鹅脂肪肝(俗称鹅肥肝)与哺乳类动物的非酒精性脂肪肝(NAFLD)虽然存在不少共同特点,但近年来的研究表明鹅肥肝的形成也有其独特性。与正常肝相比,鹅肥肝中的内质网应激(ERS)水平下降[1]、促炎症因子肿瘤坏死因子α(TNFα)的表达水平受到抑制[2-3],而脂联素受体表达水平[2]、线粒体相关基因表达水平显著增加等[4],这与人和啮齿类动物NAFLD中出现的情形相反[5]。这些差异提示,鹅在进化过程中可能形成了一套保护机制,使鹅肥肝成为一种生理性而非病理性脂肪肝。但目前鹅肥肝形成中的保护机制尚不完全明确,对其进行研究一方面有助于揭示鹅肥肝的形成机理,另一方面也可为其他畜禽和人脂肪肝的研究提供参考。
已有研究表明,ERS在NAFLD形成中扮演重要角色,一方面可以通过诱导TRIB3抑制胰岛素信号通路关键分子AKT的磷酸化,引起胰岛素抵抗[5];另一方面,ERS还参与蛋白质合成[6]和细胞凋亡的调控[7-8]。ERS会激活3个信号通路(PERK/eIF2α通路、IRE1/XBP1通路以及ATF6介导的通路)[9-10],这些信号通路可以通过一些途径与免疫/炎症信号途径(如NFκB/IKK、JNK/AP1信号途径)[11-12]和氧化应激信号途径[13-14]相连接,因此ERS可能参与免疫/炎症和氧化应激信号的调控。最近的研究表明,相对于正常肝,鹅肥肝中许多免疫/炎症相关基因的表达发生了明显的变化,比如促炎症因子Tnfα的表达在鹅肥肝中受到显著抑制[3]。与此相一致的是,鹅肥肝中ERS标记分子葡萄糖调节蛋白78 (Grp78)和Xbp1的表达也受到抑制[1]。基于这些发现以及ERS与免疫/炎症信号通路间的潜在关联,推测,ERS可能参与鹅肥肝形成中免疫/炎症相关基因的表达调控。
Grp78(或免疫球蛋白重链结合蛋白,BIP)属于应激蛋白家族。Grp78与蛋白质合成、Ca2+平衡等多种生物学功能相关。在某些情况下,细胞会发生ERS,启动未折叠蛋白反应(UPR),暂停翻译过程;同时,Grp78及有关蛋白折叠酶表达会增加,提高内质网对蛋白质的折叠能力,减轻内质网的负荷[15-16]。Grp78还参与调节内质网的钙稳定,内质网是Ca2+的重要储存场所,当机体处于静息状态的时候,内质网内储存的Ca2+量约有25%是由Grp78储存的[17]。另外,Grp78与多种疾病相关,比如:在代谢综合征、酒精性肝病和CCL4诱发的肝硬化等多种疾病发生时[18],Grp78的表达会上调。Grp78与胚胎发育的关系也有报道[19],这可能与胚胎时期容易受外界环境影响而发生应激反应有关,Grp78可以为应激状态下的胚胎提供某种自我保护。当肝因环境因素的刺激而发生应激反应时,Grp78在肝保护上发挥着重要所用。有研究表明,肝发生热应激时,Grp78的表达增强,细胞产生适应性,使细胞避免凋亡,这说明Grp78对肝具有保护作用。还有报道称,鸡的耐热能力与Grp78存在关联, 提示Grp78或可作为鸡耐热能力选择的一个候选基因[20]。
本研究拟对鹅肥肝形成过程中不同组织的Grp78表达变化规律及其所影响的信号通路和基因进行测定分析。本研究将在阐述Grp78基因功能及其与免疫/炎症信号通路关联的同时,揭示ERS在鹅肥肝形成中的生物学作用,促进鹅肥肝形成机制的阐明。
1 材料与方法 1.1 试验动物试验所用朗德鹅由淮安笠诚畜禽养殖有限公司提供,饲养以及填饲试验也在该公司进行。试验选择健康的、体重相近的70日龄朗德鹅30只(未分公母),随机分为对照组(15只)和填饲组(15只)。对照组朗德鹅自由采食,用煮熟的玉米作为其饲料;填饲组从70日龄开始进行人工填饲,填饲饲料用玉米、1%猪油和1%食盐混合组成。分别在77、84和89日龄时,从两组中分别选取4只进行屠宰取样,采集胸肌、肝和腹脂的组织样,保存在-80 ℃冰箱中待用。
