肌内脂肪是沉积在骨骼肌肌纤维和肌束间的脂肪组织,是衡量牛肉质量的重要标准之一,与牛肉的多汁性、嫩度以及风味有很大关系[1-2]。近年来在动物育种中,GWAS[3]技术在发现调控重要经济性状的基因中已被广泛应用。本团队以1 141头西门塔尔牛为材料,使用Illumina 770 K高密度芯片分型数据对肉质性状进行了GWAS研究,筛选到与大理石花纹评分性状显著相关的候选基因S100A10[4]。
S100A10(S100 calcium binding protein A10)是S100家族成员之一,大多数情况下S100A10与外周膜结合蛋白ANNEXIN A2结合形成紧密的异四聚体复合物,作用于膜结构,尤其靶向于质膜和早期核内体的膜[5-6]。Reddy等[7]在小鼠和人乳腺癌细胞中发现,当沉默掉S100A10时,抑制了Annexin A2与细胞膜的结合。目前,关于S100A10在脂肪方面的研究鲜有报道。2017年Liu等[8]发现,在人肝癌细胞中过表达S100A10,其与细胞质中脂滴的位置一致;2016年Salameh等[9]在小鼠模型中发现,ANNEXIN A2与抗增殖蛋白(PHB)、脂肪酸转运者(CD36)形成复合物参与脂肪组织中脂肪酸的跨膜转运。
本研究以S100A10基因可能调控肉牛肌内脂肪沉积为出发点,采用小鼠前体脂肪细胞体外诱导分化模型,通过敲低S100a10基因并诱导其白色/棕色成脂分化来探究S100a10基因对于前体脂肪细胞成脂分化的影响,进而研究脂肪沉积和能量消耗两个生物学过程中S100a10的功能。为进一步研究S100A10基因对于肉牛肌内脂肪沉积的影响提供理论支持。
1 材料与方法 1.1 试剂与仪器TRIzol Reagent和Lipofectamine® RNAiMAX Reagent购自Invitrogen公司;PrimeScriptTMRT Master Mix购自TaKaRa公司;Ⅳ型胶原酶、地塞米松、3-异丁基-1-甲基黄嘌呤(IBMX)、胰岛素、吲哚美辛和三碘甲状腺原氨酸(T3)购自Sigma公司;胎牛血清、青链霉素双抗和胰酶购自Gibco公司;FABP4(sc-18661)和UCP1(sc-293418)抗体购自SantaCruz公司;PPARG(16643-1-AP)和β-tubulin(10094-1-AP)抗体购自Proteintech公司;荧光定量PCR仪为ABI Q7;激光共聚焦显微镜为Leica TCS SP8。
1.2 试验方法 1.2.1 小鼠前体脂肪细胞分离和培养12周龄的C57BL/6小鼠被处死后,用75%酒精进行消毒,用眼科剪沿着小鼠背部剪开,取腹股沟两侧的白色脂肪组织置于1.5 mL离心管中,用眼科剪将其剪成1 mm3大小的肉糜颗粒,加1 mg·mL-1的Ⅳ型胶原酶(溶于低糖DMEM培养基中)1 mL,置于37 ℃恒温空气浴摇床中消化45 min,消化结束先后用孔径为100和40 μm的尼龙网筛进行过滤,得到单细胞悬液,1 500 r·min-1离心5 min,弃上清后,用生长培养基(15%胎牛血清+84%低糖培养基+1%双抗)悬浮细胞,接种后于37 ℃,5%CO2培养箱中培养。
1.2.2 小鼠前体脂肪细胞传代及诱导分化当生长的原代前体脂肪细胞融合度达到约75%时,用0.25%的胰酶进行消化传代,接种在12孔板中,每2 d更换1次培养基,细胞融合度达到约100%时进行白色/棕色诱导分化。白色诱导分化培养基为生长培养基添加0.5 mmol·L-1 IBMX、1 μmol·L-1地塞米松和10 μg·mL-1胰岛素,诱导2 d后更换为维持培养基,即生长培养基添加10 μg·mL-1胰岛素;棕色诱导分化培养基为生长培养基添加0.5 mmol·L-1 IBMX、1 μmol·L-1地塞米松、10 μg·mL-1胰岛素、125 nmol·L-1吲哚美辛和1 nmol·L-1 T3,诱导2 d后更换为维持培养基,即生长培养基添加10 μg·mL-1胰岛素和1 nmol·L-1 T3。诱导分化的同时进行转染si-S100a10 (终浓度为50 nmol·L-1),首先用无血清的培养基稀释转染试剂,之后将其与siRNA混匀,室温孵育0~15 min,缓慢加入到培养基中。每2 d更换1次培养基,诱导分化至第6天时收取样品。
