肌纤维性状是影响肌肉品质的重要因素,与肌肉嫩度、滴水损失、pH、肉色、肌内脂肪含量等密切相关[1-2]。肌纤维性状主要包括肌纤维直径、密度和类型等,肌纤维直径与密度呈负相关,肌纤维类型对直径和密度均有影响,三者之间彼此相关,共同决定肌肉品质的优劣。肌纤维类型是影响肌肉品质的重要因素之一,根据其代谢和收缩功能,骨骼肌纤维分为慢速氧化型、快速氧化型、快速酵解型和中间型4种,分别由不同的肌球蛋白重链(myosin heavy chain, MyHC)异构体MyHCⅠ、MyHCⅡa、MyHCⅡb和MyHCⅡx确定[3]。在4种类型的肌纤维中,Ⅰ型肌纤维直径最小,Ⅱb型最大,Ⅱa和Ⅱx型介于中间[4]。由于Ⅰ型肌纤维直径小,线粒体含量丰富,肌肉中Ⅰ型肌纤维含量与肉色和嫩度呈正相关,与滴水损失呈负相关[5-6]。此外,所有Ⅰ型肌纤维均包含中性脂质,而只有26%的Ⅱa型和1%的Ⅱb型含有脂质[7],因此,Ⅰ型肌纤维含量与IMF、多汁性、风味均呈正相关[7-8]。Ⅱ型肌纤维,尤其是Ⅱb型肌纤维直径大,线粒体含量低,肌肉中Ⅱb型含量与肌肉亮度、WHC、滴水损失均呈正相关[2]。此外,Ⅱb型含量高、Ⅰ型含量低的肌肉中,宰后45 min糖原和乳糖含量高,糖酵解快速,导致肉色灰白,滴水损失高,且Ⅱb型肌纤维百分比与pH45呈负相关(r=-0.33)[9]。总之,Ⅰ型肌纤维含量高、Ⅱb型含量低时,肌肉嫩度、肉色好,肌内脂肪含量高,而Ⅱ型肌纤维的增加将增加肌肉代谢率,导致肌肉品质变差[8]。肌纤维性状差异的形成与肌纤维发育过程密切相关,受到多种基因的影响和调控。因此,有必要在全基因组水平挖掘和筛选与肌纤维性状相关的候选基因和信号通路。
RNA-Seq是基于第二代测序技术的一种转录组学研究方法,可以高通量准确确定RNA的表达水平,在一定程度上能够加快对基因表达、调控以及网络的系统性理解。Xu等[10]对梅山猪65胚龄及3、60和120日龄的骨骼肌进行了转录组测序,发现338个差异表达基因,功能分析结果显示,差异表达基因主要与代谢、肌原纤维丝形成、细胞骨架、收缩活性以及信号转导等有关。Li等[11]利用基因芯片技术对梅山猪红肌和白肌样品进行分析,发现了与肌纤维类型相关的28条信号通路,包括能量代谢通路和细胞周期通路等。Ayuso等[12]对肉质较好的伊比利亚猪和产肉量较高的杜洛克与伊比利亚猪杂交F1代猪的股二头肌进行转录组测序,发现149个差异表达基因,在伊比利亚猪中,差异表达基因主要富集在脂质代谢过程,而在杂交猪中则主要富集在与肌肉生长有关的生物学过程。
马身猪是山西省地方品种,与大白猪相比适应性强,肌肉品质好,但生长速度慢,饲料转化率低。前期研究表明,马身猪和大白猪在生长速度、胴体和肌肉品质、肌纤维性状等方面存在显著差异[13-14]。本研究在分析马身猪和大白猪6月龄背最长肌肌纤维性状差异的基础上,采用RNA-Seq技术对两品种猪背最长肌进行转录组测序,通过差异表达基因(differentially expressed genes, DEGs)、基因本体论(gene ontology, GO)和京都基因与基因组百科全书(kyoto encyclopedia of genes and genomes, KEGG)等生物信息学分析,筛选影响肌纤维性状的候选基因和信号通路,研究结果将为阐明肌肉生长发育、代谢调控机理及改善肌肉品质提供理论依据。
1 材料与方法 1.1 试验动物和样品采集大白猪(Large White, LW)和马身猪(Mashen, MS)均来自大同市种猪场,在相同饲养管理条件下生长发育,6月龄时选择来源于不同家系、生长发育良好、健康无病、种内个体体重接近的大白猪((120.00±1.04) kg)和马身猪((72.50±2.04) kg)各3头,于达到日龄当天屠宰。屠宰后,立即采集背最长肌约10 g,放入15 mL离心管中,分别标记为LW61、LW62、LW63、MS61、MS62和MS63,迅速置于液氮速冻,随后转入-80℃冰箱保存备用。同时,采集左半侧胴体第9~11肋骨处背最长肌,按切面与肌纤维走向垂直的要求修成1 cm3的肌肉块,用4%多聚甲醛浸泡固定,用于肌纤维性状的测定。