1.2 组织和细胞中RNA的提纯及cDNA合成根据TRIzol试剂盒(TaKaRa Biotechnology Co., Ltd)的使用说明对样品中的总RNA进行分离和提纯。利用HiScriptTM Q RTSuperMix反转录试剂盒(诺威赞生物有限公司)将提纯的RNA样品反转录为cDNA。
1.3 目标基因相对表达量的荧光定量PCR分析根据NCBI上公布的鹅的基因序列,使用在线Primer 3.0软件设计了Grp78、Tnfsf10、Map3k7cl、Ccl20目标基因和内参GAPDH的荧光定量PCR引物(表 1)。引物的序列特异性依据Primer-Blast和熔解曲线的检测结果来确定。按照生产商的使用说明,利用Vazyme AceQ qPCR SYBR Green Master Mix试剂盒(诺威赞生物有限公司)和上述合成的cDNA样品进行荧光定量PCR分析,测定组织或细胞中各目标基因和内参基因(GAPDH)的表达量。采用2-△△CT方法计算目的基因的相对表达量。
|
|
表 1 荧光定量PCR引物的序列 Table 1 The sequences of primers for quantitative PCR |
利用孵化第22~23天正常发育的朗德鹅胚蛋进行鹅原代肝细胞的分离与培养。在标准培养基中培养过夜后,用胰岛素、棕榈酸、油酸和亚油酸处理细胞14 h。具体方法见参考文献[21]。
1.5 鹅Grp78基因的过表达将鹅Grp78基因的编码区克隆进含CMV驱动子的pcDNA3.1载体中,构建Grp78基因的过表达载体。用Lipofectamine 2000(Invitrogen)把Grp78基因的过表达载体和空载体转染到培养24 h的鹅原代肝细胞中。经32 h的转染,收集细胞用于Grp78基因和免疫相关因子mRNA表达量的检测。具体的转染方法见转染试剂盒使用说明书和参考文献[5]。
1.6 鹅原代肝细胞的转录组测序与分析利用从Grp78基因过表达载体和空载体转染的鹅原代肝细胞中分离纯化的RNA,建立cDNA文库并进行RNA-seq分析,筛选出差异表达基因和富集的信号通路。简单地说,RNA在提取纯化后进行质量检验。对于符合质量要求的RNA样品(n=3)进行接头连接,并反转录为cDNA,经PCR扩增富集和纯化后建立cDNA文库。经上机测序(在BGISEQ-500平台上进行),去除低质量序列(质量值低于15的碱基占该序列总碱基数的比例大于20%的读数),获得干净数据后,使用HISAT将干净数据比对到参考基因组序列。指定差异基因的标准为最大的RPKM值>2,处理组数值与对照组之比>2或 < 0.5,且两组平均值间差异达到显著水平(P < 0.05)。具体的转录组测序与分析方法见参考文献[3]和[21]。
1.7 统计学处理SPSS17.0分析软件被用于数据的统计分析。所有数据均以“平均值±标准误”表示。两组平均值间的差异显著性检验采用样本大小相同、总体方差不同的t检验法。P < 0.05和P < 0.01分别设为组间存在统计意义上的显著和极显著差异。
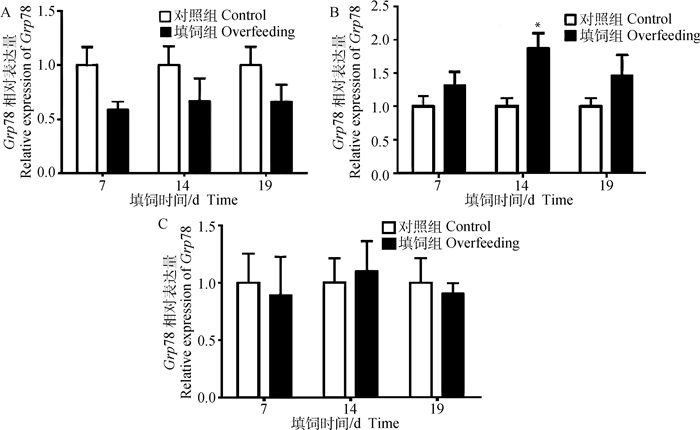
