2. 中国农业科学院北京畜牧兽医研究所, 北京 100193;
3. 河南科技大学动物科技学院, 洛阳 471023;
4. 新乡医学院生命科学技术学院, 新乡 453003
2. Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193, China;
3. College of Animal Science & Technology, Henan University of Science and Technology, Luoyang 471023, China;
4. School of Life Sciences and Technology, Xinxiang Medical University, Xinxiang 453003, China
毛囊干细胞(hair follicle stem cells,HFSCs)具有较强的自我更新和多重分化潜能,可分化为毛囊、表皮、皮脂腺等器官,在哺乳动物的毛发生长和皮肤修复过程中发挥着重要作用[1-2],同时,HFSCs也成为软骨组织工程中重要的种子细胞,构建具有毛囊等皮肤的组织工程是未来组织工程发展的方向[3]。毛囊控制毛发的生长,毛发自我更新和修复依赖于HFSCs的增殖、分化和迁移,且皮肤组织结构状态和毛囊的性状对羊毛的品质、产量有较大影响[4]。阐明影响HFSCs生物学特性的相关因素及其生长机制,有助于了解羊毛周期性的调控,促进羊毛的生产,在优化畜牧生产上也有较大的应用价值。
白细胞介素-6(interleukin-6,IL-6)是一种多效性的细胞炎症因子,参与急性炎症反应,也参与包括癌症等多种慢性炎症性疾病的发病机制[5],在许多肿瘤细胞如前列腺癌、乳腺癌中高表达,并参与肿瘤的增殖、凋亡及迁移等生物学性状的调节[6],可激活血管内皮细胞诱导产生IL-8、单核细胞趋化蛋白-1(MCP-1)、细胞间黏附分子-1(ICAM-1)等[7]。同时,IL-6也是一种主要的免疫调节剂,通过在体液免疫和细胞免疫中调节激活辅助性T细胞、抑制调节性T细胞(Treg)和分化B细胞中发挥积极的作用[8-9]。Huang等[10]利用体外培养方法发现了部分炎性因子,如IL-6可抑制克隆性角质细胞的生长,阻止毛发从生长终期向具有生长活性的生长初期的转变,促进毛乳头早发性衰老并导致毛囊的老化,IL-6对毛发的生长周期有抑制作用,此外,增加IL-6的量可延长静止期,进而抑制HFSCs的活化。Wu等[11]研究证实,毛囊热损伤后,TNF-α、IL-1b和IL-6在内的细胞因子直接参与损伤过程,与其他因子相比,IL-6对修复过程有着重要作用。
关于炎症因子对HFSCs增殖、迁移过程的影响和分子机制尚未明了,这也是目前科研工作的重要课题之一。研究报道,细胞内有多条信号通路可介导IL-6刺激信号,其中与细胞增殖、迁移关系密切的信号通路主要有MAPK/ERK以及P38等相关信号通路[12-13]。本试验着重研究了过表达IL-6对HFSCs增殖和迁移能力的影响,同时也对IL-6调控HFSCs增殖、迁移的分子机制进行初步探究,以期为干细胞组织工程的研究,毛发的移植和受损皮肤修复的临床应用提供新的思路。
1 材料与方法 1.1 试验材料山羊HFSCs由本实验室分离培养;DMEM低糖培养基、优质胎牛血清(FBS)、胰蛋白酶购自Gibco公司;1%青链霉素、细胞裂解液、蛋白酶抑制剂(PMSF)、SDS蛋白上样缓冲液、辣根过氧化物酶标记山羊抗小鼠IgG(H + L)抗体购自上海碧云天生物技术有限公司;抗体p-ERK1/2、ERK2、p-p38、p38、myc-Tag均购自Cell Signaling Technology;DNA Marker购自上海欣百诺生物科技有限公司;引物由上海桑尼生物科技有限公司合成;预染蛋白marker购自Bio-Rad公司;MTT购自于Sigma公司;PVDF膜和显色底物液均购自Millipore公司。
