2. 广东省兽医临床重大疾病综合防控重点实验室, 广州 510642;
3. 广东省宠物工程技术研究中心, 广州 510642
2. Guangdong Provincial Key Laboratory of Prevention and Control for Severe Clinical Animal Diseases, Guangzhou 510642, China;
3. Guangdong Technological Engineering Research Center for Pet, Guangzhou 510642, China
近年来犬乳腺肿瘤的发病率不断上升,已成为仅次于犬皮肤肿瘤的常见肿瘤疾病[1]。犬作为人类的伴侣动物,在生活习性、生活环境及饮食活动上均有着一定的共性[2]。犬乳腺肿瘤的研究才刚刚起步,主要研究方向集中在体外研究和抗肿瘤药物的研究,对犬乳腺肿瘤的体内发生及发展机制、致病机制等研究非常少,本试验拟为此类研究提供有价值的参考;而且合适的肿瘤动物模型在一定程度上能够复制肿瘤发生发展的过程[3],节约研究成本。
研究显示,人乳腺肿瘤与犬乳腺肿瘤在致病机制、发病机制等方面具有相似之处[4]。从生物分子层面上看,犬乳腺肿瘤具有和人家族遗传性乳腺肿瘤相同的易感基因BRCA1和BRCA2[5]。犬的基因序列图和单核苷酸阵列相关研究表明,控制犬乳腺肿瘤形态、生物学行为、临床发展进程的基因序列和与之相对应人的基因序列具有一定的相似性[6]。但目前人们对犬乳腺肿瘤的发生机制、致病机制等知之甚少,而犬乳腺肿瘤动物模型的建立可以在一定程度上帮助人们了解犬乳腺肿瘤的发生机制、致病机制等,对于犬乃至人类乳腺肿瘤的研究有着重要的意义。
活体成像检测是近几年发展起来的新技术[7],该技术可以在不处死实验动物的情况下观察肿瘤的发生及转移、干细胞示踪、抗肿瘤药物的筛选等[8]。目前主要应用于肿瘤方向研究,主要通过对实验动物体内荧光信号分子的检测从而追踪肿瘤的发生、发展及转移情况[9-10],也可以观察抗肿瘤药物在动物体内的分布及代谢等[9-10]。犬乳腺肿瘤的诊断方法主要有临床诊断、影像学诊断、病理学诊断等。病理学诊断是目前为止判断肿瘤最可靠的方法,其主要的内容包括病理切片分析、免疫组织化学检查等[11]。病理切片分析主要是指将一定大小的新鲜组织通过病理学方法制成病理切片,再通过显微镜检查判断肿瘤的类型。
因此,本研究欲通过将可稳定表达GFP基因标记的肿瘤细胞接种到模型动物体内,建立裸鼠成瘤模型并观察裸鼠成瘤的动态演变,探讨不同细胞系各自成瘤的特点与其生长特性,为早期诊断提供理论基础,最大限度保障犬的健康和功能,提高犬福利;而且可为进一步深入研究犬乳腺肿瘤的发生机制和抗肿瘤药物以及人类乳腺肿瘤研究提供有价值的参考。
1 材料与方法 1.1 材料 1.1.1 实验动物BALB/c裸鼠27只,购自北京华阜康生物科技股份有限公司,许可证号:SCXK(京)2014-0004。鼠龄:4~6周龄,性别:雌性,体重:18~22 g。饲养于华南农业大学实验动物中心,使用许可证号:SYXK(粤)2014-0136。
1.1.2 细胞系和质粒犬乳腺肿瘤细胞系CHMp与CHMm是从患乳腺肿瘤的雌性杂种犬中分离建立的,“p”代表原发病灶细胞,“m”代表转移病灶细胞。本试验中选用的犬乳腺肿瘤细胞系CHMp与CHMm均由日本东京大学农业部生命科学学科犬学院兽医外科实验室惠赠。人肾上皮细胞系293 T细胞由本教研室传代培养。
慢病毒包装和包膜质粒分别为psPAX2和pMD2.G质粒,现由华南农业大学兽医学院外科教研室保存,载体质粒是一种以PWPI为载体携带绿色荧光蛋白基因的质粒,该质粒能够在转染过程中进入细胞并在细胞中表达,在荧光显微镜下能观察到绿色荧光,该质粒由华南农业大学兽医学院外科教研室保存。Lipofectamine 2000购自Invitrogen公司。
