2. 安徽农业大学生命科学学院, 合肥 230036;
3. 天津市畜牧兽医研究所, 天津 300381
2. School of Life Sciences, Anhui Agricultural University, Hefei 230036, China;
3. Tianjin Institute of Animal Sciences, Tianjin 300381, China
动物下丘脑-垂体-性腺轴(hypothalamic-pituitary-gonadal axis,HPGA)系统调控机体生殖和发育。其中,下丘脑作为神经内分泌活动中心,其神经分泌细胞可合成下丘脑调节肽,这些调节肽运输至腺垂体,从而促进或抑制腺垂体激素的分泌。例如,下丘脑在接收外界信号或自身反馈信息后会释放促性腺激素释放激素(GnRH)来刺激垂体促性腺激素的释放[1]。此外,HPGA系统上的一系列调控因子在机体信号传递和转换过程中参与启动动物季节性繁殖活动[2-4],例如Dardente等[5-7]已发现,HPGA上游的一些基因如EYA3、TSHβ等对动物发情启动和终止有重要调控作用。
促性腺激素抑制激素(GnIH)是最早在鹌鹑下丘脑中被发现的一种十二肽[8],它以剂量依赖的模式作用于垂体前叶,并抑制GnRH及垂体促性腺激素合成和分泌。血管活性肠肽(VIP)则是一种由28个氨基酸构成的生物活性肽[9],属于胰高血糖素-胰泌素家族成员。GnIH、VIP在家禽繁殖方面的作用主要表现为下丘脑分泌的VIP经垂体门脉运输至垂体,并与垂体分泌催乳素(PRL)细胞上特异性受体VIPR结合以促进PRL分泌,同时下丘脑室旁核(PVN)分泌的GnIH也作用于垂体,抑制促性腺激素的生物合成和释放[10-11]。后来在哺乳动物上研究发现,由RFRP基因编码的GnIH肽类(如GnIH-1、GnIH-3)位于下丘脑背内侧神经元(DMN)及PVN前区,其中GnIH-3与禽类GnIH在遗传上最相近,当外界压力和季节变化时GnIH-3对机体应激、繁殖行为等起抑制作用[12-14]。目前,已有研究表明,公绵羊血清中GnIH浓度达300 pg·mL-1以上[15];通过原位杂交分析母羊下丘脑中GnIH基因的表达,发现其在排卵前降低,与促黄体素(LH)变化相关,推测GnIH作为抑制剂对绵羊繁殖状态改变有一定调节作用[16-17]。
本试验以苏尼特母羊(典型的季节性发情品种)和小尾寒羊母羊(典型的常年发情品种)为研究对象,采用实时荧光定量PCR(qPCR)技术进一步检测性腺轴各组织中GnIH和VIP基因在不同繁殖状态下的表达差异,并分析不同光照时间点季节性发情品种苏尼特羊下丘脑中GnIH和VIP基因mRNA的变化趋势,为进一步揭示或完善绵羊季节性繁殖分子调控机制奠定基础。
1 材料与方法 1.1 试验动物及样品采集不同繁殖状态下目的基因组织表达试验:本试验所需的苏尼特羊(Sunite sheep,SNT)和小尾寒羊(Small Tail Han sheep,STH)均来自天津市畜牧兽医研究所试验羊场。苏尼特羊:分别选择短光照(光照8 h,黑暗16 h,人工模拟发情配种季节)第21天和长光照(光照16 h,黑暗8 h,模拟休情季节)第49天的健康成年SNT母羊各3只,光控条件下开灯后4 h内屠宰并采样。小尾寒羊:课题组前期挑选健康状况良好的成年STH母羊6只,利用阴道孕酮栓(CIDR)进行同期发情处理,在撤栓后采用腹腔镜检测母羊卵泡发育和排卵状况以确定卵泡期取样时间,最终在撤栓后45 h(卵泡期)和第10天(黄体期)屠宰采样,2个时期各取3只羊[18]。上述所有羊只屠宰后迅速采集性腺轴组织样(下丘脑、垂体、松果体、卵巢、输卵管、子宫体),分别装入2 mL RNase-Free冻存管,立即置于液氮中。样品带回实验室后全部转置-80 ℃冰箱。
短光照转至长光照条件下丘脑中目的基因表达变化模式试验:从上述试验羊场中选择一批健康的成年SNT母羊摘除其卵巢后埋植雌激素处理(OVX+E2),短光照(光照8 h,黑暗16 h)条件饲养42 d后转至长光照(光照16 h,黑暗8 h)饲养49 d,根据文献报道[19-22]和本课题组预试验结果,分别在短光照第21天(SP-21 d)和长光照第3(LP-3 d)、15(LP-15 d)、21(LP-21 d)、42(LP-42 d)、49天(LP-49 d)共6个不同光照时间点屠宰绵羊用于后续分析,每个时间点屠宰3只羊,迅速采集下丘脑并速冻于液氮中,后转存于-80 ℃冰箱[23]。
