2. 山西农业大学动物科技学院, 太谷 030801
2. College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu 030801, China
单胎动物牛在发情周期中,一般只有1个卵泡生长发育较快并成为优势卵泡(dominant follicles, DF)[1-2],其余卵泡即为从属卵泡(subordinate follicles, SF)。DF通过各种途径抑制SF生长,除DF发育为排卵卵泡外[3],大多数卵泡走向闭锁[4-5],如何改善低排卵率一直是单胎家畜繁殖生物技术领域的热点。卵泡生长发育机制复杂,既有激素的作用,如:促性腺激素、胰岛素、雌激素、促卵泡激素,又涉及到许多生长因子的参与,如:胰岛素样生长因子[6]和血管内皮生长因子[7],它们共同调节其生长、增殖及分化。颗粒细胞(granulesa cells, GCs)作为卵泡内主要细胞成分,其生长状态在卵泡发育和闭锁过程中起重要作用,研究表明,GCs凋亡是导致卵泡闭锁的直接原因[8-9]。
Label-free定量蛋白质组学技术,对样本操作量少,检测到的低丰度蛋白较多,使其迅速成为近年来重要的质谱定量方法[10]。本研究利用Label-free蛋白质组学技术,Proteome Discover 2.0软件结合Mascot算法[11],系统分析牛DF和SF蛋白质组分差异,筛选在卵泡发育过程中起关键调控作用的蛋白质,为探索牛卵母细胞成熟机理及关键蛋白,进一步研究卵泡闭锁及优势化提供参考依据。
1 材料与方法 1.1 试验材料裂解液、蛋白酶抑制剂、考马斯亮蓝G-250染料(A-Pharmacia公司)、1 mg·mL-1 BSA、95%乙醇、88%磷酸、(NH4)2CO3、甲酸、乙腈、氨水均购自Sigma公司;碘乙酰胺(IAA)购自Vetec公司;DTT购自Genview公司;胰蛋白酶购自Promega公司。
选取3头健康的中国荷斯坦母牛,注射PGF2α(前列腺素F2α)进行同期发情处理,期间每天用B超监测2次并记录卵泡生长情况,当双侧卵巢中出现优势化卵泡(增长率显著高于其它卵泡)时,摘除双侧卵巢,投入灭菌DPBS中,用眼科剪分别剪下最大卵泡和第二大卵泡,即为DF和SF。
1.2 蛋白质提取将DF和SF用无菌DPBS漂洗2次,在加有DPBS的培养皿中,用眼科剪将卵泡剪破,沿卵泡内壁轻轻刮取GCs于培养皿中,移液枪移至1.5 mL EP管中,1 400 r·min-1离心5 min,弃上清,将GCs放入液氮中预冷。超声破碎细胞,每次运行3 s停10 s,重复20次。在样品中各加入1 mL裂解液,对应加入1%体积蛋白酶抑制剂,混匀。4 ℃ 1 400 r·min-1离心40 min,取上清,Bradford法测定蛋白质浓度,-80 ℃保存。
1.3 蛋白质酶解取50 μg蛋白样品,加入1/3体积的200 mol·L-1(NH4)2CO3,混匀;向溶液中加入终浓度10 mmol·mL-1 DTT,56 ℃孵育1 h。向溶液中加入终浓度55 mol·L-1 IAA,室温避光孵育40 min。最后向溶液中加入5 μg胰蛋白酶,37 ℃孵育14~16 h。加入适量浓缩酶解液至浓度约为1 mg·mL-1。
1.4 质谱检测取1 μg酶解后产物进行质谱分析,每个样品进样1次,每份样品采用纳升流速HPLC液相系统Eksigent 425(AB SCIEX)进行分离。液相A液为0.1%甲酸溶液,B液为0.1%甲酸乙腈水溶液。样品由自动进样器上样到捕集柱C18 trap column(3 μm×0.10 mm×20 mm),再经分析柱C18 column(5 μm×0.75 mm×150 mm)分离,色谱柱以100%的A液平衡,流速为300 nL·min-1。相关液相梯度:0~110 min,B液线性梯度从5%~80%;110~110.1 min,B液线性梯度从80%~5%;110.1~120 min,B液维持在5%。酶解产物经毛细管高效液相色谱分离后,Q-Exactive质谱仪(Thermo Scientific)进行质谱分析。过柱时长120 min,离子化后均带一个单位正电荷;母离子扫描范围:350~1 750。
1.5 Mascot非标记分析将原始文件通过Proteome Discoverer 2.0提交至Mascot服务器,数据库为Uniprot-COW FASTA。参数设定:酶解方式为胰蛋白酶,固定修饰选择烷基化修饰,可变修饰为氧化修饰和乙酰化修饰。
