese) |
| [13] | PUNTA M, COGGILL P C, EBERHARDT R Y, et al. The Pfam protein families database[J]. Nucleic Acids Res, 2012, 40(D1): D290–D301. DOI: 10.1093/nar/gkr1065 |
| [14] | BATEMAN A, COGGILL P, FINN R D. DUFs:families in search of function[J]. Acta Crystallogr Sect F Struct Biol Cryst Commun, 2010, 66(10): 1148–1152. DOI: 10.1107/S1744309110001685 |
| [15] | HOU C C, TIAN W, KLEIST T, et al. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes[J]. Cell Res, 2014, 24(5): 632–635. |
| [16] | PAVY N, BOYLE B, NELSON C, et al. Identification of conserved core xylem gene sets:conifer cDNA microarray development, transcript profiling and computational analyses[J]. New Phytol, 2008, 180(4): 766–786. DOI: 10.1111/nph.2008.180.issue-4 |
| [17] | SWARBRECK D, WILKS C, LAMESCH P, et al. The arabidopsis information resource (TAIR):gene structure and function annotation[J]. Nucleic Acids Res, 2008, 36(D1): D1009–D1014. |
| [18] | KO J H, BEERS E P, HAN K H. Global comparative transcriptome analysis identifies gene network regulating secondary xylem development in Arabidopsis thaliana[J]. Mol Genet Genomics, 2006, 276(6): 517–531. DOI: 10.1007/s00438-006-0157-1 |
| [19] | YOSHIDA A, SUZAKI T, TANAKA W, et al. The homeotic gene long sterile lemma (G1) specifies sterile lemma identity in the rice spikelet[J]. Proc Natl Acad Sci U S A, 2009, 106(47): 20103–20108. DOI: 10.1073/pnas.0907896106 |
| [20] | KROEGER T S, WATKINS K P, FRISO G, et al. A plant-specific RNA-binding domain revealed through analysis of chloroplast group Ⅱ intron splicing[J]. Proc Natl Acad Sci U S A, 2009, 106(11): 4537–4542. DOI: 10.1073/pnas.0812503106 |
| [21] | OKADA N, YAMAMOTO T, WATANABE M, et al. Identification of TMEM45B as a protein clearly showing thermal aggregation in SDS-PAGE gels and dissection of its amino acid sequence responsible for this aggregation[J]. Protein Expr Purif, 2011, 77(1): 118–123. DOI: 10.1016/j.pep.2011.01.011 |
| [22] | SUN D, CHEN J, ZHOU Z S, et al. Ectopic expression of a proteinase inhibitor I4(MtPiI4) gene from Medicago truncatula confers plant resistance to Pseudomonas syringae pv.Tomato DC3000[J]. .Plant Mol Biol Rep, 2015, 33(6): 1686–1696. |
| [23] | GERBER P A, HEVEZI P, BUHREN B A, et al. Systematic identification and characterization of novel human skin-associated genes encoding membrane and secreted proteins[J]. PLoS One, 2013, 8(6): e63949. DOI: 10.1371/journal.pone.0063949 |
| [24] | JUSTESEN J, HARTMANN R, KJELDGAARD N O. Gene structure and function of the 2′-5′-oligoadenylate synthetase family[J]. Cell Mol Life Sci, 2000, 57(11): 1593–1612. DOI: 10.1007/PL00000644 |
| [25] | LEE S, STEWART S, NAGTEGAAL I, et al. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer[J]. Cancer Res, 2012, 72(17): 4574–4586. DOI: 10.1158/0008-5472.CAN-12-0636 |
| [26] | LAN Z J, LABUS J C, HINTON B T. Regulation of gamma-glutamyl transpeptidase catalytic activity and protein level in the initial segment of the rat epididymis by testicular factors:role of basic fibroblast growth factor[J]. Biol Reprod, 1998, 58(1): 197–206. |
| [27] | FAISST S, MEYER S. Compilation of vertebrate-encoded transcription factors[J]. Nucleic Acids Res, 1992, 20(1): 3–26. DOI: 10.1093/nar/20.1.3 |
| [28] | AUDET M, BOUVIER M. Restructuring G-protein-coupled receptor activation[J]. Cell, 2012, 151(1): 14–23. |
| [29] | ARITRO S, BETTEGOWDA A, JIMENEZ-KRASSEL F, et al. Cocaine-and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells[J]. Endocrinology, 2007, 148(9): 4400–4410. DOI: 10.1210/en.2007-0332 |
| [30] | SEN A, LV L H, BELLO N, et al. Cocaine-and amphetamine-regulated transcript accelerates termination of follicle-stimulating hormone-induced extracellularly regulated kinase 1/2 and Akt activation by regulating the expression and degradation of specific mitogen-activated protein kinase phosphatases in bovine granulosa cells[J]. Mol Endocrinol, 2008, 22(12): 2655–2676. DOI: 10.1210/me.2008-0077 |
| [31] | VITALE A M, GONZALEZ O M, PARBORELL F, et al. Inhibin a increases apoptosis in early ovarian antral follicles of diethylstilbestrol-treated ratsl[J]. Biol Reprod, 2002, 67(6): 1989–1995. |