1.2 SNT和STH性腺轴组织总RNA提取及其cDNA合成采用RNAprep pure动物组织RNA提取试剂盒(天根,北京)依次提取上述性腺轴各组织总RNA,具体操作参照试剂盒说明。其中,用Trizol(Invitrogen,美国)代替细胞裂解液。NanoDrop 2000测定RNA浓度及纯度,OD值(A260 nm/A280 nm)在1.8~2.1之间。1.0%琼脂糖凝胶电泳检测RNA,无明显降解。利用cDNA快速合成试剂盒(TaKaRa,Japan)反转录获得cDNA第一条链。全程操作在冰上进行。反转录产物用RNase-Free ddH2O 5倍稀释后保存于-20 ℃冰箱[24]。
1.3 绵羊GnIH和VIP基因扩增引物设计以NCBI数据库公布的绵羊GnIH和VIP基因序列(GenBank登录号详见表 1)为参照,通过Genome Browser和Primer 3.0在线软件排列基因序列并跨外显子区域设计特异性qPCR引物各1对,以持家基因β-actin作内参。引物由北京天一辉远生物科技有限公司合成。引物具体信息见表 1。
|
|
表 1 qPCR引物信息 Table 1 The primer information for real-time PCR |
5倍稀释后的合格cDNA样按1/2浓度梯度稀释,分别获得8个浓度梯度(1、1/2、1/4、1/8、1/16、1/32、1/64、1/128)cDNA样品,以这些cDNA为模板进行qPCR反应。以浓度梯度对数值(10为底数)为横坐标,以检测所得Ct值为纵坐标,绘制目的基因和持家基因的标准曲线,上述引物扩增效率具体见表 1。各阳性样品模板反应后期熔解曲线呈单峰、峰值尖锐的形态(图略),引物扩增效率在1.90~2.05之间,符合qPCR试验要求。
1.4.2 qPCR检测GnIH和VIP基因表达水平利用Roche Light Cycler®480Ⅱ PCR仪检测以上2个目的基因的相对表达量。根据课题组前期设定的qPCR反应体系和程序[25-26],依次检测SNT和STH各组织样中2个基因的表达,每种样品做3个技术性重复,设置阴性对照(H2O为模板)。反应结束后分析熔解曲线,根据2-ΔΔCt法[27]计算目的基因的相对表达量。
1.5 数据统计使用SPSS20.0软件对数据进行统计分析。组间比较采用单因素方差分析(one-way ANOVA)检验,用最小显著差异法(least significant difference, LSD)进行多重比较,所有数据用“平均值±标准误(Mean±SEM)”表示。P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果 2.1 GnIH和VIP基因在短光照和长光照条件下苏尼特羊性腺轴组织中的表达本试验发现,GnIH和VIP基因广泛表达于被检测的各组织中(图 1)。其中,GnIH基因表达最高的3个组织分别是下丘脑、卵巢、输卵管;VIP基因在下丘脑中表达量较高。下丘脑和垂体中GnIH和VIP基因在短光照(SP)和长光照(LP)之间的表达差异极显著(P<0.01)。
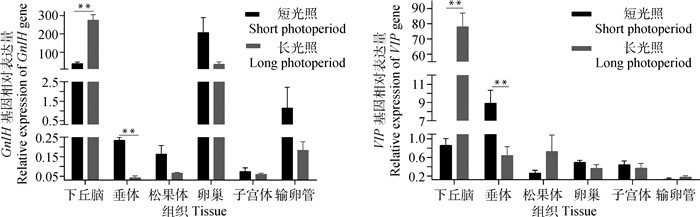
|
*表示差异显著(P<0.05),**表示差异极显著(P<0.01),下同 *Indicates significant difference(P < 0.05), ** indicates extremely significant difference (P < 0.01), the same as below 图 1 GnIH和VIP基因在不同光照条件下苏尼特羊各组织中的表达 Fig. 1 Expression of GnIH and VIP genes in detected tissues of SNT under different photoperiods |