1.6 生物信息学分析PANTHER(http://www.pantherdb.org)对成功鉴定的蛋白进行生物过程分析,对差异表达蛋白进行功能富集分析。KEGG pathway数据库对差异蛋白的信号通路筛选并分析。STRING(https://string-db.org)进行蛋白质互作分析。
2 结果 2.1 蛋白浓度测定通过测定样品吸光值,利用Bradford法标准曲线计算出样品蛋白浓度,如表 1所示。
|
|
表 1 样品蛋白浓度 Table 1 The concentration of samples |
经数据库搜索共获得3 409种蛋白质(FDR≤0.01),其中DF蛋白质组2 895种,SF蛋白质组3 102种;2 760种DF蛋白质被识别并成功注释,注释成功率为95.34%,2 962种SF蛋白质被识别并注释,注释成功率为95.49%。蛋白生物过程分析表明:除配子产生和胚胎发育外,蛋白质组分富集于细胞周期、细胞分化、MAPK级联反应、信号转导、细胞通讯、生物合成及调节过程和蛋白质代谢过程,DF与SF蛋白质组分数量相近;7种蛋白质(ILF2、ILF3、ZP3、STRBP、KHDRBS1、QKI、HCFC1)参与配子产生过程,4种蛋白质(SCRIB、RALB、RALA、CDC42BPB)参与胚胎发育过程,且以上11种蛋白质仅在DF中表达(图 1)。
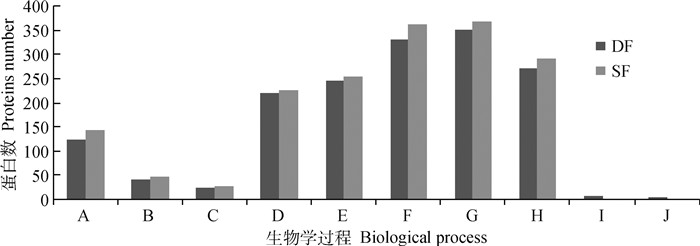
|
A.细胞周期;B.细胞分化;C.MAPK级联反应;D.信号转导;E.细胞通讯;F.生物合成;G.蛋白质代谢过程;H.生物调节过程;I.配子产生;J.胚胎发育 A.Cell cycle; B.Cell differentiation; C.MAPK cascade; D.Signal transduction; E.Cell communication; F.Biosynthetic process; G.Protein metabolic process; H.Biological regulation; I.Gamete generation; J.Embryo development 图 1 DF与SF蛋白生物学过程富集 Fig. 1 Biological process enrichment of DF and SF proteins |
数据库检索获得的2 895种DF蛋白质组中,共有236种蛋白质仅在DF中表达;而3 102种SF蛋白质组中,443种蛋白质仅在SF中表达。共获得6个DF特异表达蛋白质与卵泡发育相关,分别为EVPL、XIRP2、TGFBI、LIMA1、OLFML3和KCNK12;SF特异表达蛋白质SERPINB2与抑制卵泡发育相关,表达量前10的DF和SF特异表达蛋白质见表 2。
|
|
表 2 表达量前10的DF和SF特异表达蛋白质 Table 2 Top 10 specific expression proteins in DF and SF |
对鉴定出的3 409种蛋白设定参数:差异倍数>2,P < 0.05,共获得259种差异表达蛋白。GO分析表明:参与分子功能的蛋白分别涉及翻译调节活性、结构分子活性、催化活性等(图 2a);参与生物学过程的蛋白主要涉及代谢过程、细胞过程等(图 2b);参与细胞组分的差异表达蛋白分别位于突触、膜、胞内、细胞器和胞外区等(图 2c)。
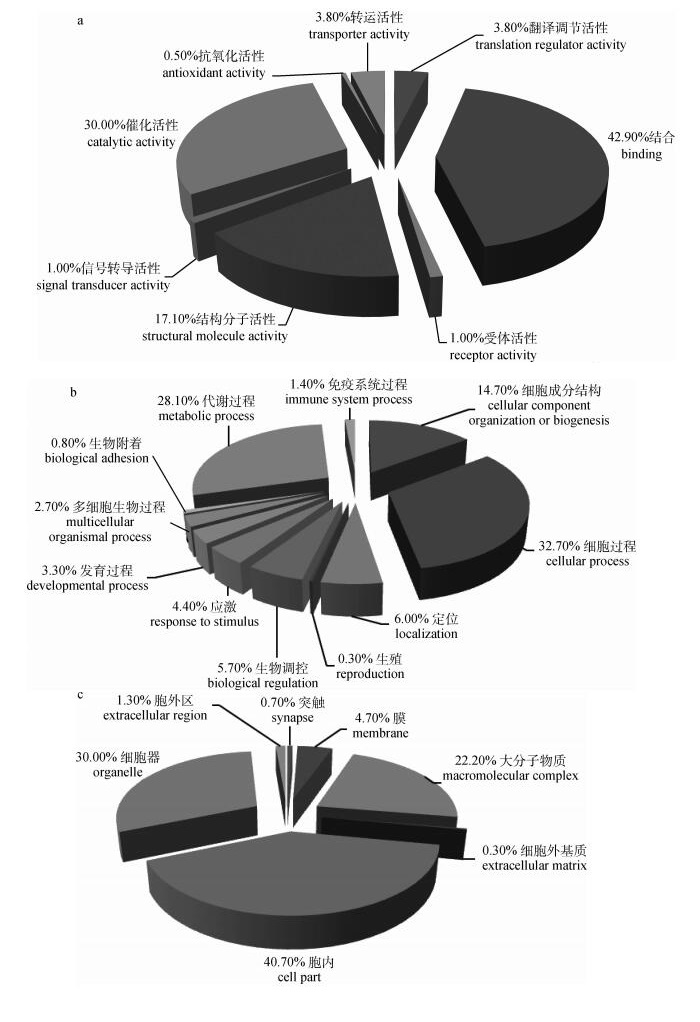
|
图 2 差异表达蛋白质功能分析 Fig. 2 Functional clustering analysis of differentially expressed proteins |
KEGG pathway分析表明,差异蛋白共涉及16条信号通路,其中有8条通路与卵泡发育相关,如:PI3K-Akt信号通路(HSP90AB1、YWHAG、YWHAH、YWHAB、YWHAQ、GNG10、RPS6、CDC37),Hippo信号通路(YWHAG、YWHAH、YWHAB、YWHAQ)(图 3)。
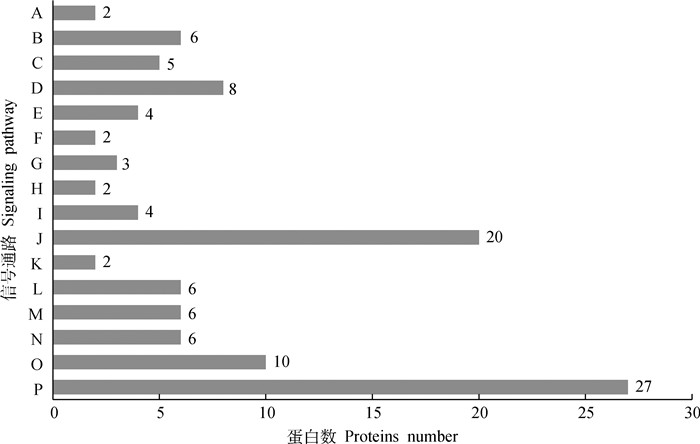
|