1.2 方法 1.2.1 山羊HFSCs分离和培养试验过程主要参照本实验室已发表改进的干细胞分离、培养方法:山羊毛囊干细胞分离培养方法研究[14]。简要过程为:取关中奶山羊耳部皮肤标本0.5 cm×0.5 cm,在净化工作台中用柳叶刀刮去皮肤表面脏物,75%酒精消毒2次,然后用D-Hank′s液冲洗3~5次,每次5 min。消毒的皮肤标本用眼科剪剪成小条,加入2.4 U·mL-1的Dispase酶,4 ℃消化15 h后,用眼科镊拔出毛囊, 在体视显微镜下切取毛囊隆突部,置于5 mL胰酶(0.5 mg·mL-1胰酶+0.2 mg·mL-1 EDTA)中37 ℃消化1.5 h,终止反应, 1 200 r·min-1离心收集细胞接种于100 μg·mL-1的Ⅳ型胶原包被的培养皿中。48 h后首次换液,去除未贴壁的细胞,此后,每2 d换1次培养液,当细胞接近90%融合,0.25%胰蛋白酶(pH 7.4)消化细胞,用新鲜生长培养基重悬细胞。显微镜下观察细胞形态及生长变化,记录细胞增殖和迁移情况。
1.2.2 目的片段的扩增及重组质粒的构建提取山羊HFSCs的总RNA,反转录成为cDNA文库,设计扩增IL-6的引物,上游引物:5′-ATGAACTCCCTCTTCACAAG-3′, 下游引物:5′-CTACTTCATCCGAATAGCT -3′, 克隆获得CDS全长序列并测序验证,扩增条件:95 ℃预变性5 min;95℃变性30 s,60 ℃退火30 s,72 ℃延伸1 min,30个循环;72 ℃延伸5 min。将测序正确的IL-6序列和pXJ40-myc空载体均使用BamH Ⅰ和Xhol Ⅰ进行双酶切,37 ℃连接2 h,将连接产物转化到受体菌DH5α,挑取单克隆抽提质粒酶切鉴定。
1.2.3 IL-6重组载体转染山羊HFSCs用含10%胎牛血清的DMEM培养基培养的HFSCs接种于6孔板中,24 h后转染。按照空载体组和IL-6载体组2组进行分别转染。转染时将1 μg质粒与0.5 μL转染试剂相混合,用生理盐水稀释至50 μL,静置20 min后加入到单孔中,4 h后换液。
1.2.4 Western blot检测山羊HFSCs中IL-6蛋白表达水平和信号通路关键激酶的磷酸化水平收集转染24 h后的细胞进行Western blot分析,收集转染细胞用预冷PBS各洗3次,加入裂解缓冲液,静置30 min,超声破碎细胞30 s,12 000 r·min-1离心20 min后取上清液。采用BCA法进行蛋白定量,经SDS-PAGE分离将蛋白转移到PVDF膜上,脱脂牛奶封闭1 h,加入一抗,室温孵育2 h,PBST漂洗5 min,重复3次,加入二抗,室温孵育1 h。再次利用PBST漂洗5 min,并重复3次。将PVDF膜用发光剂ECL显色后曝光,并进行灰度值分析。
1.2.5 MTT检测山羊HFSCs细胞增殖水平将转染的细胞株接种于96孔板中,待细胞贴壁后加入各组培养基,每组7个复孔,分别培养至第1、2、3、4、5、6、7天时加入5 g·L-1 MTT 20 μL,置于恒温箱中孵育4 h,取上清液,加入200 μL二甲基亚砜以溶解细胞形成的结晶,酶标仪检测570 nm波长处吸光度值,并分析各组细胞株增殖情况。
1.2.6 划痕试验检测山羊HFSCs细胞迁移能力HFSCs传代后重悬,用细胞计数仪测量细胞浓度,得到细胞总数,并在2个相同大小的培养皿中分别加入数量相同的细胞。于每个培养皿中各加入2 mL培养液,4 ℃培养。当细胞密度达到100%时,用10 μL枪头进行均匀划线。划线后倒掉培养液,用PBS洗2次,再重新加入DMEM培养基,每隔6 h观察划痕部位愈合情况,并置于显微镜下拍照,24 h后结束观察,整理数据并制图,使用Image J软件分析划痕后0、6、18、24 h划痕处的愈合面积。