试验结果显示,2个基因广泛表达于小尾寒羊被测各组织中(图 2)。其中,GnIH基因表达最高的3个组织分别是下丘脑、卵巢和输卵管;VIP基因在下丘脑和垂体中表达量较高。松果体中GnIH基因在黄体期的表达量极显著高于卵泡期(P<0.01)。下丘脑中VIP基因在黄体期的表达量显著高于卵泡期(P<0.05),垂体中该基因在卵泡期的表达量极显著高于黄体期(P<0.01)。
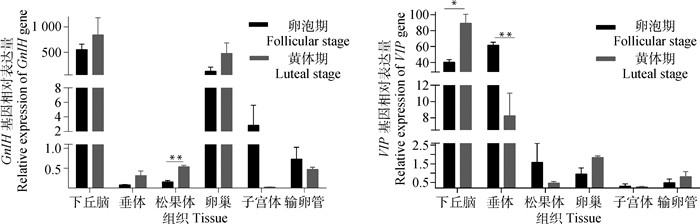
|
图 2 GnIH和VIP基因在不同繁殖时期小尾寒羊各组织中的表达 Fig. 2 Expression of GnIH and VIP genes in detected tissues of STH at different reproductive stages |
基因的表达变化趋势OVX+E2模型的成年SNT母羊在不同光照时间点其下丘脑中GnIH和VIP基因均表达(图 3),且这2个基因在SP转至LP后表达量均存在先升高的趋势。其中,GnIH基因表达在LP-3 d时达到峰值,之后逐渐降低并保持平稳表达状态;VIP基因在LP-3 d达到表达高峰,之后其表达量呈降低趋势。
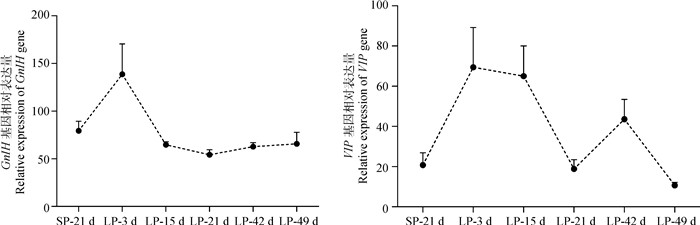
|
SP-21 d、LP-3 d、LP-15 d、LP-21 d、LP-42 d、LP-49 d分别代表短光照第21天和长光照第3、15、21、42、49天 SP-21 d, LP-3 d, LP-15 d, LP-21 d, LP-42 d, LP-49 d represent the 21th day under short photoperiod, the 3rd, 15th, 21th, 42th and 49th days under long photoperiod, respectively 图 3 SP转至LP后不同时间点苏尼特羊下丘脑中GnIH和VIP基因的表达模式 Fig. 3 Expression pattern of GnIH and VIP genes during the transition from SP to LP in hypothalamus of SNT |
天府肉鹅GnIH mRNA在下丘脑高表达,在肾上腺、输卵管、卵巢中也有一定表达[28],且在卵泡发育过程中GnIH mRNA表达量先升高后降低,卵泡直径为4~5 mm阶段GnIH表达量高于其他各级卵泡发育阶段,推测GnIH基因表达可能与卵泡选择及闭锁调控有关[29]。向体外培养的鹅卵泡颗粒细胞模型中添加外源GnIH后,颗粒细胞活性降低,雌二醇和孕酮分泌减少,LH-R和FSH-R及抑凋亡(促生存)基因Bcl-2表达下调[30]。后期Zhu等[31]研究表明,长光照条件下匈牙利白鹅下丘脑GnIH和VIP基因表达下调,GnRH-I、Dio2、OPN5、c-Fos基因表达上调,导致垂体促性腺激素基因表达并激活下游繁殖系统。刘晶[32]通过RT-PCR方法检测新西兰白兔体内GnIH及其受体基因的表达情况,结果发现,GnIH基因在兔眼球壁、下丘脑、肝、肾和肾上腺中表达量较高,在垂体、中脑、卵巢和睾丸等组织中也有一定表达。成年猪GnIH基因在下丘脑、延髓、中脑和大脑中表达量较高,在子宫、卵巢、眼、睾丸、附睾等组织中该基因呈中度表达,而在肠道中几乎不表达[33-36]。小鼠GnIH基因在下丘脑、脑桥、输卵管和卵巢组织中表达量较高,在心、肝、脾等内脏组织中其表达量相对较低[37]。山羊GnIH基因在下丘脑、垂体、卵巢中均表达且在下丘脑中表达量最高,在子宫中不表达[3]。绵羊中也是集中在下丘脑表达,其作用通路大致为:在卵泡期其下丘脑中Kisspeptin刺激GnRH分泌,通过神经内分泌形成正反馈调节,诱导LH在排卵前分泌增多以促进排卵;LH分泌过量时,则负反馈刺激下丘脑GnIH分泌,GnIH作用于GnRH细胞并执行与Kisspeptin相反的作用[38]。针对动物VIP基因表达部位的研究较少,研究发现,下丘脑视交叉上核(SCN)中VIP缺失可导致小鼠SCN节律性振荡减弱,节律因子如Per1、Per2、Bmal1等表达缺失,神经元分泌活动紊乱,最终HPGA系统活力下降[39];Beymer等[40]研究表明,哺乳动物SCN能通过VIP作用影响GnIH活动来调控繁殖轴活力变化。本试验中,GnIH和VIP基因广泛表达于绵羊的性腺轴各组织中,且这2个基因在SNT和STH绵羊下丘脑中高表达,可见绵羊中GnIH和VIP基因的表达分布特征与上述报道动物中的表达特征相似;另一方面,2个基因在动物性腺轴组织中均有分布,提示其与动物繁殖相关。