1.2 猪肌纤维直径和肌纤维密度的测定背最长肌组织块用4%多聚甲醛固定24 h后,在75%、85%、95%、100%的乙醇中各脱水1 h,然后用二甲苯透明1 h,随后石蜡包埋。使用microtome (Leica, Germany)切片机切片,厚度为5 μm。切片用二甲苯和乙醇进行连续脱蜡和脱水后进行苏木精-伊红(hematoxylin-eosin staining, HE)染色。采用DMi8 microscope (Leica, Germany)系统观察切片,拍照并测量肌纤维直径和密度。每头猪选3张切片,每张切片随机选择3个完整视野,测得每头猪所有视野内肌纤维直径,求平均值,即为样品肌纤维直径(μm);对每头猪每个视野内的肌纤维数计数并求平均数,再换算成每平方毫米的肌纤维根数,即为1.0 mm2内肌纤维密度。用SPSS22.0(IBM, NY)对马身猪和大白猪背最长肌肌纤维直径和密度的差异进行统计分析,P < 0.05表示差异显著,P < 0.01表示差异极显著。
1.3 RNA提取、cDNA文库构建及Illumina测序使用Trizol(Thero Fisher Science, Carlsbad, Canada)试剂提取组织样总RNA,所得总RNA再用RNeasy Kits(QIAGEN, GmbH, Germany)进一步纯化,并使用安捷伦2100生物分析仪(Agilent 2100, Santa Clara, America)检测RNA的完整性。按照TruSeq® Rapid Duo cBotTM Sample Loading Kit试剂盒流程构建cDNA文库,其过程为Oligo(dT)磁珠分离mRNA,RNA片段化,cDNA合成及PCR扩增等。对制备好的cDNA文库在Illumina HiSeq 2500平台上进行2×100 bp的双端测序,得到原始数据(raw reads)。
1.4 测序结果的质量控制使用Perl脚本对原始数据进行质量控制,去除接头序列、未知核酸序列信息大于10%的序列以及质量分数≤10的碱基数占到整个碱基数50%以上的冗余序列,得到clean reads。
1.5 差异表达基因筛选及功能注释在服务器上使用bowtie2 (v2.2.9)对clean reads建立索引,用tophat (v2.0.12)将这些质控后的序列片段mapping到猪基因组上(http://hgdownload.soe.ucsc.edu/goldenPath/susScr3/bigZips/susScr3.fa.gz),再利用cufflinks (v2.2.1)和cuffmerge (v3)将Reads组装为新的注释文件,用cuffdiff (v2.0.2)进行筛选得到转录本信息。利用FPKM(每百万个reads中map到外显子的每千个碱基上的reads数)算法对转录本的表达量进行标准化,最后参考P值、错误发现率(false discovery rate, FDR)和差异倍数(fold change, FC),以P<0.01、FDR<0.01和|log2(FC)|≥1为标准筛选差异表达基因。用DAVID对差异表达基因进行GO和KEGG功能分析,以P≤0.05为筛选条件,筛选差异表达基因显著富集的GO和KEGG条目。
1.6 利用实时荧光定量PCR验证RNA-Seq结果为验证RNA-Seq结果的准确性,本研究选择6个差异表达基因,根据GenBank中各基因的mRNA序列,利用NCBI网站的Primer-BLAST模块在线设计引物(表 1),18S为内参。取与RNA-Seq分析用相同的组织样品,使用Trizol (TaKaRa, Japan)试剂提取总RNA,反转录成cDNA。使用SYBR Premix Ex Taq (TaKaRa, Japan)进行qRT-PCR分析。反应体系总体积10 μL:1 μL cDNA (100 ng·μL-1),0.2 μL (10 μmol·L-1)上游引物,0.2 μL (10 μmol·L-1)下游引物,5 μL SYBR Premix Ex TaqTM(2×),3.6 μL RNase free H2O。反应程序:95 ℃预变性30 s;95 ℃变性10 s,60 ℃退火和延伸30 s,40个循环。每个样品设置3个重复。采用2-ΔΔCt法计算mRNA的相对表达量。
|
|
表 1 实时荧光定量PCR引物序列 Table 1 The primer sequences of qRT-PCR |
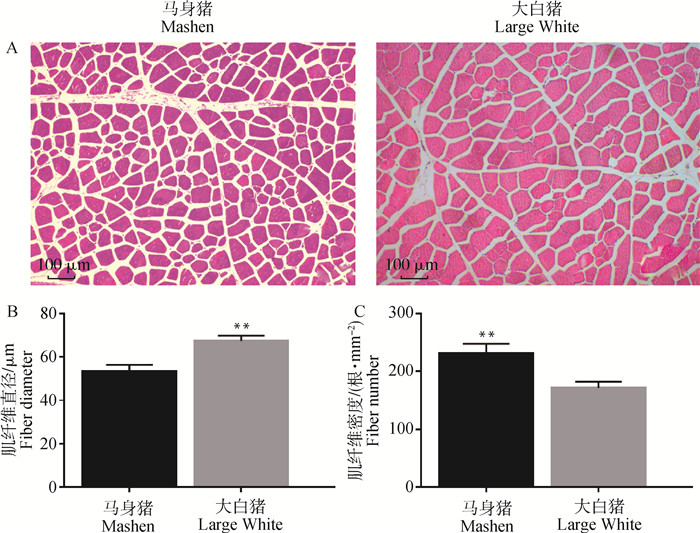
马身猪和大白猪背最长肌组织HE染色结果如图 1所示,马身猪背最长肌肌纤维直径极显著低于大白猪(P < 0.01),肌纤维密度极显著高于大白猪(P < 0.01)。
|