1.2.7 统计学分析采用SPSS 17. 0统计软件包(SPSS,美国)进行统计学处理。数据以“均数±标准差”表示,各组间观察指标的比较采用单因素方差分析和最小显著差法,检验水准α值取双侧P<0.05。
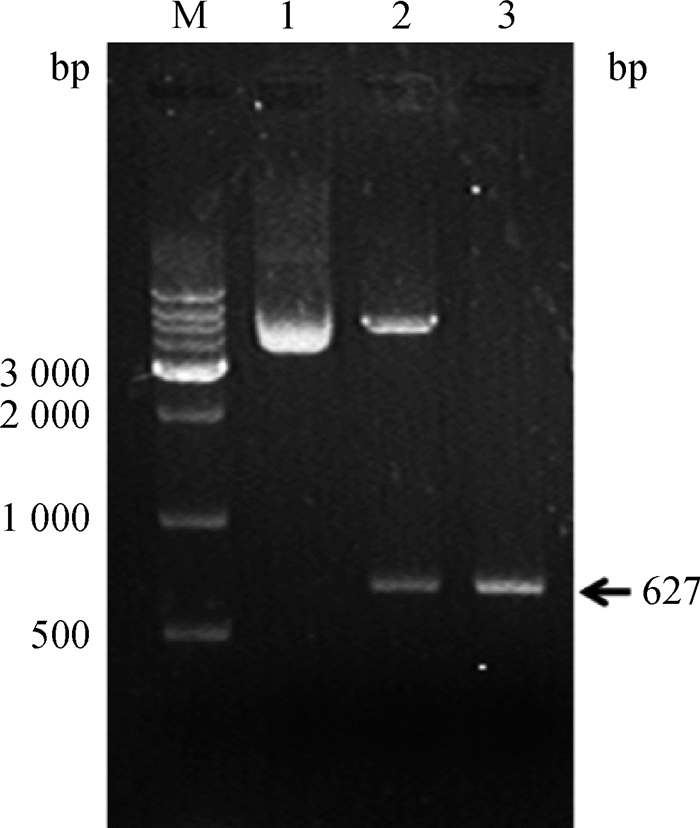
2 结果 2.1 山羊IL-6过表达重组载体的构建使用PCR技术扩增山羊HFSCs中的IL-6基因,PCR产物经1%琼脂糖凝胶电泳显示,其与预期627 bp大小一致,进行产物回收。将胶回收得到的IL-6片段和pXJ40-myc空载体均使用BamH Ⅰ和Xhol Ⅰ进行双酶切,22 ℃水浴3 h,产物胶回收后用T4DNA连接酶于16 ℃金属浴过夜连接,连接产物转化到受体菌DH5α,挑取单克隆,抽提质粒,酶切鉴定,通过DNA测序来确定阳性克隆。从图 1可以看出,泳道1空载体组片段大小约为5 000 bp,泳道2载体组出现5 000和627 bp两个条带,泳道3显示IL-6扩增片段约为627 bp,鉴定结果显示重组载体pXJ40-myc-IL-6构建成功。
|
M.DNA相对分子质量标准;1. pXJ40-myc空载体;2.重组载体pXJ40-myc-IL-6双酶切;3. IL-6基因PCR扩增产物 M.DNA marker; 1. pXJ40-myc empty vector; 2. Double digestion of recombinant vector pXJ40-myc-IL-6; 3. The PCR product of IL-6 gene 图 1 构建重组表达载体pXJ40-myc-IL-6 Fig. 1 Construction of the pXJ40-myc-IL-6 recombinant vector |
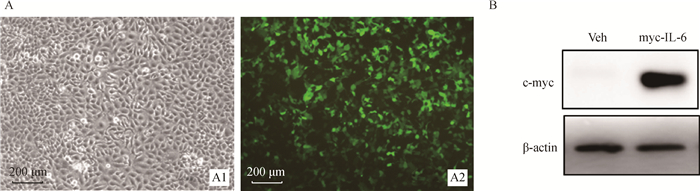
山羊HFSCs转染重组载体24 h后在荧光显微镜下观察到绿色荧光蛋白表达,转染效率约80%,结果见图 2A。同时收获转染细胞进行Western blot分析,用myc-Tag抗体检测IL-6融合蛋白的表达,以β-actin的表达水平作参照,结果发现,在转染空载体的对照组中,没有检测到融合蛋白IL-6的表达,在转染重组载体的试验组中成功检测到IL-6融合蛋白的高表达(图 2B)。
|
A.荧光显微镜观察GFP绿色荧光蛋白的表达:A1.山羊HFSCs转染重组载体后白场照片;A2.毛囊干细胞转染重组载体后395 nm激发照片。B. myc-Tag抗体检测融合蛋白myc-IL-6的表达:Veh.转染空载体;myc-IL-6.转染重组载体pXJ40-myc-IL-6,下同 A. Fluorescence microscopy was used to observe the expression of GFP green fluorescent protein: A1. Photograph of white field after transfection of goat HFSCs into recombinant vector; A2. Photograph of 395 nm excitation after transfection of goat HFSCs into recombinant vector. B. myc-Tag antibody was used to detect the expression of fusion protein myc-IL-6: Veh. Transfected with empty vector; myc-IL-6. Transfected with recombinant vector pXJ40-myc-IL-6, the same as below 图 2 重组IL-6载体转染山羊HFSCs后融合蛋白表达鉴定 Fig. 2 Identification of fusion protein expression after transfection of recombinant IL-6 vector into goat HFSCs |