3.2 不同繁殖状态下GnIH和VIP基因表达差异具有季节性发情特征的动物包括两类:短日照动物(以绵羊为主)和长日照动物(家禽、鼠等)。Zhu等[41]研究发现,扬州鹅(长日照繁殖鸟类)受到长光照刺激后,其下丘脑GnRH基因表达上升,GnIH和VIP mRNA表达下降。类似的,长光照条件下匈牙利白鹅下丘脑GnIH和VIP基因表达下调,GnRH-I、Dio2、OPN5、c-Fos基因表达上调,导致垂体促性腺激素基因表达,并激活下游繁殖系统。魏岳等[42]分析不同光照条件对兴国灰鹅性腺轴组织中FSHβ、PRL和VIP基因表达水平的影响,发现在30和80 lx光照强度条件下这3个基因在相同试验组不同组织中的表达规律一致。长日照繁殖动物与短日照繁殖动物的繁殖季节不同,长、短日照条件下发情相关通路中的基因表达变化刚好相反。针对短日照繁殖的绵羊,Dardente等[43]研究发现,在短光照转至长光照休情状态后,Soay绵羊下丘脑中GnIH基因表达上调,休情期GnIH基因表达较卵泡期高[44],下丘脑PVN及DMN区分泌至门静脉血液中的GnIH肽总量水平在非繁殖季节较繁殖季节高[45]。本研究表明,长光照条件下苏尼特羊下丘脑中GnIH和VIP基因表达量极显著高于短光照条件(P<0.01);黄体期小尾寒羊下丘脑中这2个基因表达量较卵泡期高。与长日照繁殖鸟类相反,长光照条件下,下丘脑中GnIH和VIP基因上调导致下丘脑-垂体-性腺轴基因表达下调,最终启动绵羊休情机制。该研究结果进一步阐明GnIH和VIP基因在绵羊季节性繁殖和繁殖时期转换中扮演了一定角色。
本团队前期研究发现,苏尼特羊垂体中PRLR基因在长光照条件下的表达量高于短光照条件,小尾寒羊垂体中PRLR在黄体期的表达量较卵泡期高[23]。VIP和PRLR基因都与PRL的分泌关系密切,动物下丘脑分泌的VIP到达垂体后与VIPR特异性结合促进PRL分泌,然后PRL与其受体PRLR结合调控下游通路。Wood等[6]研究发现,短光照转为长光照后的前2~3周,绵羊血浆PRL明显升高,长光照条件下绵羊PRL整体分泌水平较短光照时高。因此,上述研究表明,与PRL分泌相关的重要基因有协同变化趋势;同时也暗示,VIP、VIPR基因在绵羊季节性繁殖和繁殖时期转换过程中具有重要调控作用。
3.3 短光照转至长光照后绵羊下丘脑中GnIH和VIP基因表达变化模式在OVX+E2模型中,短光照转至长光照后苏尼特羊下丘脑中GnIH和VIP表达量均升高,都在LP-3 d达到表达高峰,之后逐渐降低。本团队前期研究证明,短光照转至长光照后苏尼特羊垂体中季节性繁殖相关基因EYA3与TSHβ表达上升,在LP-3 d时EYA3表达量最高,后逐渐下降[7]。EYA3、TSHβ的表达变化趋势与GnIH、VIP基因相似,提示下丘脑中GnIH、VIP基因与性腺轴上游其他因子协同变化共同参与动物繁殖活动调控,主要在LP-3 d之前发挥关键作用,参与启动机体内长日照机制,使绵羊成功进入长日照繁殖状态。
4 结论本试验分析了成年苏尼特母羊和小尾寒羊母羊性腺轴各组织中GnIH和VIP基因在不同繁殖状态下的定量表达变化,研究结果进一步表明,下丘脑中GnIH和VIP基因参与绵羊季节性发情调控和繁殖时期转换;在短光照转至长光照过程中GnIH和VIP主要在长光照前3 d发挥关键作用,参与长日照繁殖模式的启动。
| [1] | MIKAMI S I. Immunocytochemistry of the avian hypothalamus and adenohypophysis[J]. Int Rev Cytol, 1986, 103(6): 189–248. |