A.6月龄马身猪和大白猪背最长肌HE染色结果;B.肌纤维直径;C.肌纤维密度。**表示差异极显著(P < 0.01) A.HE staining of longissimus dorsi of Mashen and Large White pigs at 6 months old; B. Muscle fiber diameter; C. Muscle fiber number per mm2. **denotes extremely significant difference (P < 0.01) 图 1 马身猪和大白猪6月龄背最长肌肌纤维直径和密度 Fig. 1 Muscle fiber diameter and density of longissimus dorsi of Mashen and Large White pigs at 6 months old |
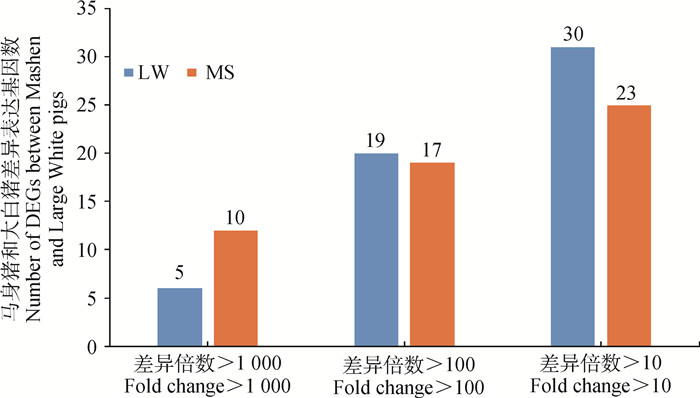
比较马身猪和大白猪背最长肌RNA-Seq结果,得到在两品种中差异表达基因的情况。设定筛选差异表达基因的条件为P<0.01、FDR<0.01和|log2(FC)|≥1,共得到105个差异表达基因,其中马身猪相对于大白猪上调的基因有55个,下调的基因有50个。差异倍数在1 000倍以上的基因,马身猪有10个,大白猪有5个;差异倍数在100倍以上的基因,马身猪有17个,大白猪有19个(图 2)。
|
图 2 马身猪和大白猪差异表达基因分布图 Fig. 2 DEGs distribution map between Mashen and Large White pigs |
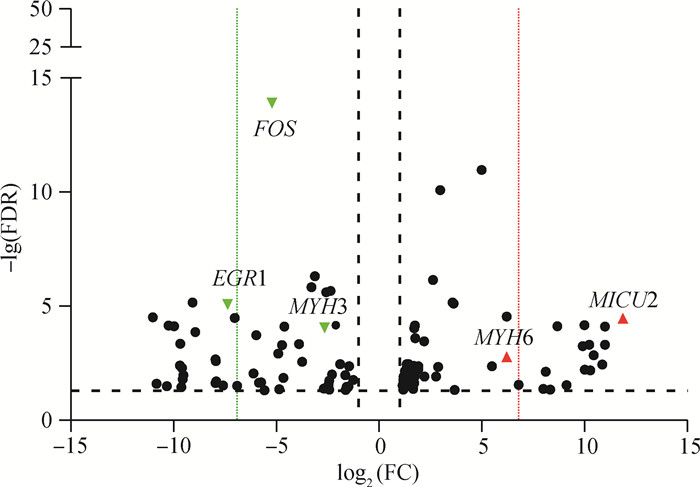
由差异基因热图(图 3)可以看出,表达量最高且在马身猪和大白猪中表达差异较大的两个基因分别为肌球蛋白轻链3 (myosin light chain 3, MYL3)基因和线粒体脱氢酶亚基6 (NADH dehydrogenase subunit 6, ND6)基因。此外,肌球蛋白重链6 (myosin heavy chain 6, MYH6)基因、早期生长应答蛋白1(early growth response gene-1, EGR1)基因、肌球蛋白重链3(myosin heavy chain 3, MYH3)、线粒体钙离子摄取蛋白2 (mitochondrial calcium uptake 2, MICU2)基因在马身猪和大白猪中表达量较高,差异倍数较大,FDR较低(图 4);原癌基因FOS (fos proto-oncogene, FOS)虽然表达丰度较低,但在两品种中的表达水平差异明显,且FDR较低(图 4),表明该基因可能也参与肌纤维的形成。

|
此图以FPKM值为依据,颜色由红色到蓝色对应FPKM值由大到小 This figure is based on the FPKM value, and the color from red to blue corresponds to the FPKM value from large to small 图 3 马身猪和大白猪差异表达基因热图 Fig. 3 DEGs heat map between Mashen and Large White pigs |
|
红色代表上调,绿色代表下调, 红色和绿色虚线用于区分两品种的特异基因 Red denotes upregulated genes; green denotes downregulated genes, red and green dotted lines are used to distinguish the specific genes in Mashen and Large White pigs 图 4 马身猪和大白猪差异表达基因火山图 Fig. 4 Volcano plot for DEGs between Mashen and Large White pigs |
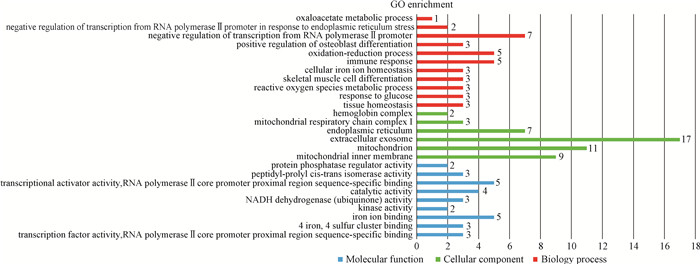
与KEGG分析GO分析可以用来更好地对差异表达基因进行注释和基因功能分类,它分为3个组分,即生物过程(biology process, BP)、细胞组分(cellular component, CC)和分子功能(molecular function, MF)。GO分析结果显示,差异表达基因显著富集的条目有26个,其中生物过程11个,细胞组分6个,分子功能9个(图 5,表 2)。与生物过程有关的主要是骨骼肌分化过程,在该过程中富集到了火山图中差异显著的基因EGR1;与细胞构成有关的主要有细胞组分和线粒体,肌纤维类型与线粒体紧密相关,在该条目中显著富集到了差异表达基因热图中丰度很高的基因ND6;与分子功能有关的主要是分子绑定相关的转录因子活性条目,暗示EGR1和FOS等基因编码的转录因子可能参与调控肌纤维性状的形成。