2 结果 2.1 填饲对朗德鹅肝、脂肪和肌肉组织中Grp78基因表达的影响与对照组正常肝相比,Grp78基因在填饲第7、14和19天的鹅肥肝中,其mRNA表达水平均呈现降低趋势,而且降低程度并未随着填饲时间的延长而发生明显的变化(图 1A,P>0.05)。与肝的情形相反,填饲鹅腹脂中Grp78基因的mRNA表达水平则高于对照鹅腹脂中的表达水平,而在填饲第14天时甚至达到了统计显著水平(图 1B,P < 0.05)。虽然Grp78在肝和脂肪组织中的表达均受到填饲的影响,但是在肌肉组织中的表达几乎不受填饲所影响(图 1C,P>0.05)。以上结果表明,填饲可能通过调控肝和脂肪组织中Grp78的表达来影响鹅肥肝的形成。
|
用荧光定量PCR测定Grp78在常规饲养鹅(对照组)和填饲7、14和19天鹅肝(A)、腹脂(B)和胸肌(C)中的表达量,内参基因为GAPDH。以同期常规饲养鹅为对照,n=4。*表示差异达到统计显著水平,即P < 0.05。所有表达值用“平均值±标准误”表示,下同 The expression of Grp78 in the liver (A), abdominal fat (B) and pectoral muscle (C) of the geese normally fed (the control group) and the geese overfed for 7, 14 and 19 d were determined by real-time PCR. The reference gene was GAPDH. The geese conventionally raised in the same period were used as control, n = 4. * indicates that the difference was significant at P < 0.05 level. All the data were expressed as the "mean±standard error", the same as below 图 1 不同填饲阶段Grp78在朗德鹅肝、腹脂和胸肌中的mRNA表达 Fig. 1 Expression of Grp78 in the liver, abdominal fat and pectoral muscle of Landaise geese overfed for different time |
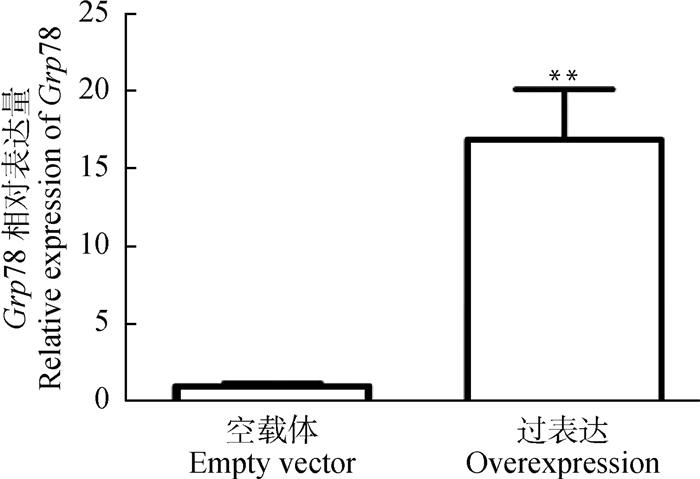
为了进一步明晰ERS/ Grp78在鹅肥肝形成进程中的作用,本研究在鹅原代肝细胞中过表达Grp78基因。通过对转染Grp78基因过表达载体和空载体(对照组)32 h后的原代肝细胞进行定量PCR分析,数据显示,Grp78过表达载体转染的细胞中Grp78基因的mRNA水平极显著地高于对照组(P < 0.01,图 2)。
|
用荧光定量PCR测定Grp78在Grp78过表达或空载体(对照)转染的鹅原代肝细胞中的mRNA表达量,内参基因为GAPDH。n=3。**表示差异达到统计极显著水平,即P < 0.01 The mRNA expression of Grp78 in goose primary hepatocytes transfected with Grp78 overexpression vectors or empty vectors (the control) were determined by real-time PCR, and the reference gene was GAPDH. n=3. ** indicates that the difference was extremely significant at P < 0.01 level 图 2 鹅原代肝细胞中Grp78过表达的检测 Fig. 2 Detection of Grp78 overexpression in goose primary hepatocytes |