| [2] | GREIVES T J, MASON A O, SCOTTI M A L, et al. Environmental control of kisspeptin:implications for seasonal reproduction[J]. Endocrinology, 2007, 148(3): 1158–1166. DOI: 10.1210/en.2006-1249 |
| [3] |
黄冬维, 储明星, 狄冉, 等. 山羊繁殖季节性相关基因KiSS-1与RFRP的表达研究[J]. 畜牧兽医学报, 2015, 46(6): 924–931.
HUANG D W, CHU M X, DI R, et al. Study on expression of KiSS-1 and RFRP genes related to reproductive seasonality in goats[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(6): 924–931. (in Chinese) |
| [4] | DALLMANN R, BROWN S A, GACHON F. Chronopharmacology:new insights and therapeutic implications[J]. Annu Rev Pharmacol Toxicol, 2014, 54(1): 339–361. DOI: 10.1146/annurev-pharmtox-011613-135923 |
| [5] | DARDENTE H, LOMET D, ROBERT V, et al. Seasonal breeding in mammals:from basic science to applications and back[J]. Theriogenology, 2016, 86(1): 324–332. DOI: 10.1016/j.theriogenology.2016.04.045 |
| [6] | WOOD S H, CHRISTIAN H C, MIEDZINSKA K, et al. Binary switching of calendar cells in the pituitary defines the phase of the circannual cycle in mammals[J]. Curr Biol, 2015, 25(20): 2651–2662. DOI: 10.1016/j.cub.2015.09.014 |
| [7] |
夏青, 张效生, 刘秋月, 等. EYA3和TSHβ在季节性发情和常年发情绵羊中的表达模式[J]. 畜牧兽医学报, 2018, 49(2): 263–269.
XIA Q, ZHANG X S, LIU Q Y, et al. Expression patterns of EYA 3 and TSH β in seasonal estrous and year-round estrous sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(2): 263–269. (in Chinese) |
| [8] | TSUTSUI K, SAIGOH E, UKENA K, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release[J]. Biochem Biophys Res Commun, 2000, 275(2): 661–667. DOI: 10.1006/bbrc.2000.3350 |
| [9] | SAID S I, MUTT V. Polypeptide with broad biological activity:isolation from small intestine[J]. Science, 1970, 169(3951): 1217–1218. DOI: 10.1126/science.169.3951.1217 |
| [10] |
孔鸽萍.鹅催乳素受体基因的克隆和表达[D].南京: 南京农业大学, 2006.