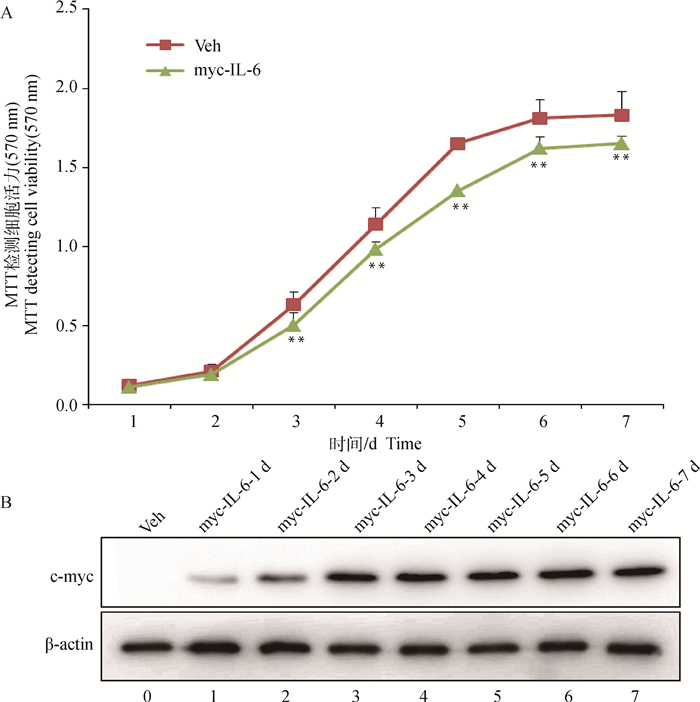
MTT法检测连续培养7 d HFSCs的增殖活力,发现与空载体组相比,过表达IL-6组山羊HFSCs的增殖活力在第3天时出现抑制效果,增殖活力下降了约23% (P < 0.01),并持续抑制,在第6和7天时增殖活力下降速度基本趋于稳定,为约13%(P < 0.01),结果见图 3A,且具有显著统计学意义。Western blot检测转染pXJ40-myc空载体和IL-6重组载体中myc-IL-6的表达,结果显示,第1~3天IL-6蛋白的表达量逐渐增加,第4~7天IL-6蛋白的表达量处于稳定的表达水平,如图 3B所示。结果提示IL-6过表达对山羊HFSCs的增殖活力具有抑制作用。
|
A. MTT法检测羊HFSCs转染IL-6重组载体后细胞增殖活力; B. Western blot检测转染IL-6重组载体后IL-6蛋白连续7 d的表达水平;0. myc-Tag抗体检测转染pXJ40-myc空载体后融合蛋白myc-IL-6的表达;1~7. myc-Tag抗体检测转染pXJ40-myc-IL-6重组载体后连续7 d融合蛋白myc-IL-6的表达。**. P < 0.01,下同 A. MTT assay was used to detect the proliferation of goat HFSCs transfected with IL-6 recombinant vector; B. Western blot was used to detect the expression level of IL-6 protein for 7 days after transfecting with IL-6 recombinant vector; 0. myc-Tag antibody was used to detect the expression of myc-IL-6 fusion protein after transfecting with pXJ40-myc empty vector; 1-7. myc-Tag antibody was used to detect the expression of fusion protein myc-IL-6 for 7 days after transfecting with pXJ40-myc-IL-6 recombinant vector. **. P < 0.01, the same as below 图 3 MTT法检测羊HFSCs转染IL-6重组载体后细胞增殖活力 Fig. 3 Detection of the cell proliferation activity of goat HFSCs by MTT assay |
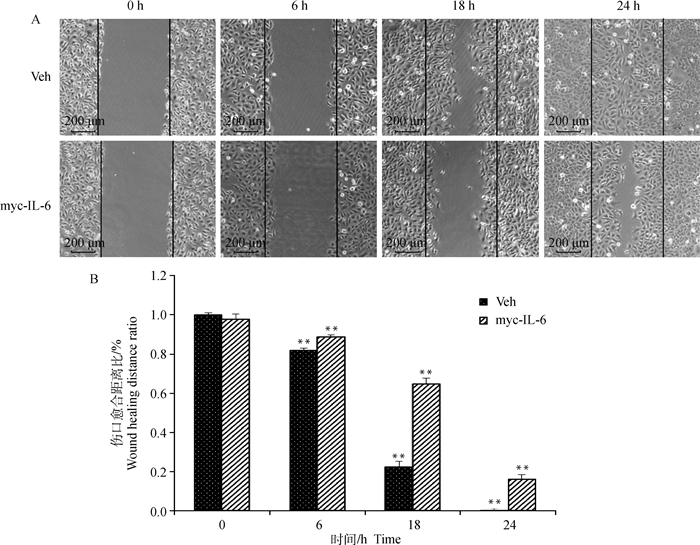
划痕试验后分别统计6、18、24 h山羊HFSCs的划痕部位愈合程度,空载体组划痕部位的愈合率均明显高于过表达IL-6组,结果见图 4A;使用Image J软件对划痕试验后伤口的愈合程度进行统计,24 h时空载体组划痕部位与0 h相比未愈合率为1.5%,愈合率达到约98.5%,已基本愈合;过表达IL-6组划痕部位伤口未愈合率为15.8%,愈合率为84.2%,结果显示,山羊HFSCs过表达IL-6后愈合率显著降低且具有统计学意义(P < 0.01),如图 4B所示,提示过表达IL-6后对伤口的愈合具有显著的抑制作用。
|