A.纤维母细胞生长因子信号通路;B.细胞周期;C.卵母细胞减数分裂;D.PI3K-Akt信号通路;E. Hippo信号通路;F.MAPK信号通路;G.细胞凋亡;H.表皮生长因子受体信号通路;I.剪接体;J.代谢途径;K.DNA复制;L.病毒致癌作用;M.亨廷顿氏疾病;N.巴尔病毒感染;O.RNA运输;P.核糖体 A.FGF signaling pathway; B.Cell cycle; C.Oocyte meiosis; D.PI3K-Akt signaling pathway; E.Hippo signaling pathway; F.MAPK signaling pathway; G.Apoptosis; H.EGF receptor signaling pathway; I.Spliceosome; J.Metabolic pathways; K.DNA replication; L.Viral carcinogenesis; M.Huntingto's disease; N.Epstein-Barr virus infection; O.RNA transport; P.Ribosome 图 3 差异表达蛋白质信号通路分析 Fig. 3 Pathway analysis of differentially expressed proteins |
在259种差异表达蛋白中,26种蛋白在DF中表达上调,233种蛋白在DF中表达下调。对差异蛋白中表达量前10的蛋白质进行筛选(表 3),结果显示:表达量高的差异蛋白全部为上调蛋白。
|
|
表 3 表达量前10的差异蛋白 Table 3 Top 10 high expression level of differential proteins |
利用STRING软件对鉴定出的259个差异蛋白,进行蛋白质互作网络分析,如图 4所示,两个蛋白之间,线条数量越多,表明联系越紧密。由图 4可知,热休克蛋白HSP90AB1与SKP1、ST13、TCEB2、EDF1、YWHAH、SIL1、CACYBP、CCT6A、SUGT1、OGN、CDC37、SOD2、CYCT、CFL1、RAB11B、EEF1D、EEF1A1、RBM3和NUP107联系较为紧密,其中OGN和NUP107为表达量较高的差异蛋白。
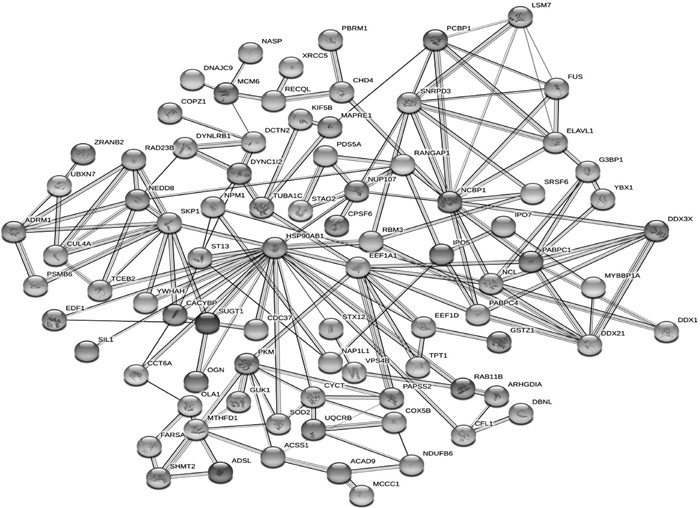
|
图 4 差异表达蛋白质互作网络图 Fig. 4 Protein-protein interaction network diagram of differentially expressed proteins |
本研究应用Label-free高通量蛋白质组分析技术,比较在牛卵泡发育过程中DF与SF蛋白组分差异,结果表明,获得的DF与SF蛋白质组种类数量相近,但由于DF与SF不同的生理状态,这些蛋白质的表达量也存在差异,共鉴定出259种差异表达蛋白质。通过对本研究所获得的蛋白质组数据与李鹏飞等[12]研究所获得的转录组测序结果进行比较分析,发现一些在转录组测序中被检测到的差异基因,同时在蛋白质组结果中也被发现。如:ACTA2、OGN、CCT5和DAZAP1等。另外,ACTA2和OGN在DF中表达量均比SF中高(差异倍数>2),CCT5和DAZAP1在SF中表达量均比DF中高(差异倍数>2),也表明本次测序结果的可信度较高。
参与细胞调控生物过程的蛋白数量在DF和SF中分别为247种和253种,GCs通过与卵母细胞、内膜细胞相互作用,从而调控卵泡的发育过程。11种DF蛋白(ILF2、ILF3、ZP3、STRBP、KHDRBS1、QKI、HCFC1、SCRIB、RALB、RALA、CDC42BPB)参与配子产生和胚胎发育过程,SF中未检测到参与这两种途径的蛋白,说明这11种蛋白可能与DF优势化有关。研究表明,ILF2与ILF3(NF90)相互作用,参与有丝分裂、转录调控、DNA修复、microRNA和病毒复制过程[13]。此外,ILF2通过调节Bcl-2、Bok、BAX和cIAP1,从而抑制肝癌细胞的凋亡[14]。RALA和RALB,在人癌症细胞的增殖、存活和转移中扮演着重要角色,包括肺癌、结肠癌、胰腺癌、前列腺癌、皮肤癌和膀胱癌等[15]。
鉴定出的3 409种蛋白质中,共有236种蛋白质在DF中特异表达,443种蛋白质在SF中特异表达。其中,表达量较高的DF特异蛋白TGFBI、EVPL、XIRP2、LIMA1、OLFML3和KCNK12都与细胞增殖相关[16-21],推测这6种蛋白可能是影响DF生长及优势化的关键蛋白。而SF特异表达蛋白质SERPINB2[22]表达水平的升高对细胞增殖有抑制作用,故SERPINB2可能与SF闭锁有关。
KEGG pathway分析结果显示,涉及到细胞凋亡信号通路的有3种蛋白质(CYCT、TUBA1C、TUBA1B),且这3种蛋白质在SF中表达量高于DF中表达量,推测CYCT、TUBA1C、TUBA1B可能与卵泡闭锁有关。有8条信号通路与卵泡增殖和闭锁相关,胞外产生的表皮生长因子和纤维母细胞生长因子通过相应的作用刺激卵泡生长。研究表明,表皮生长因子与促卵泡激素协同可能会更好的抑制GCs凋亡[23]。STMN1和CRK涉及MAPK信号通路,该途径与细胞增殖、分化、迁移、衰老和凋亡有关[24]。促卵泡激素通过激活MAPK途径,从而促进GCs增殖[25]。YWHAG、YWHAH、YWHAB、YWHAQ参与Hippo信号通路,该信号通路抑制细胞增殖[26-28],这4种蛋白属于14-3-3家族蛋白。研究证明,14-3-3蛋白对神经细胞的增殖和分化起着重要作用;与特定蛋白互作,参与细胞周期调控、细胞存活与胞间黏附[29]。