1.2 实验方法 1.2.1 GFP标记犬乳腺肿瘤细胞与筛选采用三质粒包装系统(psPAX2+pMD2.G+目的基因PWPI)和lipofectamine2000进行病毒包装,将绿色荧光蛋白(GFP)导入到犬乳腺肿瘤CHMm系和CHMp系细胞。之后采用有限稀释法筛选出能稳定表达GFP的犬乳腺肿瘤细胞。
1.2.2 裸鼠成瘤模型的建立随机将裸鼠分为两个组(CHMm-GFP与CHMp-GFP裸鼠成瘤组),每组12只,且两组又各分成a、b、c、d四小组;体外培养犬乳腺肿瘤细胞CHMm-GFP与CHMp-GFP,待细胞生长状态稳定后收集细胞,用PBS调整细胞浓度为1×107个·mL-1且活细胞数>95%,加入等量Matrigel基质胶及少量双抗配制细胞悬液,细胞悬液按0.2 mL·只-1,统一注射于裸鼠右侧第二对乳头处。
1.2.3 裸鼠活体成像 1.2.3.1 活体成像裸鼠用异氟烷气体麻醉后放入活体成像仪内成像观察;参数如下,发射波长535 nm,激发波长480 nm,曝光时间5 s。试验结束待裸鼠状态稳定后将裸鼠放入普通级环境饲养,两组成瘤模型均分别随机挑选4、3、3只裸鼠在第15、25、35天进行活体成像检测。
1.2.3.2 离体器官成像待裸鼠活体成像检测完成后,麻醉状态下颈椎脱臼处死裸鼠,切开裸鼠接种部位皮肤且充分暴露肿块,放入活体成像仪内,进行成像观察;之后剖解裸鼠摘取肿块、心、肝、脾、肺、肾、淋巴结组织,放入活体成像仪内进行离体脏器成像观察,检测裸鼠体内肿瘤细胞是否出现转移。两组成瘤模型裸鼠分别在第25、35天进行离体器官的成像检测。
1.2.4 裸鼠解剖与病理学检测两组成瘤模型的a、b、c、d四小组,分别在第25、35、45和60天摘取两组成瘤模型裸鼠的肿块、心、脾、肝、肺、肾和淋巴结,并按常规方法进行石蜡包埋切片、HE染色和光镜观察。
1.2.5 统计分析试验组数据均采用SPSS 20.0软件分析。图中*代表 0.01<P<0.05;**代表P<0.01。
2 结果 2.1 绿色荧光蛋白标记犬乳腺肿瘤细胞利用慢病毒将GFP导入犬乳腺肿瘤细胞CHMp和CHMm中,经有限稀释法筛选后两株细胞均发出绿色荧光,分别命名为CHMm-GFP和CHMp-GFP。倒置荧光显微镜观察结果见图 1。
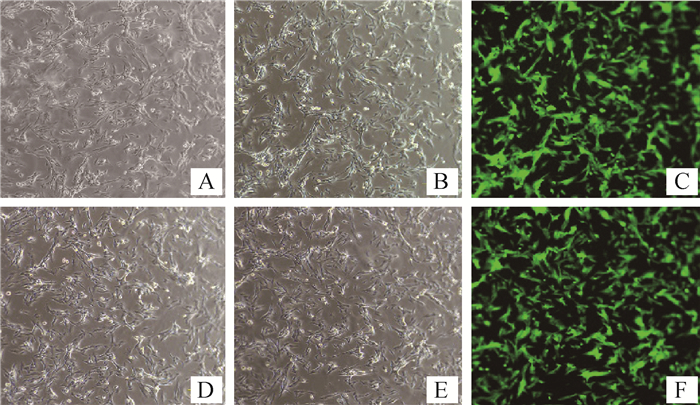
|
图A为白光条件下未感染的CHMm细胞;图B为白光条件下CHMm-GFP细胞;图C为荧光条件下CHMm-GFP细胞;图D为白光条件下未感染的CHMp细胞;图E为白光条件下CHMp-GFP细胞;图F为荧光条件下CHMp-GFP细胞。图A和D对应的荧光图片只有一片漆黑,观察不到任何东西,故没有给出。图中放大倍数为200× Fig. A is uninfected CHMm cells under white light; Fig. B is CHMm-GFP cells under white light; Fig. C is CHMm-GFP cells under fluorescence; Fig. D is uninfected CHMp cells under white light; Fig. E is CHMp-GFP cells under white light; Fig.F diagram shows CHMp-GFP cells under fluorescence condition. Fig. A and Fig. D are only completely dark under fluorescent conditions, and nothing is observed, so they are not given. The magnification is 200× 图 1 筛选后同一视野下细胞荧光与白光对照 Fig. 1 Comparison of cell fluorescence and white light in the same field after screening |