A.划痕试验检测HFSCs迁移能力;B. Image J软件计算伤口愈合程度。Veh.转染空载体;myc-IL-6.转染重组载体pXJ40-myc-IL-6 A. The wound healing assay was used to detect the migration ability of HFSCs; B. Image J software was used to calculate wound healing degree 图 4 划痕试验检测HFSCs的迁移能力 Fig. 4 Detection of HFSCs migration by wound healing assay |
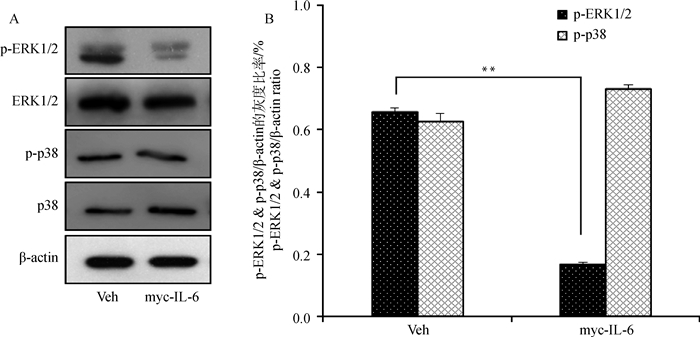
为进一步探究过表达IL-6抑制HFSCs迁移的内在可能分子机制,本研究尝试探究介导此现象的相关信号通路。山羊HFSCs成功转染IL-6重组表达载体24 h后,收获细胞,Western blot检测相关信号通路关键激酶的磷酸化水平,结果发现,在IL-6过表达后,MAPK/ERK信号通路中的关键激酶ERK1/2的磷酸化水平(p-ERK1/2)显著下降了76%(P < 0.01),P38信号通路中的关键激酶p38的磷酸化水平(p-p38)表达量上调了17%(P=0.15),但差异无统计学意义,结果如图 5所示。结果提示,MAPK/ERK信号通路可能在IL-6抑制山羊HFSCs增殖和迁移过程中发挥了一定的调控作用。
|
A.在空载体组和IL-6重组载体组中检测p-ERK1/2和p-p38的磷酸化水平;B. Image J软件计算p-ERK1/2 & p-p38和β-actin的灰度比值 A. The phosphorylation level of p-ERK1/2 & p-p38 in transfected empty vector and recombinant vector pXJ40-myc-IL-6; B. Image J software was used to calculate p-ERK1/2 & p-p38/β-actin ratio 图 5 Western blot检测HFSCs中MAPK/REK和P38信号通路相关蛋白表达水平 Fig. 5 Detection of the expression level of MAPK/REK and P38 signaling pathway-related proteins in HFSCs by Western blot |
HFSCs具有干细胞的一般特性,即具有多向分化潜能等广泛的生物学功能,易获得、易培养,与胚胎干细胞相比,拥有使用时不受伦理学问题限制的优点[15-16]。同时研究显示,毛囊干细胞与神经元细胞具有较好的亲和性,因此,探究细胞增殖、迁移和分化的微环境,使用细胞-支架组合植入,对毛发的移植和受损皮肤修复有临床应用价值,也是再生医学体外技术发展的一个潜在方向[17-18]。而毛囊对于产毛动物(如羊)具有非常重要的作用,因此近年来对HFSCs生物学应用的探索成为了科研热点。
有研究显示,秃顶人群的真皮乳头(dermal papilla)细胞与未秃顶的真皮乳头细胞相比,炎症因子IL-6的表达量上调。分离培养人的毛囊细胞,加入外源性重组IL-6能够增加细胞基质中IL-6受体和gp130的表达量,并对毛囊细胞的增殖有明显的抑制作用[19]。毛囊能够保持周期性的休息(休眠期)、再生(生长期)和退化(退化期)取决于毛囊干细胞的更新能力。在毛发生长初期,凸起的毛囊干细胞被接收到的信号激活真皮乳头,干细胞退出隆起并向下扩散,形成一条小径成为外根鞘[20-21]。HFSC的休眠期受许多内在和外在机制的调节,能够在新的毛发周期中很快被激活分裂[22]。