1.2.3 小鼠脂肪细胞进行油红O染色细胞培养至终末分化阶段,加入37%甲醛溶液至终浓度4%,室温固定细胞30 min,用PBS清洗,之后加入0.5%油红O溶液浸染30 min,在显微镜下观察脂滴融合情况,拍照后,用异丙醇将油红溶出来进行比色,并通过酶标仪在490 nm波长处测定样品吸光度。
1.2.4 总RNA的提取和荧光定量PCR依照TRIzol试剂说明书的操作步骤来提取总RNA,使用分光光度计检测总RNA浓度和纯度。取一部分RNA进行电泳,鉴定RNA的完整度,使用TaKaRa反转录试剂盒进行反转。利用Primer 5.0软件设计PCR扩增引物,由生工生物公司合成。引物序列见表 1。
|
|
表 1 qRT-PCR引物序列 Table 1 qRT-PCR primer sequence |
按照KAPA SYBR® FAST qPCR Kit Master Mix(2×)Universal试剂盒说明书,以18S为内参基因进行荧光定量PCR。PCR反应体系总体积为15 μL:Mix 7.5 μL,Rox 0.3 μL,cDNA 1 μL,上下游引物(10 μmol·L-1)各0.3 μL,ddH2O 5.6 μL。反应条件:95 ℃预变性3 min;95 ℃变性2 s,60 ℃退火20 s,72 ℃延伸1 min,40个循环。
1.2.5 蛋白提取和蛋白免疫印迹检测将细胞裂解后提取总蛋白,进行SDS-PAGE电泳;电泳结束后转移到NC膜上;转膜后用5%的脱脂奶粉进行室温封闭1 h;加入一抗,4 ℃过夜孵育,第2天用TBST室温摇洗3次,每次5 min;加入HRP标记的二抗,室温孵育1 h,用TBST室温摇洗3次,每次5 min;使用ECL试剂盒显色之后进行曝光,获取目的图像,针对目的基因和内参基因进行比较分析。
1.2.6 小鼠脂肪细胞免疫荧光检测培养好的细胞用冷的甲醇室温固定15 min,固定后用冷的PBS室温摇洗3次,每次5 min;加1% TritonX-100透化10 min,用PBS摇洗3次,每次5 min;加入一抗,4 ℃过夜孵育,第2天用PBS摇洗3次,每次5 min;室温避光孵育二抗1 h,用PBS摇洗3次,每次5 min;DAPI孵育1 min,PBS清洗后封片拍照。
1.2.7 数据分析所有定量样品以18S作为参照基因,通过2-△△CT法对定量的结果进行计算得到目的mRNA的相对表达量。所有检测的蛋白水平样品以β-tubulin为参照。数据均以“平均值±标准误(mean±SE)”表示。采用SPSS18.0统计软件进行独立样本t检验(one sample t test),*表示差异显著(P < 0.05);**表示差异极显著(P < 0.01)。
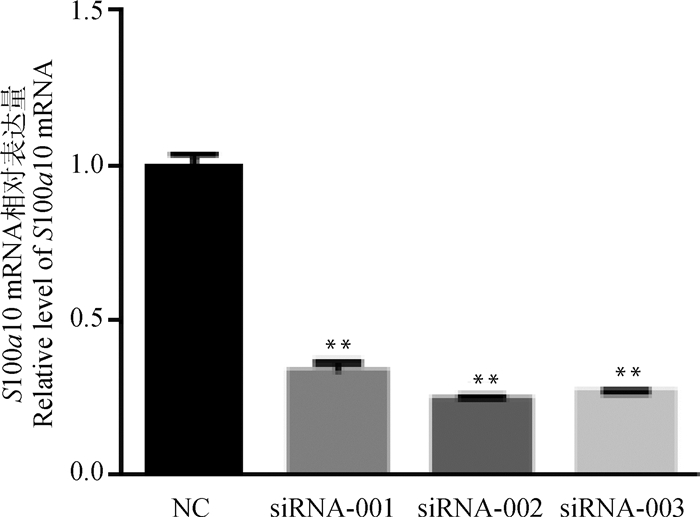
2 结果 2.1 小鼠S100a10干扰效率检测针对小鼠S100a10基因设计了3条干扰序列,分别为si-S100a10-001: GGCTCACCATTGCATGCAA;si-S100a10-002:CCAGAGCTTTCTATCACTA;si-S100a10-003:TGGACAAAATAATGAAGGA,由广州锐博生物公司合成。利用脂质体转染前体脂肪细胞2 d后,与对照组相比,si-S100a10-002干扰效率最高,达到75%以上(P < 0.01)(图 1),因此选择si-S100a10-002开展后续试验。
|
图 1 S100a10的干扰效率检测 Fig. 1 S100a10 interference efficiency detection |
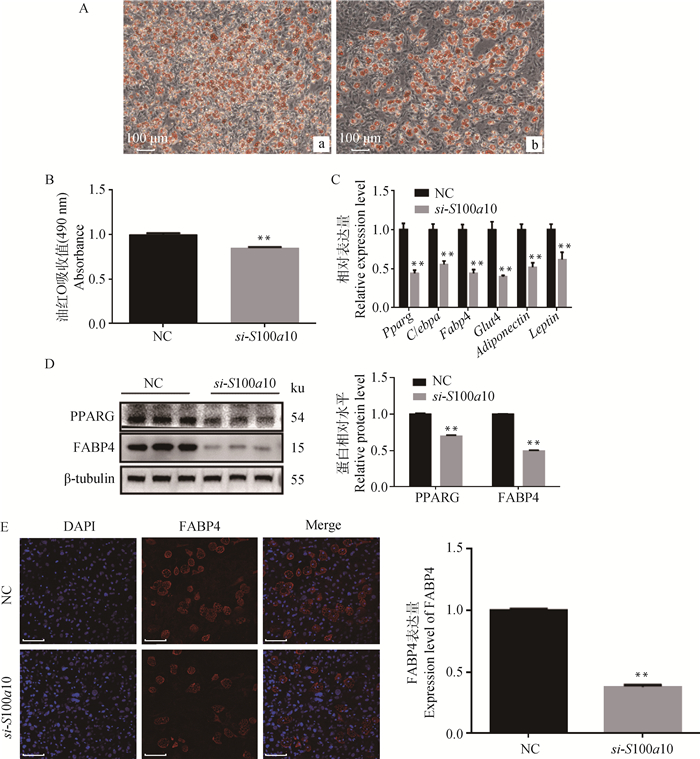