此外,利用流式细胞仪对已构建细胞系中表达了GFP受体的细胞进行定量检测,结果如图 2所示。表明犬乳腺肿瘤细胞系能表达GFP的细胞数比例在99%左右。将该细胞系连续传到十几代,通过荧光显微镜观察,比例没有下降,绿色荧光仍明显。
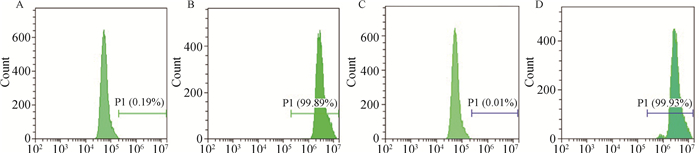
|
图A为亲本细胞CHMm;图B为CHMm-GFP;图C为亲本细胞CHMp;图D为CHMp-GFP Fig. A is the parent cell CHMm; Fig. B is CHMm-GFP; Fig. C is the parent cell CHMp; Fig. D is CHMp-GFP 图 2 流式细胞仪检测构建的细胞系中表达GFP受体的转染效率 Fig. 2 Flow cytometry to detect transfection efficiency of GFP receptors in constructed cell lines |
犬乳腺肿瘤细胞CHMm-GFP裸鼠成瘤模型活体成像检测,试验结果如图 3所示。明显观察到在试验前期活体成像检测有不同程度的杂质干扰,试验后期干扰消失但裸鼠接种部位荧光信号强度减弱,且出现荧光信号的范围非常小。
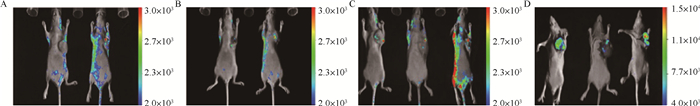
|
图A、B为接种CHMm-GFP细胞第15天进行活体成像检测时4只裸鼠GFP表达状态;图C为接种CHMm-GFP细胞第25天进行活体成像检测时3只裸鼠GFP表达状态;图D为接种CHMm-GFP细胞第35天进行活体成像检测时3只裸鼠的GFP表达状态 Fig. A and Fig. B are the GFP expression status of 4 nude mice when inoculated with CHMm-GFP cells 15 days biopsy; Fig. C is the GFP expression of 3 nude mice when inoculated with CHMm-GFP cells 25 days biopsy state; Fig. D shows the GFP expression status of 3 nude mice when in vivo imaging was performed 35 days inoculation of CHMm-GFP cells 图 3 犬乳腺肿瘤细胞CHMm-GFP裸鼠活体荧光检测 Fig. 3 Fluorescence detection of canine mammary tumor cells CHMm-GFP nude mice |
犬乳腺肿瘤细胞CHMp-GFP裸鼠成瘤模型活体成像检测,试验结果如图 4所示。可以明显观察到随着时间的增长,肿瘤接种部位的荧光面积逐渐变大且信号强度越来越强。
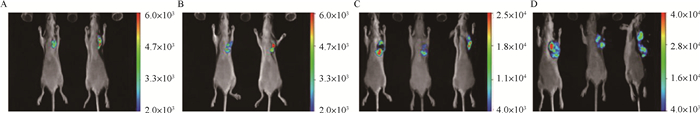
|