OGN、ACTA2、纤维蛋白原(FGA、FGB和FGG)均是差异表达倍数前10的上调蛋白。纤维蛋白原是止血系统的重要组成部分,哺乳动物正常妊娠与凝血状态密切相关,研究表明,纤维蛋白原在妊娠过程中逐渐增加[30],暗示FGA、FGB和FGG可能在卵泡发育阶段起重要作用。OGN在DF中表达量最高,不同类型肿瘤中OGN表达缺失,且OGN促进细胞凋亡[31],ACTA2抑制细胞增殖[32]。推测OGN和ACTA2可能与卵泡闭锁有关。
在哺乳动物配子形成过程中,热休克蛋白的表达是最基本的生物过程,不同的热休克蛋白在卵巢中陆续被发现[33]。为保证卵子的正常形成,需要多种热休克蛋白共同完成该过程[34]。结合蛋白质互作网络分析结果,热休克蛋白HSP90B1与其他高表达蛋白联系较为紧密,其可能在卵泡生长过程中起关键作用。
4 结论本研究共获得259种差异表达蛋白质,17种蛋白质(ILF2、ILF3、ZP3、STRBP、KHDRBS1、QKI、HCFC1、SCRIB、RALB、RALA、CDC42BPB、TGFBI、EVPL、XIRP2、LIMA1、OLFML3和KCNK12)可能参与牛卵泡优势化过程;SERPINB2可能调控牛卵泡闭锁。
| [1] | FORTUNE J E, RIVERA G M, EVANS A C O, et al. Differentiation of dominant versus subordinate follicles in cattle[J]. Biol Reprod, 2001, 65(3): 648–654. DOI: 10.1095/biolreprod65.3.648 |
| [2] | ADAMS G P, MATTERI R L, KASTELIC J P, et al. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers[J]. J Reprod Fertil, 1992, 94(1): 177–188. |
| [3] | FIELD S L, DASGUPTA T, CUMMINGS M, et al. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation[J]. Mol Reprod Dev, 2014, 81(4): 284–314. DOI: 10.1002/mrd.22285 |
| [4] | SUNDERLAND S J, CROWE M A, BOLAND M P, et al. Selection, dominance and atresia of follicles during the oestrous cycle of heifers[J]. J Reprod Fertil, 1994, 101(3): 547–555. DOI: 10.1530/jrf.0.1010547 |
| [5] | JOHNSON A L. Intracellular mechanisms regulating cell survival in ovarian follicles[J]. Anim Reprod Sci, 2003, 78(3-4): 185–201. DOI: 10.1016/S0378-4320(03)00090-3 |
| [6] | KAFI M, MESBAH S F, DAVOODIAN N, et al. Fine structures of the oocyte in relation to serum, follicular fluid steroid hormones and IGF-I in the ovulatory-sized follicles in one-humped camel (Camelus dromedarius)[J]. Avicenna J Med Biotechnol, 2014, 6(1): 57–61. |