HFSCs的增殖、迁移对毛发的周期性增长和调控以及更新有重要作用,尽管关于成体干细胞已经有不少报道,但较多集中于对其培养、分离方法等的研究[23-24],关于调控HFSCs增殖、迁移的作用机制尚不清楚。有研究显示,IL-6可与CD5低亲和力结合,激活STAT3(transcription 3)、MAPKs、丝裂原活化蛋白激酶来调控细胞的行为[25]。Osorio等[26]研究显示,人类胚胎皮肤前体细胞Runx1通过Wnt信号通路调控成人HFSCs的活化和增殖。在本研究中,从羊背部皮肤成功提取、分离并培养了羊HFSCs,通过体外试验发现,过表达IL-6对HFSCs的增殖和迁移具有显著的抑制作用。此外,还尝试探索了HFSCs中MAPK/REK和P38信号通路[27]中关键激酶的活化水平,发现MAPK/REK信号通路中的关键激酶ERK1/2的磷酸化水平显著下调,P38信号通路中的关键激酶p38的磷酸化水平上调,但在统计学上无显著性差异,因此,推测过表达IL-6可能是通过影响MAPK/REK信号通路进而影响HFSCs的增殖和迁移过程。
本试验是通过构建重组IL-6载体,着重研究过表达IL-6对HFSCs增殖和迁移过程的影响,而IL-6是否确实通过MAPK/REK信号通路负调控HFSCs增殖和迁移的,具体分子机制仍有待深入研究,这也将成为本实验室后续研究的重点和方向,对揭示山羊HFSCs的迁移及组织修复机制的研究具有重要意义。
4 结论本研究构建的IL-6重组载体成功转染到羊的HFSCs中,并检测到IL-6蛋白的过表达,发现过表达IL-6对HFSCs的增殖和迁移具有抑制效果,并可能是通过MAPK/REK信号通路发挥负性调控作用。
| [1] | MILLAR S E. Committing to a hairy fate:epigenetic regulation of hair follicle stem cells[J]. Cell Stem Cell, 2011, 9(3): 183–184. DOI: 10.1016/j.stem.2011.08.009 |
| [2] | LIEN W H, POLAK L, LIN M Y, et al. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators[J]. Nat Cell Biol, 2014, 16(2): 179–190. |
| [3] | LIN C L, XU R J, YI J K, et al. Alkaline ceramidase 1 protects mice from premature hair loss by maintaining the homeostasis of hair follicle stem cells[J]. Stem Cell Rep, 2017, 9(5): 1488–1500. DOI: 10.1016/j.stemcr.2017.09.015 |
| [4] | CHEN C C, WANG L, PLIKUS M V, et al. Organ-level quorum sensing directs regeneration in hair stem cell populations[J]. Cell, 2015, 161(2): 277–290. DOI: 10.1016/j.cell.2015.02.016 |
| [5] | FISHER D T, APPENHEIMER M M, EVANS S S. The two faces of IL-6 in the tumor microenvironment[J]. Semin Immunol, 2014, 26(1): 38–47. DOI: 10.1016/j.smim.2014.01.008 |
| [6] | ALMURAIKHY S, KAFIENAH W, BASHAH M, et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance[J]. Diabetologia, 2016, 59(11): 2406–2416. DOI: 10.1007/s00125-016-4031-3 |