|
图 5 差异表达基因的GO富集分析 Fig. 5 GO analysis of differentially expressed genes |
|
|
表 2 马身猪和大白猪差异表达基因GO富集分析结果 Table 2 GO analysis of differentially expressed genes between Mashen and Large White pigs |
KEGG有助于从分子水平上了解更高级的功能和生物系统。以Q < 0.05为条件,共有50个差异表达基因显著富集到11个通路中(图 6),主要有代谢、氧化磷酸化和一些与疾病相关的通路(帕金森、疟疾)(表 3)。ND6基因显著富集在代谢和氧化磷酸化通路中,从KEGG角度再次暗示线粒体相关基因确实在调节肌纤维性状上发挥重要作用。
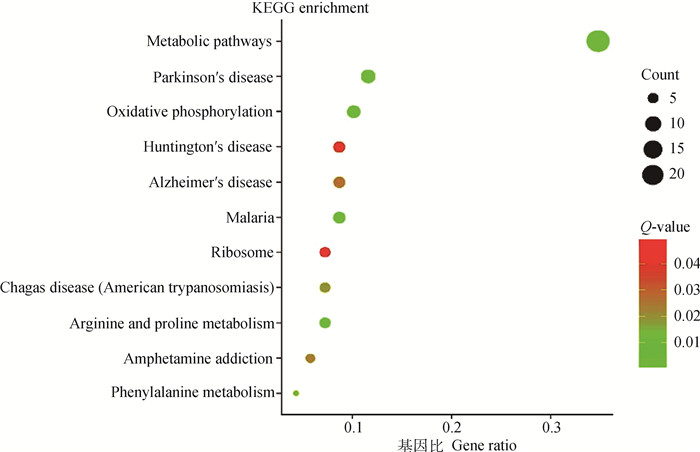
|
横坐标是gene ratio,为对应通路差异表达基因占总差异表达基因的比例 The abscissa is gene ratio, which is the ratio of the DEGs in the corresponding pathway to the total DEGs 图 6 差异表达基因KEGG富集分析 Fig. 6 KEGG pathway analysis of differentially expressed genes |
|
|
表 3 差异表达基因KEGG富集分析结果 Table 3 KEGG enrichment analysis results of differentially expressed genes |
GO和KEGG分析结果可以对差异表达基因参与的功能进行更全面的理解。结合火山图、热图、GO和KEGG分析结果,可见MYL3、MYH6、MYH3、ND6、MICU2、EGR1和FOS参与到肌纤维性状的形成,是影响肌纤维性状的候选基因。
2.4 qRT-PCR验证RNA-Seq结果为了验证RNA-Seq结果的可靠性,选取6个差异表达基因进行qRT-PCR分析。结果显示,虽然个别差异基因的荧光定量结果和RNA-Seq结果的差异倍数之间存在偏差,但是两者之间的上调和下调的表达趋势相同(图 7),荧光定量PCR的结果与RNA-seq结果相关系数达到0.990 1(P < 0.01),表明RNA-Seq结果准确可靠。
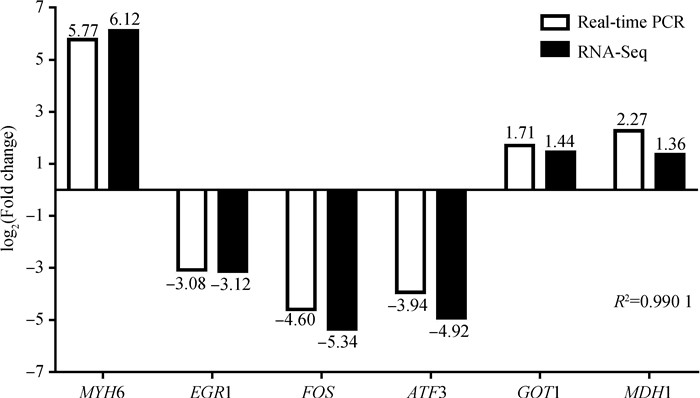
|
图中白色代表荧光定量结果,黑色代表RNA-Seq结果,数字表示差异倍数,R2=0.990 1 White represents qRT-PCR results, black represents RNA-Seq results, number is difference multiple, R2=0.990 1 图 7 差异表达基因荧光定量与RNA-Seq对比结果 Fig. 7 Comparison results between DEGs expression detected by qRT-PCR and RNA-Seq |
肌纤维性状与肉色、系水力、嫩度、肌内脂肪含量、pH等性状密切相关[8]。本研究结果表明,马身猪肌纤维直径极显著低于大白猪(P < 0.01),肌纤维密度极显著高于大白猪(P < 0.01),这与马身猪肉质细嫩、肉香味浓的食用品质相一致。肌纤维类型与肌纤维直径及肌肉品质密切相关[6, 15-16]。不同类型的肌纤维在代谢(氧化和糖酵解能力)和生物物理特性(纤维大小、纤维颜色、糖原和脂肪含量)等方面均存在差异[17-18]。目前普遍将肌纤维类型分为Ⅰ型、Ⅱ型肌纤维,Ⅱ型肌纤维又可细分为Ⅱa、Ⅱb和Ⅱx。Ⅰ型肌纤维为氧化型纤维,富含线粒体,主要利用氧化磷酸化来进行能量的生产;Ⅱ型肌纤维线粒体含量较少,主要通过糖酵解途径产生ATP[19]。Ⅰ型肌纤维直径较细,Ⅱ型肌纤维尤其是Ⅱb型肌纤维直径较粗[6, 15]。当Ⅰ型肌纤维比例较高时,肌肉肉色和大理石评分较高,肉质细嫩且风味较好[8, 20-21]。