KONG G P.Cloning and expression of prolactin receptor gene of goose[D].Nanjing: Nanjing Agricultural University, 2006.(in Chinese) http://d.wanfangdata.com.cn/Thesis/Y1009879 |
| [11] |
褚晓红, 徐宁迎, 胡锦平, 等. 浙东白鹅催乳素基因表达特点[J]. 遗传, 2008, 30(8): 1021–1025.
CHU X H, XU N Y, HU J P, et al. Expression characteristics of prolactin gene in eastern Zhejiang White geese[J]. Hereditas, 2008, 30(8): 1021–1025. DOI: 10.3321/j.issn:0253-9772.2008.08.012 (in Chinese) |
| [12] | UBUKA T, INOUE K, FUKUDA Y, et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone[J]. Endocrinology, 2012, 153(1): 373–385. DOI: 10.1210/en.2011-1110 |
| [13] | PAPARGIRIS M M, RIVALLAND E T A, CLARKE I J, et al. Evidence that RF-amide related peptide-3 is not a mediator of the inhibitory effects of psychosocial stress on gonadotrophin secretion in ovariectomised ewes[J]. J Neuroendocrinol, 2011, 23(3): 208–215. DOI: 10.1111/jne.2011.23.issue-3 |
| [14] | TSUTSUI K, UBUKA T, BENTLEY G E, et al. Gonadotropin-inhibitory hormone (GnIH):discovery, progress and prospect[J]. Gen Comp Endocrinol, 2012, 177(3): 305–314. DOI: 10.1016/j.ygcen.2012.02.013 |
| [15] |
姚晓磊, 黄欣爱, 肖慎华, 等. 不同发育阶段湖羊生殖器官脂联素受体与睾酮分泌关键基因表达模式及相关性研究[J]. 畜牧兽医学报, 2018, 49(8): 1642–1650.
YAO X L, HUANG X A, XIAO S H, et al. Expression patterns and correlation of adiponectin receptors and testosterone secretion related-genes expression in male reproductive organs at different developmental stages of Hu sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(8): 1642–1650. (in Chinese) |
| [16] | CLARKE I J, SMITH J T. The role of kisspeptin and gonadotropin inhibitory hormone (GnIH) in the seasonality of reproduction in sheep[J]. Soc Reprod Fertil Suppl, 2010, 67(5): 159–169. |
| [17] | CLARKE I J, SMITH J T, HENRY B A, et al. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding[J]. Neuroendocrinology, 2012, 95(4): 305–316. DOI: 10.1159/000332822 |
| [18] |
曹晓涵, 刘秋月, 王翔宇, 等. 小尾寒羊黄体期和卵泡期生殖轴凋亡基因的表达研究及其与发情的关系[J]. 畜牧兽医学报, 2017, 48(11): 2091–2097.
CAO X H, LIU Q Y, WANG X Y, et al. Expression levels of apoptotic genes in HPG axis during luteal and follicular periods and their association with estrus in Small Tail Han sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(11): 2091–2097. (in Chinese) |
| [19] | DARDENTE H, WYSE C A, BIRNIE M J, et al. A molecular switch for photoperiod responsiveness in mammals[J]. Curr Biol, 2010, 20(24): 2193–2198. DOI: 10.1016/j.cub.2010.10.048 |
| [20] | MASUMOTO K H, UKAI-TADENUMA M, KASUKAWA T, et al. Acute induction of EYA3 by late-night light stimulation triggers TSHβ expression in photoperiodism[J]. Curr Biol, 2010, 20(24): 2199–2206. DOI: 10.1016/j.cub.2010.11.038 |
| [21] | WOOD S, LOUDON A. Clocks for all seasons:unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary[J]. J Endocrinol, 2014, 222(2): R39–R59. DOI: 10.1530/JOE-14-0141 |
| [22] | TSUJINO K, NARUMI R, MASUMOTO K H, et al. Establishment of TSHβ real-time monitoring system in mammalian photoperiodism[J]. Genes Cells, 2013, 18(7): 575–588. DOI: 10.1111/gtc.2013.18.issue-7 |
| [23] |
李晓雨, 贺小云, 刘秋月, 等. TAC1和PRLR基因在绵羊不同繁殖状态下的表达模式分析[J]. 畜牧兽医学报, 2018, 49(2): 253–262.