图A、B为接种CHMp-GFP细胞第15天进行活体成像检测时4只裸鼠的GFP表达状态;图C为接种CHMp-GFP细胞第25天进行活体成像检测时3只裸鼠的GFP表达状态;图D为接种CHMp-GFP细胞第35天进行活体成像检测时3只裸鼠的GFP表达状态 Fig. A and Fig. B are the GFP expression status of 4 nude mice when in vivo imaging was performed 15 days inoculation of CHMp-GFP cells; Fig. C shows the GFP expression of 3 nude mice after inoculation of CHMp-GFP cells 25 days inoculation state; Fig. D shows the GFP expression status of 3 nude mice when in vivo imaging was performed 35 days inoculation of CHMp-GFP cells 图 4 犬乳腺肿瘤细胞CHMp-GFP裸鼠活体荧光检测 Fig. 4 Fluorescence detection of canine mammary tumor cells CHMp-GFP nude mice |
犬乳腺肿瘤细胞CHMm-GFP裸鼠成瘤模型离体脏器成像检测,试验结果如图 5所示。可以观察到肿块离体后荧光强度明显强于在裸鼠体内;在接种的第25天发现有1只裸鼠的淋巴结出现荧光信号;在接种的第35天3只裸鼠的淋巴结均出现荧光信号。且荧光强度在接种的第35天较第25天大幅减弱。
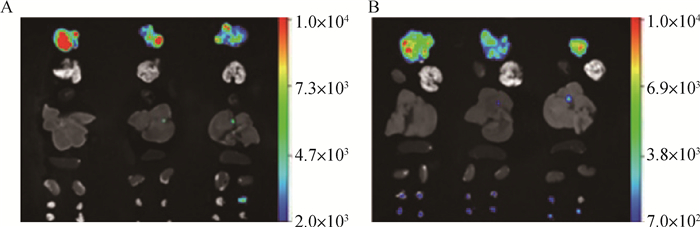
|
图A为接种犬乳腺肿瘤CHMm-GFP细胞第25天进行活体成像检测时3只裸鼠离体脏器GFP的表达状态;图B为接种犬乳腺肿瘤CHMm-GFP细胞第35天进行活体成像检测时3只裸鼠离体脏器GFP的表达状态,图中从上往下分别为肿块、心、肺、肝、脾、肾、淋巴结 Fig. A shows the expression of GFP in isolated organs of 3 nude mice in vivo imaging of CHMm-GFP cells inoculated with canine mammary tumors 25 days; Fig. B shows the in vivo imaging of 35 days inoculation of canine breast tumor CHMm-GFP cells. At the same time, the expression status of GFP in three organs of isolated nude mice was tumor, heart, lung, liver, spleen, kidney and lymph nodes from top to bottom 图 5 犬乳腺肿瘤细胞CHMm-GFP裸鼠离体器官荧光检测 Fig. 5 Fluorescence detection of isolated organs of CHMm-GFP nude mice in canine mammary tumor cells |
犬乳腺肿瘤细胞CHMp-GFP裸鼠成瘤模型离体脏器成像检测,结果如图 6所示。在接种的第25天时,发现有1只裸鼠的淋巴结出现荧光信号;接种的第35天,3只裸鼠的淋巴结均出现荧光信号,且荧光强度在接种的第35天较第25天明显增强。
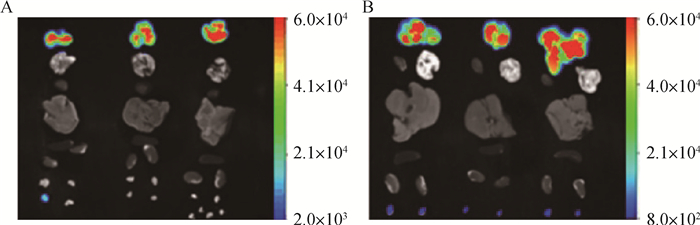
|
图A为接种犬乳腺肿瘤CHMp-GFP细胞第25天进行活体成像检测时3只裸鼠离体脏器GFP的表达状态;图B为接种犬乳腺肿瘤CHMp-GFP细胞第35天进行活体成像检测时3只裸鼠离体脏器GFP的表达状态,图中从上往下分别为肿块、心、肺、肝、脾、肾、淋巴结 Fig. A shows the expression of GFP in isolated organs of 3 nude mice in vivo imaging of CHMp-GFP cells inoculated with canine mammary