ND6是线粒体呼吸链的重要组成成分,催化NADH脱氢和电子转移[22]。本研究中,ND6在马身猪中高表达,且与显著富集的氧化磷酸化通路有关,表明马身猪骨骼肌中富含线粒体的Ⅰ型氧化型肌纤维含量高于大白猪,这解释了马身猪肌纤维直径显著小于大白猪的原因,也与贾静敏[14]的研究结果相一致。贾静敏[14]采用qRT-PCR技术检测了马身猪和大白猪中与肌纤维类型相关的肌球蛋白重链(myosin heavy chains, MyHCs)异构体编码基因的表达量,发现马身猪背最长肌中决定氧化型纤维Ⅰ、Ⅱa的基因MyHC Ⅰ和MyHC Ⅱa的表达极显著高于大白猪(P < 0.01),而大白猪酵解型和中间型的编码基因MyHC Ⅱb和MyHC Ⅱx的表达量极显著高于马身猪(P < 0.01)[14]。
MYL3是肌球蛋白轻链家族的成员,主要参与慢肌纤维Ⅰ的形成[23]。MYL3蛋白质可以结合钙离子,促进肌肉发育,并参与横纹肌的收缩[24]。当人类卫星细胞在缺氧环境中培养时,增加MYL3基因的表达可以促进卫星细胞分化为成肌细胞[25]。本研究中,马身猪MYL3的表达量显著高于大白猪,这与马身猪肌纤维直径显著小于大白猪的表型特征相一致,也与马身猪中Ⅰ型肌纤维含量高的特征相一致[14]。MYH3和MYH6是肌球蛋白重链(myosin heavy chain, MyHC)家族的成员,MYH3主要在胎儿肌肉中表达量较高,同时参与了肌肉的再生过程[26],MYH3的表达量与肌纤维粗细成正相关[27-28]。MYH6主要在心房肌中表达,参与肌肉细胞骨架结构,与骨骼肌发育密切相关,该基因的表达量与纤维的密度呈极显著正相关[29]。本研究中,马身猪MYH6基因表达量显著高于大白猪,MYH3基因表达量显著低于大白猪,这两个基因的表达规律与马身猪肌纤维直径显著低于大白猪、肌纤维密度显著高于大白猪的表型特征相吻合。
MICU2基因编码的蛋白为线粒体钙离子摄取蛋白,位于线粒体膜间隙,可作为调节亚基调节线粒体Ca2+的吸收[30],维持细胞的正常生理功能。线粒体钙转运主通道主要由MICU1同源二聚体调控,MICU1同源二聚体又受到MICU1-MICU2异源二聚体调控[31]。Patron等[32]发现,上调MICU2的表达可增加MICU1-MICU2异质二聚体的含量,降低MICU1同源二聚体含量,从而降低细胞中线粒体Ca2+浓度;相反,沉默MICU2基因后,减少了MICU1-MICU2异质二聚体的含量,增加了MICU1同源二聚体含量,导致线粒体Ca2+吸收增加。马身猪中MICU2表达量明显大于大白猪,说明马身猪线粒体调度与利用率明显大于大白猪,也间接说明马身猪骨骼肌中富含线粒体,可能是其骨骼肌中Ⅰ型肌纤维含量高造成的。
FOS是调节细胞增殖、分化、凋亡等多种细胞过程的转录因子,作为启动基因转录的分子开关,通过细胞应激、刺激等来调节基因的表达[33-34]。EGR1作为转录因子在细胞增殖、分化及凋亡等许多生物学过程中都扮演着重要角色。研究表明,EGR1基因能够促进C2C12细胞分化[35]。EGR1基因还可以促进FOS基因的表达,在研究子宫平滑肌瘤细胞时发现,诱导EGR1后FOS的表达量显著增加[36]。本研究中,FOS和EGR1在大白猪中高表达,这解释了大白猪由于肌细胞增长导致肌纤维直径增大,肌纤维密度降低的原因。
4 结论马身猪和大白猪在肌纤维直径和密度等性状上存在极显著差异。在6月龄大白猪和马身猪背最长肌中共检测到105个差异表达基因,其中,MYL3、MYH3、MYH6基因通过影响肌纤维的组成而影响肌纤维特性,ND6基因通过参与线粒体氧化磷酸化过程影响肌纤维类型,MICU2基因通过维持线粒体Ca2+浓度的稳态影响细胞功能,编码转录因子的EGR1和FOS基因通过调节细胞增殖、分化和凋亡等过程影响肌肉发育。本研究初步揭示了造成马身猪和大白猪肌纤维性状差异的主要原因,为猪肉品质的改善提供了相应的理论依据。
| [1] | ŞIRINE, AKSOYY, UǦURLUM, 等. The relationship between muscle fiber characteristics and some meat quality parameters in Turkish native sheep breeds[J]. Small Ruminant Res, 2017, 150: 46–51. |
| [2] | JOO S T, KIM G D, HWANG Y H, et al. Control of fresh meat quality through manipulation of muscle fiber characteristics[J]. Meat Sci, 2013, 95(4): 828–836. DOI: 10.1016/j.meatsci.2013.04.044 |