| [7] | BABITHA V, PANDA R P, YADAV V P, et al. Amount of mRNA and localization of vascular endothelial growth factor and its receptors in the ovarian follicle during estrous cycle of water buffalo (Bubalus bubalis)[J]. Anim Reprod Sci, 2013, 137(3-4): 163–176. DOI: 10.1016/j.anireprosci.2013.01.004 |
| [8] | PEDERSEN H G, WATSON E D, TELFER E E. Analysis of atresia in equine follicles using histology, fresh granulosa cell morphology and detection of DNA fragmentation[J]. Reproduction, 2003, 125(3): 417–423. DOI: 10.1530/rep.0.1250417 |
| [9] | WONGSRIKEAO P, KANESHIGE Y, OOKI R, et al. Effect of the removal of cumulus cells on the nuclear maturation, fertilization and development of porcine oocytes[J]. Reprod Domest Anim, 2005, 40(2): 166–170. DOI: 10.1111/rda.2005.40.issue-2 |
| [10] | MARX H, MINOGUE C E, JAYARAMAN D, et al. A proteomic atlas of the legume Medicago truncatula and its nitrogen-fixing endosymbiont Sinorhizobium meliloti[J]. Nat Biotechnol, 2016, 34(11): 1198–1205. DOI: 10.1038/nbt.3681 |
| [11] | WEBB-ROBERTSON B J, WIBERG H K, MATZKE M M, et al. Review, evaluation, and discussion of the challenges of missing value imputation for mass spectrometry-based label-free global proteomics[J]. J Proteome Res, 2015, 14(5): 1993–2001. DOI: 10.1021/pr501138h |
| [12] |
李鹏飞, 孟金柱, 郝庆玲, 等. PDF2和ODF1转录组测序筛选牛卵泡发育相关基因[J]. 畜牧兽医学报, 2018, 49(2): 300–309.
LI P F, MENG J Z, HAO Q L, et al. Screening and analysing of genes associated with follicular development in bovine ODF1 and PDF2 transcriptome[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(2): 300–309. (in Chinese) |
| [13] | SHAMANNA R A, HOQUE M, LEWIS-ANTES A, et al. The NF90/NF45 complex participates in DNA break repair via nonhomologous end joining[J]. Mol Cell Biol, 2011, 31(23): 4832–4843. DOI: 10.1128/MCB.05849-11 |
| [14] | CHENG S B, JIANG X, DING C F, et al. Expression and critical role of interleukin enhancer binding factor 2 in hepatocellular carcinoma[J]. Int J Mol Sci, 2016, 17(8): 1373. DOI: 10.3390/ijms17081373 |