tumors 25 days; Fig.B shows the in vivo imaging of 35 days inoculation of canine breast tumor CHMp-GFP cells. At the same time, the expression status of GFP in three organs of isolated nude mice was tumor, heart, lung, liver, spleen, kidney and lymph nodes from top to bottom 图 6 犬乳腺肿瘤细胞CHMp-GFP裸鼠离体器官荧光检测 Fig. 6 Fluorescence detection of isolated organs of CHMp-GFP nude mice in canine mammary tumor cells |
犬乳腺肿瘤细胞CHMm-GFP裸鼠成瘤模型病理组织学检测,结果如图 7所示。四组裸鼠的乳腺肿块病理切片均可见乳腺导管,导管中被覆多层细胞,乳腺间质中可见坏死的细胞碎片与乳腺间质组织,可见肿瘤细胞,肿瘤细胞核质比高,细胞大,核仁明显,细胞质淡,细胞核染色淡,可见淋巴细胞、中性粒细胞与单核细胞等炎性细胞。四组裸鼠的淋巴结病理切片,a组中仅有一只裸鼠可见肿瘤转移,其余三组全部裸鼠淋巴结病理切片均可见肿瘤转移。此外,a、b、c、d四组裸鼠的其他器官(心、肝、脾、肺、肾)病理切片出现了不同程度的炎症反应,但均没有观察到肿瘤细胞,这说明肿瘤没有转移到上述器官。
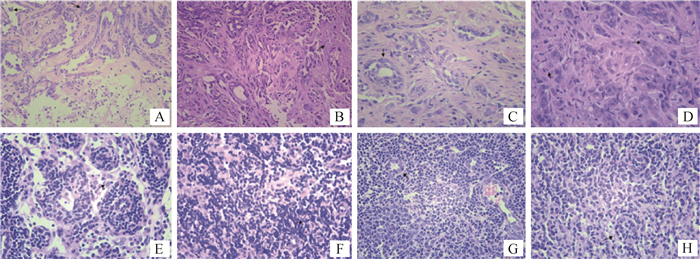
|
图A、B、C、D均为肿瘤HE染色石蜡切片,其中A图为a组10号裸鼠,B图为b组6号裸鼠,C图为c组5号裸鼠,图D为d组9号裸鼠;图E、F、G和H均为淋巴结HE染色石蜡切片,其中图E为a组10号裸鼠,图F为b组12号裸鼠,图G为c组11号裸鼠,图H为d组2号裸鼠;图A和B放大倍数为200×;图C、D、E、F、G和H放大倍数为400× Fig. A, B, C, D are paraffin sections of tumor stained by HE, in which Fig. A shows No.10 nude mouse of group a, Fig. B shows No. 6 nude mouse of group b, Fig. C for the No. 5 nude mouse of group c, Fig. D for the No. 9 nude mouse of group d; E, F, G and H are paraffin sections of lymphatic node stained by HE, in which Fig. E shows No. 10 nude mouse of group a, Fig. F shows No. 12 nude mouse of group b, Fig. G shows the No. 11 nude mouse of group c, Fig. H shows the No. 2 nude mouse of group d; The magnification of Fig. A and B is 200 ×; The magnification of Fig. C, D, E, F, G and H is 400 × 图 7 犬乳腺肿瘤细胞CHMm-GFP裸鼠乳腺肿块与淋巴结病理切片 Fig. 7 Canine mammary tumor CHMm-GFP nude mice mammary gland and lymph node pathological section |