| [7] | NAGASHIMA H, ISHⅡ N, SO T. Regulation of interleukin-6 receptor signaling by TNF receptor-associated factor 2 and 5 during differentiation of inflammatory CD4+ T cells[J]. Front Immunol, 2018, 9: 1986. DOI: 10.3389/fimmu.2018.01986 |
| [8] | KAMPAN N C, MADONDO M T, MCNALLY O M, et al. Interleukin 6 present in inflammatory ascites from advanced epithelial ovarian cancer patients promotes tumor necrosis factor receptor 2-expressing regulatory T cells[J]. Front Immunol, 2017, 8: 1482. DOI: 10.3389/fimmu.2017.01482 |
| [9] | QU Z X, SUN F, ZHOU J J, et al. Interleukin-6 prevents the initiation but enhances the progression of lung cancer[J]. Cancer Res, 2015, 75(16): 3209–3215. DOI: 10.1158/0008-5472.CAN-14-3042 |
| [10] | HUANG W Y, HUANG Y C, HUANG K S, et al. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction[J]. J Dermatol Sci, 2017, 86(2): 114–122. |
| [11] | WU Y F, WANG S H, WU P S, et al. Enhancing hair follicle regeneration by nonablative fractional laser:assessment of irradiation parameters and tissue response[J]. Lasers Surg Med, 2015, 47(4): 331–341. DOI: 10.1002/lsm.v47.4 |
| [12] | CHAN L P, LIU C, CHIANG F Y, et al. IL-8 promotes inflammatory mediators and stimulates activation of p38 MAPK/ERK-NF-κB pathway and reduction of JNK in HNSCC[J]. Oncotarget, 2017, 8: 56375–56388. |
| [13] | YU S T, ZHONG Q, CHEN R H, et al. CRLF1 promotes malignant phenotypes of papillary thyroid carcinoma by activating the MAPK/ERK and PI3K/AKT pathways[J]. Cell Death Dis, 2018, 9(3): 371. DOI: 10.1038/s41419-018-0352-0 |
| [14] |
史明艳, 杨学义, 窦忠英. 山羊毛囊干细胞分离培养方法研究[J]. 畜牧兽医学报, 2006, 37(5): 436–440.