| [15] | YAN C, THEODORESCU D, YE R D, et al. RAL GTPases:biology and potential as therapeutic targets in cancer[J]. Pharmacol Rev, 2018, 70(1): 1–11. |
| [16] | GUO S K, SHEN M F, YAO H W, et al. Enhanced expression of TGFBI promotes the proliferation and migration of glioma cells[J]. Cell Physiol Biochem, 2018, 49(3): 1138–1150. |
| [17] | SIGURDSON A J, BRENNER A V, ROACH J A, et al. Selected single-nucleotide polymorphisms in FOXE1, SERPINA5, FTO, EVPL, TICAM1 and SCARB1 are associated with papillary and follicular thyroid cancer risk:replication study in a German population[J]. Carcinogenesis, 2016, 37(7): 677–684. DOI: 10.1093/carcin/bgw047 |
| [18] | WANG Q C, LIN L C, REINKING B E, et al. Essential roles of an intercalated disc protein, mXinβ, in postnatal heart growth and survival[J]. Circ Res, 2010, 106(9): 1468–1478. DOI: 10.1161/CIRCRESAHA.109.212787 |
| [19] | COLLINS R J, JIANG W G, HARGEST R, et al. EPLIN:a fundamental actin regulator in cancer metastasis?[J]. Cancer Metastasis Rev, 2015, 34(4): 753–764. DOI: 10.1007/s10555-015-9595-8 |
| [20] | NEIDERT N, VON EHR A, ZÖLLER T, et al. Microglia-specific expression of Olfml3 is directly regulated by transforming growth factor β1-induced Smad2 signaling[J]. Front Immunol, 2018, 9: 1728. DOI: 10.3389/fimmu.2018.01728 |
| [21] | DOOKERAN K A, ZHANG W, STAYNER L, et al. Associations of two-pore domain potassium channels and triple negative breast cancer subtype in The Cancer Genome Atlas:systematic evaluation of gene expression and methylation[J]. BMC Res Notes, 2017, 10(1): 475. DOI: 10.1186/s13104-017-2777-4 |
| [22] | LEE N H, CHO A, PARK S R, et al. SERPINB2 is a novel indicator of stem cell toxicity[J]. Cell Death Dis, 2018, 9(7): 724. DOI: 10.1038/s41419-018-0748-x |
| [23] | BABU P S, KRISHNAMURTHY H, CHEDRESE P J, et al. Activation of extracellular-regulated kinase pathways in ovarian granulosa cells by the novel growth factor type 1 follicle-stimulating hormone receptor.Role in hormone signaling and cell proliferation[J]. J Biol Chem, 2000, 275(36): 27615–27626. |