敲低S100a10后,通过油红O染色观察,脂滴明显变少(图 2A);在490 nm波长处测定样品吸光度,相对定量值显著下降(P < 0.01,图 2B);qRT-PCR结果显示,Pparg、C/ebpa、Fabp4、Glut4、Adiponectin、Leptin的表达量显著下降(P < 0.01,图 2C);Western blot结果显示,PPARG和FABP4的相对表达量显著下降(P < 0.01,图 2D);免疫荧光结果显示,FABP4表达量下降(图 2E)。说明敲低S100a10后抑制前体脂肪细胞的白色成脂分化。
|
A.油红O染脂滴(标尺:100 μm):a.阴性对照组,b.试验组,敲低S100a10;B.油红O含量检测;C.成脂分化标志基因的mRNA相对表达水平;D.通过蛋白免疫印迹检测PPARG和FABP4的相对表达量;E.通过免疫荧光检测FABP4的表达量(标尺:100 μm) A. Oil Red O staining(bar:100 μm): a. negative control group, b. experimental group, S100a10 knockdown; B. Oil Red O contents were determined colorimetrically; C. The relative mRNA expression level of adipocyte-specific genes; D. The relative protein expression level of PPARG and FABP4 detected by Western blot; E. The expression of FABP4 detected by immunofluorescence(bar:100 μm) 图 2 敲低S100a10后对小鼠前体脂肪细胞白色诱导分化的影响 Fig. 2 The effect of S100a10 knockdown on white adipogenic differentiation of mice preadipocytes |
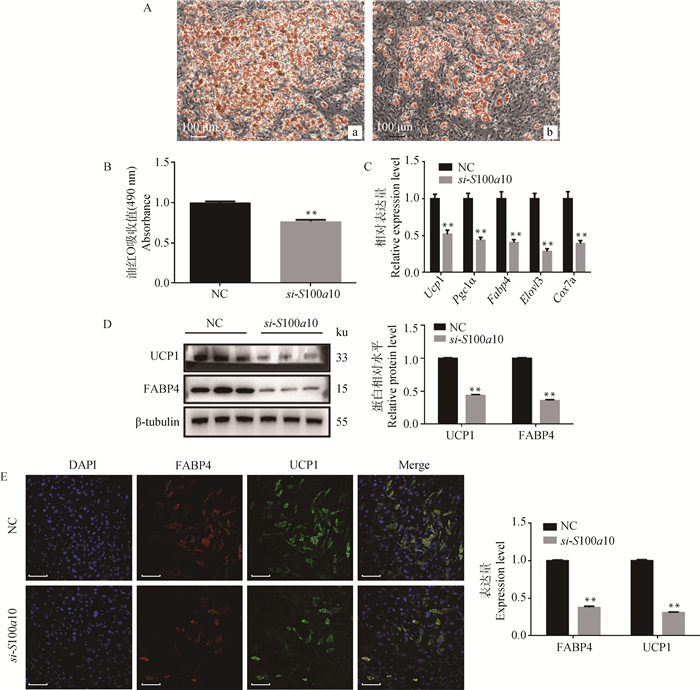
敲低S100a10后,通过油红O染色观察脂滴明显变少(图 3A);通过酶标仪在490 nm波长处测定样品吸光度,相对定量值显著下降(P < 0.01,图 3B);qRT-PCR结果显示,Ucp1、Pgc1a、Fabp4、Elovl3、Cox7a的表达量显著下降(P < 0.01,图 3C);Western blot(图 3D)和免疫荧光(图 3E)结果显示,UCP1和FABP4的表达量下降。说明敲低S100A10后抑制前体脂肪细胞的棕色成脂分化。
|
A.油红O染脂滴(标尺:100 μm):a.阴性对照组,b.试验组,敲低S100a10;B.油红O含量检测;C.成脂分化标志基因的mRNA相对表达水平;D.通过蛋白免疫印迹检测PPARG和FABP4的相对表达量;E.通过免疫荧光检测FABP4的相对表达量(标尺:100 μm) A. Oil Red O staining(bar:100 μm): a. negative control group, b. experimental group, S100a10 knockdown; B. Oil Red O contents were determined colorimetrically; C. The relative mRNA expression level of adipocyte-specific genes; D, E. The relative expression level of UCP1 and FABP4 detected by Western blot and immunofluorescence(bar:100 μm) 图 3 敲低S100a10后对小鼠前体脂肪细胞棕色诱导分化的影响 Fig. 3 The effect of S100a10 knockdown on brown adipogenic differentiation in mice preadipocytes |