| [3] | LEFAUCHEUR L, GERRARD D. Muscle fiber plasticity in farm mammals[J]. J Anim Sci, 2000, 77(Suppl_E): 1–19. |
| [4] | MALTIN C A, WARKUP C C, MATTHEWS K R, et al. Pig muscle fibre characteristics as a source of variation in eating quality[J]. Meat Sci, 1997, 47(3-4): 237–248. DOI: 10.1016/S0309-1740(97)00055-7 |
| [5] | OZAWA S, MITSUHASHI T, MITSUMOTO M, et al. The characteristics of muscle fiber types of longissimus thoracis muscle and their influences on the quantity and quality of meat from Japanese Black steers[J]. Meat Sci, 2000, 54(1): 65–70. DOI: 10.1016/S0309-1740(99)00072-8 |
| [6] | CHOI Y M, NAM K W, CHOE J H, et al. Growth, carcass, fiber type, and meat quality characteristics in Large White pigs with different live weights[J]. Livest Sci, 2013, 155(1): 123–129. DOI: 10.1016/j.livsci.2013.02.009 |
| [7] | KARLSSON A H, KLONT R E, FERNANDEZ X. Skeletal muscle fibres as factors for pork quality[J]. Livest Prod Sci, 1999, 60(2-3): 255–269. DOI: 10.1016/S0301-6226(99)00098-6 |
| [8] | YANG Y L, LIANG G M, NIU G L, et al. Comparative analysis of DNA methylome and transcriptome of skeletal muscle in lean-, obese-, and mini-type pigs[J]. Sci Rep, 2017, 7: 39883. DOI: 10.1038/srep39883 |
| [9] | RYU Y C, KIM B C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle[J]. Meat Sci, 2005, 71(2): 351–357. DOI: 10.1016/j.meatsci.2005.04.015 |
| [10] | XU Y J, QIAN H, FENG X T, et al. Differential proteome and transcriptome analysis of porcine skeletal muscle during development[J]. J Proteomics, 2012, 75(7): 2093–2108. DOI: 10.1016/j.jprot.2012.01.013 |
| [11] | LI Y, XU Z Y, LI H Y, et al. Differential transcriptional analysis between red and white skeletal muscle of Chinese Meishan pigs[J]. Int J Biol Sci, 2010, 6(4): 350–360. |
| [12] | AYUSO M, FERNÁNDEZ A, NÚÑEZ Y, et al. Comparative analysis of muscle transcriptome between pig genotypes identifies genes and regulatory mechanisms associated to growth, fatness and metabolism[J]. PLoS One, 2015, 10(12): e0145162. DOI: 10.1371/journal.pone.0145162 |