对Grp78过表达或空载体转染的鹅原代肝细胞进行转录组分析,一共筛选到61个差异表达基因(DEG),包括46个上调基因和15个下调基因(表 2)。
|
|
表 2 在Grp78过表达或空载体转染的鹅原代肝细胞中筛选到的差异表达基因 Table 2 Differentially expressed genes identified in the goose primary hepatocytes transfected with Grp78 overexpression vs. empty vectors |
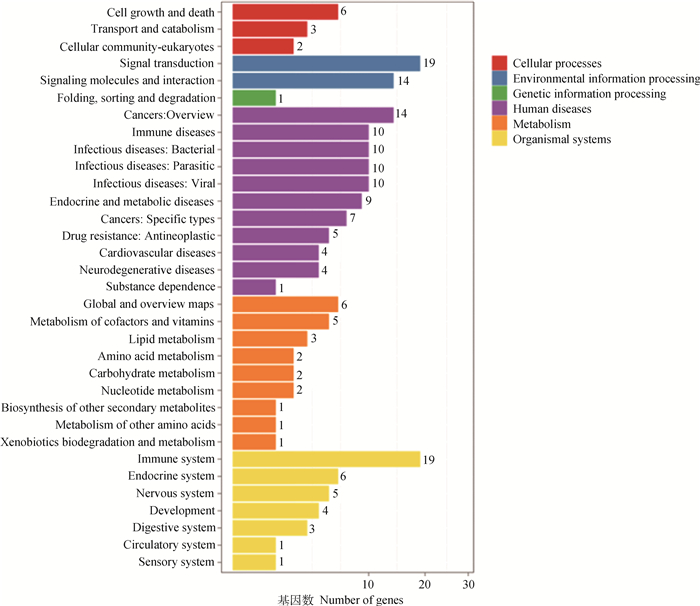
对差异表达基因进行KEGG Pathway分类以及富集分析,结果显示,差异表达基因主要归于两大类:一种是信号转导类(19个差异表达基因),另一种是免疫系统类(19个差异基因)(图 3)。此外也有一些差异表达基因归于信号分子与互作类、癌症类、免疫与传染疾病类、内分泌与代谢病类、细胞生长和凋亡类、内分泌系统类等。其中,代谢病和细胞凋亡是已知与ERS相关的类别。这里值得说明的是,差异表达基因中非编码RNA的功能是依据其上下游可能具有顺式作用的基因功能预测的。
|
图 3 差异表达基因的KEGG通路分类分析 Fig. 3 The KEGG pathway analysis on the differentially expressed genes |
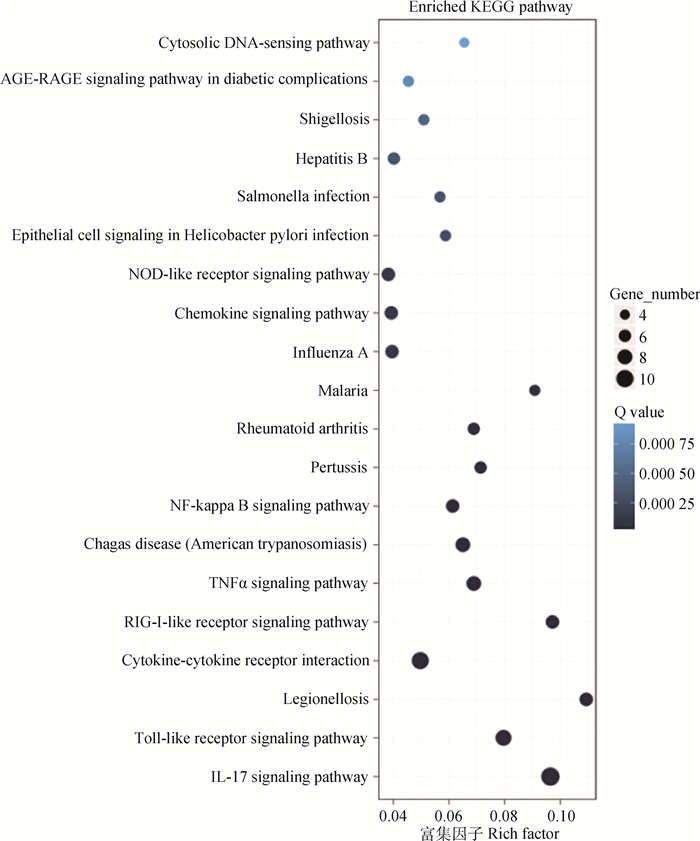
差异表达基因的KEGG通路富集分析表明,免疫/炎症相关通路是受Grp78过表达影响的主要富集通路(图 4)。在免疫/炎症相关通路中,炎症相关通路“IL-17 signaling pathway”、“Toll-like receptor signaling pathway”、“TNFα signaling pathway”、“NF-kappa B signaling pathway”最为突出(图 4)。表 3列出了与免疫/炎症有关的差异表达基因,其中包括在Grp78过表达时上调的16个和下调的3个差异表达基因。
|
图 4 差异表达基因富集的KEGG信号通路 Fig. 4 Enriched signaling pathways from KEGG pathway analysis with the differentially expressed genes |
|
|
表 3 与免疫/炎症有关的差异表达基因 Table 3 Differentially expressed genes related to immunity/inflammation |
在与免疫/炎症有关的差异表达基因中,本研究对Tnfsf10、Map3k7cl和Ccl20基因的表达水平进行了验证。这3个基因在Grp78过表达载体和空载体转染(对照组)的原代肝细胞中的表达趋势与转录组分析结果一致(表 3,图 5A-5C)。即,相对于对照组,Tnfsf10基因在Grp78过表达载体转染的细胞中mRNA表达水平极显著地升高(P < 0.01,图 5A),不过,与之相反,Grp78过表达反而极显著地抑制了Map3k7cl、Ccl20基因的表达(P < 0.01,图 5B、5C)。
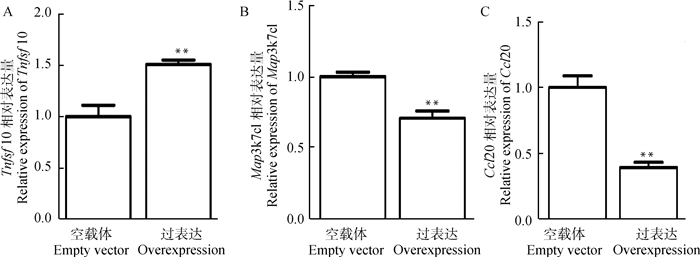
|