近20年的研究已经证实,脂肪组织是一个维持机体能量平衡的动态器官[10]。在哺乳动物中主要包括2种脂肪组织,即白色脂肪组织和棕色脂肪组织。两者位于不同的解剖学位置,有着不同的形态学结构和生物学功能[11]。白色脂肪组织由大量单泡脂肪细胞集聚而成,脂肪细胞呈圆形或多边形,中央有一个大的脂滴,胞质呈薄层,位于细胞周缘,包绕脂滴。其主要功能是将剩余的能量以甘油三酯的形式储存于脂滴中,维持能量平衡,调节胰岛素信号和内分泌活动,成年动物中含量较多;棕色脂肪组织由大量多泡脂肪细胞组成,其特点是组织中有丰富的毛细血管,脂肪细胞内散在许多小脂滴,线粒体大而丰富,核圆形,位于细胞中央。其主要功能是在寒冷和应激的情况下动员脂滴分解,进行适应性的产热来维持体温,在新出生的哺乳动物中较多[10],后来发现,在成年人的胸部和锁骨处也存在棕色脂肪组织[12-13]。近年来,在白色脂肪组织中发现了棕色样的脂肪细胞,被称为米色脂肪细胞[14],在基础状态下和白色脂肪细胞很相似,但在产热刺激后会提高产热基因的表达和加快呼吸率[15-19],能够加快能量消耗。以上理论为研究肉牛脂肪沉积提供新的思路。随着人们需求量的增加,对牛肉肉质的要求也越来越高,主要包括肉色、嫩度、风味和多汁性等。在肉牛育肥中,生产者希望得到肌内脂肪沉积丰富,饲料消耗少的牛只以获得更高的利润。有研究发现,某些生物活性物质如共轭亚油酸在提高肌内脂肪含量的同时,却降低或维持了皮下脂肪的沉积量,由此推测,肌内脂肪可能具有与皮下脂肪不同的分子生物学功能以及分子调控机制[20-21]。影响肌内脂肪含量的因素有很多,比如品种、性别、营养和肌内脂肪候选基因等。目前,对影响牛肌内脂肪沉积的候选基因在国内研究不多,对于猪的相关研究较成熟。本研究以S100A10基因可能调控肉牛肌内脂肪沉积为出发点来探讨脂肪沉积和能量消耗两个过程中S100A10的功能。
脂肪沉积是一个复杂过程,是脂肪细胞中脂类和一些脂溶性物质积累的结果,涉及能量物质的摄入、吸收、转运、沉积等多个生物过程,并且与包括毛细血管发育在内的多个组织的发育相关[22-25]。脂肪引起的很多代谢疾病都与血管功能紊乱相关,例如过多的血管形成也会导致糖尿病、肾并发症、心病等的发生[26-28]。脂肪组织血管化程度较高,每个脂肪细胞都由广泛的毛细血管网络所滋养,血管生成抑制剂已经被证明可以抑制老鼠体内脂肪的扩增[29-32]。Zhao等[33]发现,在局部缺血的小鼠视网膜中,血管内皮生长因子能够上调ANNEXIN A2的表达,ANNEXIN A2可能通过血管内皮生长因子-血管内皮生长因子受体2信号通路促进血管生成。血浆纤溶酶原在血液流动中起着抗凝血和溶栓的作用,由组织激活物(t-PA)和尿激酶(u-PA)等激活物激活成纤溶酶,降解纤维蛋白原、纤维蛋白和多种凝血因子,促进血管内皮细胞迁移,进而促进血管的形成,此反应是通过血管内皮细胞表面的纤维蛋白溶解酶原受体调控,此受体即为ANNEXIN A2与S100A10复合物[34-35]。u-PA通过减弱富脯氨酸同源结构域蛋白转录因子活性和去抑制血管内皮生长因子受体2的表达来促进血管生成[36]。由此和前文所述推测,S100A10与ANNEXIN A2复合物可能通过调节血管形成和脂肪酸转运等两个方面来影响脂肪的形成。本研究以小鼠白色脂肪的前体脂肪细胞为模型,通过分子生物学手段对S100a10在白色/棕色分化上的影响进行验证,并获得了较显著的表型,为研究S100A10基因在肉牛肌内脂肪沉积方面的影响提供理论依据。脂肪沉积是一个复杂的代谢过程,S100a10调控成脂分化的机制以及在肉牛脂肪沉积和代谢上的生物学功能尚需更为深入的研究。
4 结论本研究中,体外培养小鼠前体脂肪细胞,敲低S100a10基因后抑制前体脂肪细胞的白色成脂分化和棕色成脂分化中特征基因的表达,脂滴显著变少,因此敲低S100a10基因后抑制前体脂肪细胞分化。本研究结果为进一步研究S100A10基因的调控机制及其对肉牛肌内脂肪沉积的影响提供理论支持。
| [1] | DODSON M V, JIANG Z H, CHEN J, et al. Allied industry approaches to alter intramuscular fat content and composition in beef animals[J]. J Food Sci, 2010, 75(1): R1–R8. |