LI X Y, HE X Y, LIU Q Y, et al. Expression pattern analysis of TAC1 and PRLR genes in different reproductive states of sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(2): 253–262. (in Chinese) |
| [24] |
董新龙, 胡文萍, 贺小云, 等. 绵羊BMP15组织表达特征及FecXGr、FecXO和G971A突变的检测[J]. 农业生物技术学报, 2016, 24(12): 1810–1819.
DONG X L, HU W P, HE X Y, et al. Tissue expression and detection of the FecXGr, FecXO and G971A mutations of BMP15 gene in sheep (Ovis aries)[J]. Journal of Agricultural Biotechnology, 2016, 24(12): 1810–1819. (in Chinese) |
| [25] |
汤继顺, 狄冉, 刘秋月, 等. 精卵融合相关基因CD9和CD81在绵羊组织中的表达分析[J]. 农业生物技术学报, 2018, 26(1): 87–95.
TANG J S, DI R, LIU Q Y, et al. Tissue expression analysis of CD9 and CD81 genes associated with sperm-egg fusion in sheep (Ovis aries)[J]. Journal of Agricultural Biotechnology, 2018, 26(1): 87–95. (in Chinese) |
| [26] |
周梅, 曹晓涵, 贺小云, 等. 绵羊FGF7基因组织表达及其多态性与产羔数之间的关系[J]. 畜牧兽医学报, 2018, 49(3): 525–533.
ZHOU M, CAO X H, HE X Y, et al. Tissue expression and polymorphism of sheep FGF 7 gene and their association with litter size[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(3): 525–533. (in Chinese) |
| [27] | SCHMITTGEN T D, LIVAK K J. Analyzing real-time PCR data by the comparative CT method[J]. Nat Protoc, 2008, 3(6): 1101–1108. DOI: 10.1038/nprot.2008.73 |
| [28] |
甘超, 胡深强, 苟华, 等. 鹅GnIH受体基因的克隆及组织表达特性研究[J]. 中国家禽, 2014, 36(1): 7–11.
GAN C, HU S Q, GOU H, et al. Cloning of gonadotropin-inhibiting hormne receptors and their exprssion patterns in tissues of goose[J]. China Poultry, 2014, 36(1): 7–11. DOI: 10.3969/j.issn.1004-6364.2014.01.004 (in Chinese) |
| [29] |
董霞, 肖其海, 刘贺贺, 等. 鹅GnIH基因克隆及其在卵泡发育中的表达规律研究[J]. 中国家禽, 2013, 35(7): 5–8.
DONG X, XIAO Q H, LIU H H, et al. Cloning of GnIH gene and its expression pattern in follicles development of goose[J]. China Poultry, 2013, 35(7): 5–8. DOI: 10.3969/j.issn.1004-6364.2013.07.003 (in Chinese) |
| [30] |
甘超.鹅GnIH及其受体基因的克隆、组织表达以及在颗粒细胞中的研究[D].雅安: 四川农业大学, 2014.
GAN C.Cloning of goose GnIH and its receptor, the gene expression and research in granulosa cells[D].Ya'an: Sichuan Agricultural University, 2014.(in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10626-1016048364.htm |
| [31] | ZHU H X, LIU X Q, HU M D, et al. Endocrine and molecular regulation mechanisms of the reproductive system of Hungarian white geese investigated under two artificial photoperiodic programs[J]. Theriogenology, 2018, 123(2019): 167–176. |
| [32] |
刘晶.兔GnIH及其受体基因的克隆和mRNA在组织器官的表达[D].南京: 南京农业大学, 2011.
LIU J.The cloning and mRNA expression of GnIH and its receptors in rabbit[D].Nanjing: Nanjing Agricultural University, 2011.(in Chinese) http://d.wanfangdata.com.cn/Thesis/Y2360375 |
| [33] | LI X, SU J, LEI Z H, et al. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig:cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle[J]. Peptides, 2012, 36(2): 176–185. DOI: 10.1016/j.peptides.2012.05.008 |
| [34] |
霍孔林, 胡浩, 李建华, 等. GnIH在陆川仔公猪中枢神经系统的分布与定位[J]. 中国兽医科学, 2015, 45(10): 1072–1077.