| [13] | ZHAO Y Y, GAO P F, LI W, et al. Study on the developmental expression of Lbx1 gene in Longissimus dorsi of Mashen and Large White pigs[J]. Ital J Anim Sci, 2015, 14(1): 109–113. |
| [14] |
贾静敏.马身猪和大白猪肌球蛋白重链(MyHC)的发育性变化及肌纤维组织学观察[D].太谷: 山西农业大学, 2012.
JIA J M.Developmental changes of myosin heavy chain (MyHC) in horses and large white pigs and histological observation of muscle fibers[D].Taigu: Shanxi Agricultural University, 2012.(in Chinese) |
| [15] | GIL M, DELDAY M I, GISPERT M, et al. Relationships between biochemical characteristics and meat quality of Longissimus thoracis and Semimembranosus muscles in five porcine lines[J]. Meat Sci, 2008, 80(3): 927–933. DOI: 10.1016/j.meatsci.2008.04.016 |
| [16] | CHOI Y M, KIM B C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality[J]. Livest Sci, 2009, 122(2-3): 105–118. DOI: 10.1016/j.livsci.2008.08.015 |
| [17] | KIM J M, LEE S H, RYU Y C. Comparisons of meat quality and muscle fibre characteristics on multiple pig breeds and sexes using principal component analysis[J]. Anim Prod Sci, 2018, 58(11): 2091–2099. DOI: 10.1071/AN16223 |
| [18] | SCHIAFFINO S, REGGIANI C. Fiber types in mammalian skeletal muscles[J]. Physiol Rev, 2011, 91(4): 1447–1531. DOI: 10.1152/physrev.00031.2010 |
| [19] | CHOI Y M, RYU Y C, KIM B C. Influence of myosin heavy-and light chain isoforms on early postmortem glycolytic rate and pork quality[J]. Meat Sci, 2007, 76(2): 281–288. DOI: 10.1016/j.meatsci.2006.11.009 |
| [20] |
束婧婷, 章明, 宋迟, 等. 基于表达谱芯片挖掘清远麻鸡和科宝肉鸡比目鱼肌差异表达基因[J]. 畜牧兽医学报, 2016, 47(1): 25–33.
SHU J T, ZHANG M, SONG C, et al. Identification of differentially expressed genes for myofiber characteristics in soleus muscles between Qingyuan partridge chickens and Cobb broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(1): 25–33. (in Chinese) |
| [21] |
孟宪然, 杜琛, 王静, 等. 基于RNA-Seq识别山羊肉品质候选基因[J]. 畜牧兽医学报, 2015, 46(8): 1300–1307.