| [2] | LISTRAT A, LEBRET B, LOUVEAU I, et al. How muscle structure and composition influence meat and flesh quality[J]. Sci World J, 2016, 2016: 3182746. |
| [3] | VISSCHER P M, BROWN M A, MCCARTHY M I, et al. Five years of GWAS discovery[J]. Am J Hum Genet, 2012, 90(1): 7–24. |
| [4] | XIA J W, QI X, WU Y, et al. Genome-wide association study identifies loci and candidate genes for meat quality traits in Simmental beef cattle[J]. Mamm Genome, 2016, 27(5-6): 246–255. DOI: 10.1007/s00335-016-9635-x |
| [5] | BHARADWAJ A, BYDOUN M, HOLLOWAY R, et al. Annexin A2 heterotetramer:structure and function[J]. Int J Mol Sci, 2013, 14(3): 6259–6305. DOI: 10.3390/ijms14036259 |
| [6] | RESCHER U, GERKE V. S100A10/p11:family, friends and functions[J]. Pflügers Archiv - Eur J Phys, 2008, 455(4): 575–582. |
| [7] | REDDY T R K, LI C, FISCHER P M, et al. Three-dimensional pharmacophore design and biochemical screening identifies substituted 1, 2, 4-triazoles as inhibitors of the annexin A2-S100A10 protein interaction[J]. ChemMedChem, 2012, 7(8): 1435–1446. DOI: 10.1002/cmdc.v7.8 |
| [8] | LIU M W, GE R, LIU W L, et al. Differential proteomics profiling identifies LDPs and biological functions in high-fat diet-induced fatty livers[J]. J Lipid Res, 2017, 58(4): 681–694. DOI: 10.1194/jlr.M071407 |
| [9] | SALAMEH A, DAQUINAG A C, STAQUICINI D I, et al. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue[J]. JCI Insight, 2016, 1(10). DOI: 10.1172/jci.insight.86351 |
| [10] | SARJEANT K, STEPHENS J M. Adipogenesis[J]. Cold Spring Harb Perspect Biol, 2012, 4(9): a008417. |
| [11] | LOWE C E, O'RAHILLY S, ROCHFORD1 J J. Adipogenesis at a glance[J]. J Cell Sci, 2011, 124: 2681–2686. DOI: 10.1242/jcs.079699 |