| [24] | PALMERINI M G, NOTTOLA S A, TUNJUNG W A S, et al. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes[J]. Biochem Biophys Res Commun, 2016, 481(1-2): 159–164. DOI: 10.1016/j.bbrc.2016.10.151 |
| [25] | SUN Y, LIU W Z, LIU T, et al. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis[J]. J Recept Signal Transduct Res, 2015, 35(6): 600–604. DOI: 10.3109/10799893.2015.1030412 |
| [26] | ZHANG L, YUE T, JIANG J. Hippo signaling pathway and organ size control[J]. Fly(Austin), 2009, 3(1): 68–73. |
| [27] | HALDER G, JOHNSON R L. Hippo signaling:growth control and beyond[J]. Development, 2011, 138(1): 9–22. |
| [28] | HERGOVICH A. Mammalian hippo signalling:a kinase network regulated by protein-protein interactions[J]. Biochem Soc Trans, 2012, 40(1): 124–128. DOI: 10.1042/BST20110619 |
| [29] | SHIMADA T, FOURNIER A E, YAMAGATA K. Neuroprotective function of 14-3-3 proteins in neurodegeneration[J]. BioMed Res Int, 2013, 2013: 564534. |
| [30] | RÉGER B, PÉTERFALVI Á, LITTER I, et al. Challenges in the evaluation of D-dimer and fibrinogen levels in pregnant women[J]. Thromb Res, 2013, 131(4): 183–187. DOI: 10.1016/j.thromres.2013.02.005 |
| [31] | TASHEVA E S, MAKI C G, CONRAD A H, et al. Transcriptional activation of bovine mimecan by p53 through an intronic DNA-binding site[J]. Biochim Biophys Acta, 2001, 1517(3): 333–338. DOI: 10.1016/S0167-4781(00)00288-8 |
| [32] | LEE H W, PARK Y M, LEE S J, et al. Alpha-smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma[J]. Clin Cancer Res, 2013, 19(21): 5879–5889. DOI: 10.1158/1078-0432.CCR-13-1181 |
| [33] | HRISTOVA I. Role of heat shock proteins (Hsp) in human and mammalian fertilization and pregnancy.Part Ⅰ[J]. Akush Ginekol (Sofiia), 2012, 51(5): 45–49. |
| [34] | HRISTOVA I. Role of heat shock proteins (Hsp) in human and mammalian fertilization and pregnancy.Part Ⅱ[J]. Akush Ginekol (Sofiia), 2012, 51(6): 37–40. |