犬乳腺肿瘤细胞CHMp-GFP裸鼠成瘤模型病理组织学检测,结果如图 8所示。四组裸鼠的乳腺肿块病理切片乳腺导管结构基本消失,导管被肿瘤细胞填满,乳腺间质中可见坏死的细胞碎片与乳腺间质组织,肿瘤细胞核质比高,细胞大,核仁明显,细胞质淡,细胞核染色淡,细胞分化程度低,可见大量的成纤维细胞分布,可见淋巴细胞、中性粒细胞与单核细胞等炎性细胞,且随着时间的增长,细胞分化度低,异型性明显。四组裸鼠的淋巴结病理切片,a组中仅有一只裸鼠可见肿瘤转移,其余三组全部裸鼠淋巴结病理切片均可见肿瘤细胞。此外,a、b、c、d四组裸鼠的其他器官(心、肝、脾、肺、肾)病理切片出现了不同程度的炎症反应,但均没有观察到肿瘤细胞,这说明肿瘤没有转移到上述器官。
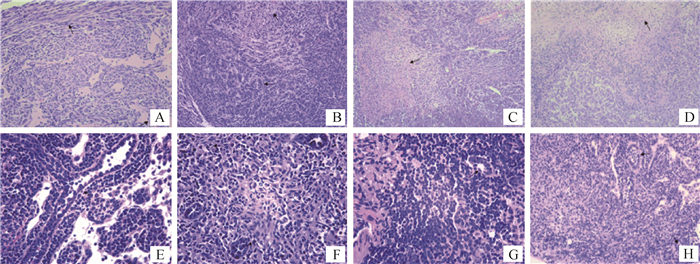
|
图A、B、C、D均为肿瘤HE染色石蜡切片,其中图A为a组8号裸鼠,图B为b组1号裸鼠,图C为c组10号裸鼠,图D为d组12号裸鼠;图E、F、G和H均为淋巴结HE染色石蜡切片,其中图E为a组4号裸鼠,图F为b组9号裸鼠,图G为c组5号裸鼠,图H为d组7号裸鼠;图B、C和D放大倍数为100×;图A、H放大倍数为200×;图E、F和G放大倍数为400× Fig. A, B, C, D are paraffin sections of tumor stained by HE, in which Fig. A shows No.8 nude mouse of group a, Fig. B shows No. 1 nude mouse of group b, Fig. C for the No. 10 nude mouse of group c, Fig. D for the No. 12 nude mouse of group d; E, F, G and H are paraffin sections of lymphatic node stained by HE, in which Fig. E shows No. 4 nude mouse of group a, Fig. F shows No. 9 nude mouse of group b, Fig. G shows the No. 5 nude mouse of group c, Fig. H shows the No. 7 nude mouse of group d; The magnification of Fig. B, C and D is 100 ×; The magnification of Fig. A and H is 200 ×; The magnification of Fig. E, F and G is 400 × 图 8 犬乳腺肿瘤细胞CHMp-GFP裸鼠乳腺肿块与淋巴结病理切片 Fig. 8 Canine mammary tumor CHMp-GFP nude mice mammary gland and lymph node pathological section |
两组成瘤模型在接种肿瘤细胞后,每天观察裸鼠状态并纪录裸鼠肿瘤体积大小和裸鼠体重,取每组裸鼠肿瘤大小,裸鼠体重的平均值来绘制裸鼠的肿瘤生长曲线,结果如图 9。
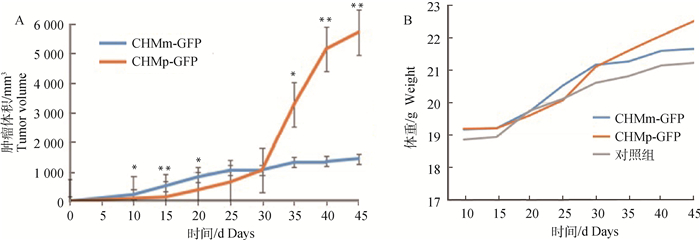
|
图A为裸鼠肿瘤体积的生长曲线,图B为注射犬肿瘤细胞系与对照组裸鼠体重的生长曲线。*.0.01<P<0.05;**. P<0.01 Fig. A is a growth curve of tumor volume in nude mice, and Fig. B is a growth curve of body weight of a canine tumor cell line and a control group. *. 0.01 < P < 0.05; **. P < 0.01 图 9 裸鼠体内肿瘤与裸鼠体重生长曲线 Fig. 9 Tumor growth and nude mouse body weight growth curve in nude mice |