SHI M Y, YANG X Y, DOU Z Y. Study on the isolation and culture of goat hair follicle stem cells[J]. Acta Veterinaria et Zootechnica Sinica, 2006, 37(5): 436–440. DOI: 10.3321/j.issn:0366-6964.2006.05.004 (in Chinese) |
| [15] | ZHANG H S, ZHANG S B, ZHAO H S, et al. Ovine hair follicle stem cells derived from single vibrissae reconstitute haired skin[J]. Int J Mol Sci, 2015, 16(8): 17779–17797. DOI: 10.3390/ijms160817779 |
| [16] |
肖平, 仲涛, 刘占发, 等. 绵、山羊毛囊发育与毛发弯曲机制研究进展[J]. 畜牧兽医学报, 2018, 49(8): 1567–1576.
XIAO P, ZHONG T, LIU Z F, et al. Research progress of mechanism of hair follicle development and hair curvature in sheep and goat[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(8): 1567–1576. (in Chinese) |
| [17] | QUAN R F, DU W B, ZHENG X, et al. VEGF165 induces differentiation of hair follicle stem cells into endothelial cells and plays a role in in vivo angiogenesis[J]. J Cell Mol Med, 2017, 21(8): 1593–1604. DOI: 10.1111/jcmm.2017.21.issue-8 |
| [18] | YANG R F, ZHENG Y, BURROWS M, et al. Generation of folliculogenic human epithelial stem cells from induced pluripotent stem cells[J]. Nat Commun, 2014, 5: 3071. DOI: 10.1038/ncomms4071 |
| [19] | KWACK M H, AHN J S, KIM M K, et al. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice[J]. J Invest Dermatol, 2012, 132(1): 43–49. |
| [20] | HSU Y C, PASOLLI H A, FUCHS E. Dynamics between stem cells, niche, and progeny in the hair follicle[J]. Cell, 2011, 144(1): 92–105. DOI: 10.1016/j.cell.2010.11.049 |
| [21] | WANG L, SIEGENTHALER J A, DOWELL R D, et al. Foxc1 reinforces quiescence in self-renewing hair follicle stem cells[J]. Science, 2016, 351(6273): 613–617. DOI: 10.1126/science.aad5440 |
| [22] | FLORES A, SCHELL J, KRALL A S, et al. Lactate dehydrogenase activity drives hair follicle stem cell activation[J]. Nat Cell Biol, 2017, 19(9): 1017–1026. DOI: 10.1038/ncb3575 |
| [23] | GE W, WANG S H, SUN B, et al. Melatonin promotes cashmere goat (Capra hircus) secondary hair follicle growth:a view from integrated analysis of long non-coding and coding RNAs[J]. Cell Cycle, 2018, 17(10): 1255–1267. DOI: 10.1080/15384101.2018.1471318 |
| [24] | ZHU B, GUO Z L, JIN M Z, et al. Establishment of dermal sheath cell line from Cashmere goat and characterizing cytokeratin 13 as its novel biomarker[J]. Biotechnol Lett, 2018, 40(5): 765–772. DOI: 10.1007/s10529-018-2532-5 |
| [25] | TANAKA T, NARAZAKI M, KISHIMOTO T. Interleukin (IL-6) immunotherapy[J]. Cold Spring Harb Perspect Biol, 2018, 10(8): a028456. DOI: 10.1101/cshperspect.a028456 |
| [26] | OSORIO K M, LILJA K C, TUMBAR T. Runx1 modulates adult hair follicle stem cell emergence and maintenance from distinct embryonic skin compartments[J]. J Cell Biol, 2011, 193(1): 235–250. DOI: 10.1083/jcb.201006068 |
| [27] |
孙晓林, 杜方原, 刘淑英. 外源性绵羊肺腺瘤病毒囊膜蛋白激活MAPK信号通路的分析[J]. 畜牧兽医学报, 2016, 47(8): 1658–1666.
SUN X L, DU F Y, LIU S Y. Recombinant plasmid pcDNA4/myc-His/exJSRV-env transiently transfect A549 cells and detect the activation of MAPK signal transduction pathway[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(8): 1658–1666. (in Chinese) |