| [12] | BRENDLE C, STEFAN N, STEF I, et al. Impact of diverse chemotherapeutic agents and external factors on activation of brown adipose tissue in a large patient collective[J]. Sci Rep, 2019, 9: 1901. DOI: 10.1038/s41598-018-37924-6 |
| [13] | DA SILVAC P V, HERNÁNDEZ-SAAVEDRA D, WHITE J D, et al. Cold and exercise:therapeutic tools to activate brown adipose tissue and combat obesity[J]. Biology, 2019, 8(1): 9. |
| [14] | BARBATELLI G, MURANO I, MADSEN L, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation[J]. Am J Phys-Endocrinol Metab, 2010, 298(6): E1244–E1253. DOI: 10.1152/ajpendo.00600.2009 |
| [15] | PARK A, KIM W K, BAE K H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells[J]. World J Stem Cells, 2014, 6(1): 33–42. |
| [16] | FRONTINI A, VITALI A, PERUGINI J, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma[J]. Biochim Biophys Acta-Mol Cell Biol Lipids, 2013, 1831(5): 950–959. |
| [17] | SCHULZ T J, HUANG P, HUANG T L, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat[J]. Nature, 2013, 495(7441): 379–383. DOI: 10.1038/nature11943 |
| [18] | FISHER F M, KLEINER S, DOURIS N, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis[J]. Genes Dev, 2012, 26(3): 271–281. |
| [19] | SEALE P, CONROE H M, ESTALL J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice[J]. J Clin Invest, 2011, 121(1): 96–105. |
| [20] |
尹靖东, 李德发. 猪肉质形成的分子机制与营养调控[J]. 动物营养学报, 2014, 26(10): 2979–2985.
YIN J D, LI D F. Molecular mechanism underlying meat quality formation and its nutritional regulation in pigs[J]. Chinese Journal of Animal Nutrition, 2014, 26(10): 2979–2985. DOI: 10.3969/j.issn.1006-267x.2014.10.009 (in Chinese) |
| [21] |
李嫒, 张秀秀, 黄万龙, 等. 大白猪和莱芜猪肌内脂肪组织circRNAs的鉴定与分析[J]. 畜牧兽医学报, 2018, 49(7): 1343–1353.