肿瘤动物模型的建立能够使人类进一步了解肿瘤的发生机制及发生、发展等,人类利用实验动物建立肿瘤模型已有多年历史[12],常见的实验动物有裸鼠[13]和重症联合免疫缺陷(SCID)小鼠等[14],如Sonzogni-desautels等[15]利用CMT-9细胞系建立裸鼠肿瘤模型,是本篇文章的重要理论依据。绿色荧光蛋白(GFP)标记的犬乳腺肿瘤细胞是指将GFP整合到犬乳腺肿瘤细胞的染色体上,主要方法有阳离子脂质体法和病毒介导法两种[16]。本试验中笔者选择病毒介导法将GFP导入犬乳腺肿瘤细胞,通过有限稀释法挑取细胞单克隆筛选细胞,从而得到GFP标记的犬乳腺肿瘤细胞CHMm-GFP和CHMp-GFP,主要原因为本实验室病毒介导法操作方法成熟、操作便捷,实验成功率高。试验后将GFP标记的CHMm-GFP和CHMp-GFP细胞放在倒置荧光显微镜下观察,均能看到发出绿色荧光的细胞,说明GFP已成功导入细胞内,细胞经多次传代后荧光信号表达稳定,细胞形态和生长速度与未标记细胞无明显差异。但CHMm-GFP细胞发出荧光的强度低于CHMp-GFP,且GFP的表达率也低于CHMp-GFP,这可能与试验时细胞的状态或细胞本身特性有关,也可能与试验时因实验条件有限,细胞筛选时未加入抗性筛选试剂进行筛选有关[17]。活体成像试验结果显示,裸鼠接种肿瘤细胞部位均能发出绿色荧光,表明了本试验导入GFP至犬乳腺肿瘤细胞成功。病理切片试验结果证明活体成像检测中荧光团块即为肿瘤,说明可以利用活体成像技术观察肿瘤生长动态过程[18]。
本试验通过病理学检测与活体成像检测双重方法验证了裸鼠右侧肿物即为肿瘤,成功建立了犬乳腺肿瘤细胞CHMm-GFP裸鼠成瘤模型与犬乳腺肿瘤细胞CHMp-GFP裸鼠成瘤模型,试验过程中裸鼠精神状态正常,但CHMm-GFP裸鼠成瘤模型中小鼠体右侧出现强荧光,可能与体外原因有关,如裸鼠基因敲除不完全生出毛发,或裸鼠身体沾有垫料可能会产生与GFP类似的波长。而且肿瘤出现了破溃及血痂等问题,可能是裸鼠肿瘤周围存在血管分布,血管由于各种原因破裂后产生分泌物和瘀血[19],使得肉眼观察裸鼠时出现黑色物质,使右侧出现强荧光。
本试验在进行活体成像时未观察到裸鼠淋巴结出现荧光,但是在进行离体脏器成像检测与病理组织学检查中均发现肿瘤已转移至淋巴结,这可能与裸鼠淋巴结解剖位置与肿瘤相近,肿瘤荧光信号遮挡淋巴结荧光信号或GFP信号弱受到干扰有关[20],也可能与试验时裸鼠肿瘤部位发生破溃、瘀血从而影响了荧光信号的接收有关。试验过程中发现CHMp-GFP成瘤模型与CHMm-GFP成瘤模型中肿瘤均能转移至淋巴结,这表明虽然CHMp-GFP成瘤模型接种的是原发病灶分离建立的肿瘤细胞,但由于肿瘤细胞有转移的特性,使该细胞仍具有转移侵袭能力[21]。试验时两组成瘤模型均在肿瘤细胞接种第25天发现1只裸鼠出现淋巴结转移,第35天3只裸鼠出现淋巴结转移,这可能与实验动物数量少、试验间隔时间较长有关。此外,CHMm-GFP裸鼠成瘤模型在进行活体成像检测时荧光信号较弱且易受干扰,但是否真正与CHMm-GFP细胞状态或者肿瘤发生腐坏有关,仍需进一步试验加以证明。
4 结论通过活体成像和病理学检测两种方法观察,发现分别接种GFP标记犬乳腺肿瘤细胞系CHMp、CHMm的两组裸鼠成瘤模型随着肿瘤细胞接种时间的增长,肿瘤能转移至裸鼠淋巴结内,成功建立裸鼠成瘤模型,为今后深入研究犬乳腺肿瘤提供了理想的动物模型。
| [1] |
孔蓓. HE染色和免疫组化染色在犬乳腺肿瘤诊断中的应用[D].杨凌: 西北农林科技大学, 2015.