HUO K L, HU H, LI J H, et al. Distribution and localization of gonadotropin-inhibitory hormone (GnIH) in the central nervous system of male Luchuan piglets[J]. Chinese Veterinary Science, 2015, 45(10): 1072–1077. (in Chinese) |
| [35] |
霍孔林, 李昀, 王晓晔, 等. GnIH在陆川仔公猪泌尿生殖系统及内分泌免疫系统中的分布[J]. 西北农林科技大学学报:自然科学版, 2016, 44(12): 16–20.
HUO K L, LI Y, WANG X H, et al. Distribution of gonadotropin-inhibitory hormone (GnIH) in urinary, reproductive, endocrine and immune systems of Luchuan male piglets[J]. Journal of Northwest A&F University:Natural Science Edition, 2016, 44(12): 16–20. (in Chinese) |
| [36] |
王晓晔, 霍孔林, 肖凯, 等. GnIH在陆川仔公猪消化系统及气管的分布定位[J]. 华中农业大学学报, 2016, 35(3): 89–93.
WANG X H, HUO K L, XIAO K, et al. Distribution and expression of gonadotropin-inhibitory hormone (GnIH) indigestion system and trachea of Luchuan male piglets[J]. Journal of Huazhong Agricultural University, 2016, 35(3): 89–93. (in Chinese) |
| [37] |
李卉, 罗明秀, 宋辉. GnIH基因在小鼠各个组织中的表达[J]. 宁夏农林科技, 2017, 58(10): 24–26.
LI H, LUO M X, SONG H. Expression of GnIH in different tissues of mice[J]. Ningxia Journal of Agriculture and Forestry Science and Technology, 2017, 58(10): 24–26. DOI: 10.3969/j.issn.1002-204X.2017.10.012 (in Chinese) |
| [38] | CLARKE I J, BARTOLINI D, CONDUCTIER G, et al. Stress increases gonadotropin inhibitory hormone cell activity and input to GnRH cells in ewes[J]. Endocrinology, 2016, 157(11): 4339–4350. DOI: 10.1210/en.2016-1513 |
| [39] | LOH D H, DRAGICH J M, KUDO T, et al. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs[J]. J Biol Rhythms, 2011, 26(3): 200–209. DOI: 10.1177/0748730411401740 |
| [40] | BEYMER M, HENNINGSEN J, BAHOUGNE T, et al. The role of kisspeptin and RFRP in the circadian control of female reproduction[J]. Mol Cell Endocrinol, 2016, 438(19): 89–99. |
| [41] | ZHU H X, CHEN Z, SHAO X B, et al. Reproductive axis gene regulation during photostimulation and photorefractoriness in Yangzhou goose ganders[J]. Front Zool, 2017, 14(1): 11–25. |
| [42] |
魏岳, 武艳平, 刘林秀, 等. 不同光照条件对兴国灰鹅FSHβ、PRL和VIP基因mRNA表达水平的影响[J]. 江西农业大学学报, 2016, 38(5): 947–953.
WEI Y, WU Y P, LIU L X, et al. Effects of light exposure on mRNA expression of FSHβ, PRL and VIP of Xingguo gray geese[J]. Acta Agriculturae Universitatis Jiangxiensis, 2016, 38(5): 947–953. (in Chinese) |
| [43] | DARDENTE H, BIRNIE M, LINCOLN G A, et al. RFamide-related peptide and its cognate receptor in the sheep:cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod[J]. J Neuroendocrinology, 2008, 20(21): 1252–1259. |
| [44] | CLARKE I J, PARKINGTON H C. Gonadotropin inhibitory hormone (GnIH) as a regulator of gonadotropes[J]. Mol Cell Endocrinol, 2014, 385(1-2): 36–44. DOI: 10.1016/j.mce.2013.08.017 |
| [45] | SMITH J T, YOUNG I R, VELDHUIS J D, et al. Gonadotropin-inhibitory hormone (GnIH) secretion into the ovine hypophyseal portal system[J]. Endocrinology, 2012, 153(7): 3368–3375. DOI: 10.1210/en.2012-1088 |