MENG X R, DU C, WANG J, et al. RNA-Seq approach for identifying candidate genes of meat quality in goats[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(8): 1300–1307. (in Chinese) |
| [22] | YUAN Y, WANG W X, LI H Z, et al. Nonsense and missense mutation of mitochondrial ND6 gene promotes cell migration and invasion in human lung adenocarcinoma[J]. BMC Cancer, 2015, 15: 346. DOI: 10.1186/s12885-015-1349-z |
| [23] | ZHANG C L, WANG J M, WANG G Z, et al. Molecular cloning and mRNA expression analysis of sheep MYL3 and MYL4 genes[J]. Gene, 2016, 577(2): 209–214. DOI: 10.1016/j.gene.2015.11.041 |
| [24] | GRABAREK Z. Structural basis for diversity of the EF-hand calcium-binding proteins[J]. J Mol Biol, 2006, 359(3): 509–525. DOI: 10.1016/j.jmb.2006.03.066 |
| [25] | KONING M, WERKER P M N, VAN LUYN M J, et al. Hypoxia promotes proliferation of human myogenic satellite cells:a potential benefactor in tissue engineering of skeletal muscle[J]. Tissue Eng Part A, 2011, 17(13-14): 1747–1758. DOI: 10.1089/ten.tea.2010.0624 |
| [26] | TAJSHARGHI H, OLDFORS A. Myosinopathies:pathology and mechanisms[J]. Acta Neuropathol, 2013, 125(1): 3–18. DOI: 10.1007/s00401-012-1024-2 |
| [27] |
张莺莺, 昝林森, 王洪宝. 利用基因芯片技术筛选秦川牛公牛与阉牛肌肉组织差异表达基因[J]. 遗传, 2010, 32(11): 1166–1174.
ZHANG Y Y, ZAN L S, WANG H B. Genome array on differentially expressed genes of muscle tissue in intact male and castrated Qinchuan cattle[J]. Hereditas (Beijing), 2010, 32(11): 1166–1174. (in Chinese) |
| [28] | XIONG X W, LIU X X, ZHOU L S, et al. Genome-wide association analysis reveals genetic loci and candidate genes for meat quality traits in Chinese Laiwu pigs[J]. Mamm Genome, 2015, 26(3-4): 181–190. DOI: 10.1007/s00335-015-9558-y |
| [29] |
胡帅帅, 王文洲, 闫晓荣, 等. 新西兰白兔中Myh6基因的表达及与肌纤维性状的相关性[J]. 华南农业大学学报, 2017, 38(2): 12–17.
HU S S, WANG W Z, YAN X R, et al. Myh6 gene expression and its correlation with muscle fiber traits of New Zealand white rabbits[J]. Journal of South China Agricultural University, 2017, 38(2): 12–17. (in Chinese) |
| [30] | KIRICHOK Y, KRAPIVINSKY G, CLAPHAM D E. The mitochondrial calcium uniporter is a highly selective ion channel[J]. Nature, 2004, 427(6972): 360–364. DOI: 10.1038/nature02246 |
| [31] | DE LA FUENTE S, MATESANZ-ISABEL J, FONTERIZ R I, et al. Dynamics of mitochondrial Ca2+ uptake in MICU1-knockdown cells[J]. Biochem J, 2014, 458(1): 33–40. DOI: 10.1042/BJ20131025 |
| [32] | PATRON M, CHECCHETTO V, RAFFAELLO A, et al. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity[J]. Mol Cell, 2014, 53(5): 726–737. DOI: 10.1016/j.molcel.2014.01.013 |
| [33] | WAGNER E. Functions of AP1(Fos/Jun) in bone development[J]. Ann Rheum Dis, 2002, 61(Suppl 2): ⅱ40–ⅱ42. |
| [34] | SHAULIAN E, KARIN M. AP-1 as a regulator of cell life and death[J]. Nat Cell Biol, 2002, 4(5): E131–E136. DOI: 10.1038/ncb0502-e131 |
| [35] |
崔亚凤, 严云勤, 李树峰, 等. Egr1对小鼠成肌细胞C2C12分化的影响[J]. 中国细胞生物学学报, 2018, 40(1): 25–32.
CUI Y F, YAN Y Q, LI S F, et al. The effect of Egr1 on C2C12 differentiation[J]. Chinese Journal of Cell Biology, 2018, 40(1): 25–32. (in Chinese) |
| [36] | ISHIKAWA H, SHOZU M, OKADA M, et al. Early growth response gene-1 plays a pivotal role in down-regulation of a cohort of genes in uterine leiomyoma[J]. J Mol Endocrinol, 2007, 39(5): 333–341. DOI: 10.1677/JME-06-0069 |