KONG B. The application of HE staining and immunohistochemical staining in the diagnosis of canine mammary tumors[D]. Yangling: Northwest A & F University, 2015. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10712-1015335046.htm |
| [2] | RUGGERI B A, CAMP F, MIKNYOCZKI S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery[J]. Biochem Pharmacol, 2014, 87(1): 150–161. DOI: 10.1016/j.bcp.2013.06.020 |
| [3] | LIU D L, XIONG H, ELLIS A E, et al. Molecular homology and difference between spontaneous canine mammary cancer and human breast cancer[J]. Cancer Res, 2014, 74(18): 5045–5056. DOI: 10.1158/0008-5472.CAN-14-0392 |
| [4] | QUEIROGA F L, RAPOSO T, CARVALHO M I, et al. Canine mammary tumours as a model to study human breast cancer: most recent findings[J]. In Vivo, 2011, 25(3): 455–465. |
| [5] | OZMEN O, KUL S, RISVANLI A, et al. Somatic SNPs of the BRCA2 gene at the fragments encoding RAD51 binding sites of canine mammary tumors[J]. Vet Comp Oncol, 2017, 15(4): 1479–1486. DOI: 10.1111/vco.2017.15.issue-4 |
| [6] | RIVERA P, VON EULER H. Molecular biological aspects on canine and human mammary tumors[J]. Vet Pathol, 2011, 48(1): 132–146. DOI: 10.1177/0300985810387939 |
| [7] | QIN C H, ZHU S P, TIAN J. New optical molecular imaging systems[J]. Curr Pharm Biotechnol, 2010, 11(6): 620–627. DOI: 10.2174/138920110792246519 |
| [8] | PYSZ M A, GAMBHIR S S, WILLMANN J K. Molecular imaging: current status and emerging strategies[J]. Clin Radiol, 2010, 65(7): 500–516. DOI: 10.1016/j.crad.2010.03.011 |
| [9] | XIN J, ZHAN Y H, LIU M H, et al. ApoG2 induces ER stress-dependent apoptosis in gastric cancer cells in vitro and its real-time evaluation by bioluminescence imaging in vivo[J]. Cancer Lett, 2013, 336(2): 260–269. DOI: 10.1016/j.canlet.2013.03.019 |
| [10] | THURBER G M, YANG K S, REINER T, et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo[J]. Nat Commun, 2013, 4: 1504. DOI: 10.1038/ncomms2506 |
| [11] | NISHIMURA R, OKAMOTO N, SATOU M, et al. HER 2 immunohistochemistry for breast cancer cell blocks can be used in the same way as that used for histological specimens[J]. Diagn Cytopathol, 2016, 44(4): 274–279. DOI: 10.1002/dc.v44.4 |
| [12] | RASHID O M, TAKABE K. Animal models for exploring the pharmacokinetics of breast cancer therapies[J]. Expert Opin Drug Metab Toxicol, 2015, 11(2): 221–230. DOI: 10.1517/17425255.2015.983073 |
| [13] |
殷小涛, 李方龙, 金一鹏, 等. 稳定表达hSPRY/Luc的人膀胱癌细胞株裸鼠皮下移植瘤模型的建立[J]. 细胞与分子免疫学杂志, 2017, 33(3): 337–341.
YIN X T, LI F L, JIN Y P, et al. Establishment of a human bladder cancer cell line stably co-expressing hSPRY2 and luciferase genes and its subcutaneous tumor xenograft model in nude mice[J]. Chinese Journal of Cellular and Molecular Immunology, 2017, 33(3): 337–341. (in Chinese) |
| [14] | SAMUEL R. Spontaneous development of neoplasms in severe combined immunodeficient mice[J]. SAGE Open Med Case Rep, 2015, 3: 2050313X14568698. |
| [15] | SONZOGNI-DESAUTELS K, KNAPP D W, SARTIN E, et al. Effect of cyclooxygenase inhibitors in a xenograft model of canine mammary tumours[J]. Vet Comp Oncol, 2011, 9(3): 161–171. DOI: 10.1111/vco.2011.9.issue-3 |
| [16] | QI B X, CHEN Y J, SU R, et al. Establishment of insect cell lines expressing green fluorescent protein on cell surface based on AcMNPV GP64 membrane fusion characteristic[J]. Cytotechnology, 2017, 69(5): 775–783. DOI: 10.1007/s10616-017-0086-3 |
| [17] | HUANG C, LAN W J, WANG F F, et al. Establishment of a human breast cancer model by fusion PCR for in vivo and in vitro fluorescence imaging of human breast cancer[J]. DNA Cell Biol, 2017, 36(1): 50–57. DOI: 10.1089/dna.2016.3531 |
| [18] | BURD C E, SORRENTINO J A, CLARK K S, et al. Monitoring tumorigenesis and senescence in vivo with a p16INK4a-luciferase model[J]. Cell, 2013, 152(1-2): 340–351. DOI: 10.1016/j.cell.2012.12.010 |
| [19] | TSENG C E, CHEN C H, CHEN S J, et al. Tumor rupture as an initial manifestation of malignant mesonephric mixed tumor:a case report and review of the literature[J]. Int J Clin Exp Pathol, 2014, 7(3): 1212–1217. |
| [20] | CACERES G, ZHU X Y, JIAO J A, et al. Imaging of luciferase and GFP-transfected human tumours in nude mice[J]. Luminescence, 2003, 18(4): 218–223. DOI: 10.1002/(ISSN)1522-7243 |
| [21] | OLIVIER T, GASTAUD L, MASCHI C, et al. Metastasis to unusual sites[J]. Bull Cancer, 2014, 101(2): 203–210. |