LI Y, ZHANG X X, HUANG W L, et al. Identification and analysis of circRNAs in intramuscular adipose tissues between Large White and Laiwu pigs[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(7): 1343–1353. (in Chinese) |
| [22] | YU Z Y, CAI Y Z, DENG M W, et al. Fat extract promotes angiogenesis in a murine model of limb ischemia:a novel cell-free therapeutic strategy[J]. Stem Cell Res Ther, 2018, 9: 294. DOI: 10.1186/s13287-018-1014-y |
| [23] | WANG Y L, LIU X Y, HOU L M, et al. Fibroblast growth factor 21 suppresses adipogenesis in pig intramuscular fat cells[J]. Int J Mol Sci, 2015, 17(1): E11. DOI: 10.3390/ijms17010011 |
| [24] | YAMADA T, KAWAKAMI S, NAKANISHI N. Fat depot-specific differences in angiogenic growth factor gene expression and its relation to adipocyte size in cattle[J]. J Vet Med Sci, 2010, 72(8): 991–997. DOI: 10.1292/jvms.10-0031 |
| [25] | YONEKURA S, TOKUTAKE Y, HIROTA S, et al. Proliferating bovine intramuscular preadipocyte cells synthesize leptin[J]. Domest Anim Endocrinol, 2013, 45(1): 33–37. DOI: 10.1016/j.domaniend.2013.03.004 |
| [26] | CARMELIET P. Angiogenesis in life, disease and medicine[J]. Nature, 2005, 438(7070): 932–936. DOI: 10.1038/nature04478 |
| [27] | BOSMA E K, VAN NOORDEN C J F, SCHLINGEMANN R O, et al. The role of plasmalemma vesicle-associated protein in pathological breakdown of blood-brain and blood-retinal barriers:potential novel therapeutic target for cerebral edema and diabetic macular edema[J]. Fluids Barriers CNS, 2018, 15: 24. DOI: 10.1186/s12987-018-0109-2 |
| [28] | LEFERE S, VAN DE VELDE F, HOORENS A, et al. Angiopoietin-2 promotes pathological angiogenesis and is a therapeutic target in murine nonalcoholic fatty liver disease[J]. Hepatology, 2019, 69(3): 1087–1104. DOI: 10.1002/hep.v69.3 |
| [29] | SUN F, SHAIKH A S, WANG J, et al. Higher anti-angiogenesis activity, better cellular uptake and longer half-life of a novel glyco-modified endostatin by polysulfated heparin[J]. Curr Pharm Biotechnol, 2018, 19(12): 996–1004. |
| [30] | PODKALICKA P, MUCHA O, DULAK J, et al. Targeting angiogenesis in Duchenne muscular dystrophy[J]. Cell Mol Life Sci, 2019. DOI: 10.1007/s00018-019-03006-7 |
| [31] | GAO Y F, ZHOU S, PANG L Z, et al. Celastrol suppresses nitric oxide synthases and the angiogenesis pathway in colorectal cancer[J]. Free Radic Res, 2019. DOI: 10.1080/10715762.2019.1575512 |
| [32] | HOSSEINI S, BEHJATI F, RAHIMI M, et al. Relationship between PIK3CA amplification and P110α and CD34 tissue expression as angiogenesis markers in Iranian Women with sporadic breast cancer[J]. Iran J Pathol, 2018, 13(4): 447–453. |
| [33] | ZHAO S H, HUANG L N, WU J H, et al. Vascular endothelial growth factor upregulates expression of annexin A2 in vitro and in a mouse model of ischemic retinopathy[J]. Mol Vis, 2009, 15: 1231–1242. |
| [34] | MADUREIRA P A, SURETTE A P, PHIPPS K D, et al. The role of the annexin A2 heterotetramer in vascular fibrinolysis[J]. Blood, 2011, 118(18): 4789–4797. DOI: 10.1182/blood-2011-06-334672 |
| [35] | SURETTE A P, MADUREIRA P A, PHIPPS K D, et al. Regulation of fibrinolysis by S100A10 in vivo[J]. Blood, 2011, 118(11): 3172–3181. DOI: 10.1182/blood-2011-05-353482 |
| [36] | STEPANOVA V, JAYARAMAN P, ZAITSEV S V, et al. Urokinase-type plasminogen activator (uPA) promotes angiogenesis by attenuating proline-rich homeodomain protein (PRH) transcription factor activity and de-repressing vascular endothelial growth factor (VEGF) receptor expression[J]. J Biol Chem, 2016, 291(29): 15029–15045. DOI: 10.1074/jbc.M115.678490 |