用荧光定量PCR测定Grp78过表达载体和空载体转染的鹅原代肝细胞中Tnfsf10、Map3k7cl和Ccl20基因mRNA表达量。内参基因为GAPDH。各基因mRNA相对表达量表示为对照组的比值或倍数,n=3。**, ***分别表示差异达到统计极显著水平,即P < 0.01,P < 0.001,下同 The mRNA expression of Tnfsf10, Map3k7cl and Ccl20 genes in goose primary hepatocytes transfected with Grp78 overexpression vectors and empty vectors were determined by real-time PCR. The reference gene was GAPDH. The relative mRNA expression of each gene was expressed as fold change over the control group, n=3. **, *** indicate the difference was statistically significant at P < 0.01, P < 0.001 levels, respectively, the same as below 图 5 鹅原代肝细胞中Grp78过表达对3个特定基因表达的影响 Fig. 5 Effect of Grp78 overexpression on the expression of 3 specified genes in goose primary hepatocytes |
为了明晰这3个基因的表达是否也与鹅肝中Grp78的表达水平存在关联,本研究利用定量PCR测定了填饲鹅和对照鹅肝在填饲第19天时各基因mRNA的表达水平。数据显示,伴随鹅肥肝中Grp78基因mRNA的表达抑制,Tnfsf10基因在鹅肥肝中的表达水平也受到极显著地抑制(P < 0.01,图 6A)。不过,Map3k7cl基因在鹅肥肝中的表达水平却极显著地增加(图 6B)。由此可见,Tnfsf10和Map3k7cl基因在鹅肝中的表达情形刚好与Grp78过表达载体转染细胞中的表达情形相反,提示,Grp78/ERS很可能参与鹅肥肝这2个基因的表达调控。对于鹅肝中Ccl20基因的表达,数据显示该基因在鹅肥肝中的表达与对照组相比极显著地降低(P < 0.01,图 6C),这与Grp78过表达显著抑制其表达不一致。
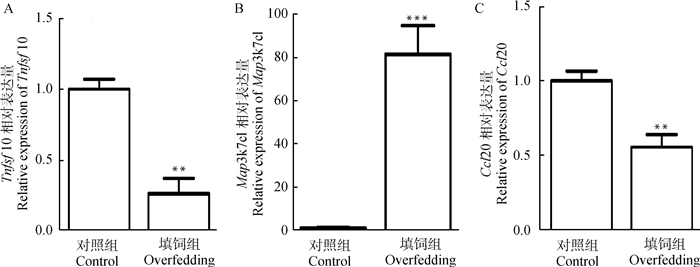
|
用荧光定量PCR测定填饲第19天时在填饲鹅(overfed)和常规饲喂鹅(control)肝中Tnfsf10、Map3k7cl和Ccl20基因的mRNA表达量。内参基因为GAPDH。各基因mRNA相对表达量表示为对照组的比值或倍数,n=4 The mRNA expression of Tnfsf10, Map3k7cl and Ccl20 genes in the livers of the overfed geese and the control geese on the 19th day of overfeeding were determined by real-time PCR. The reference gene is GAPDH. The relative mRNA expression of each gene was expressed as fold change over the control group, n=4 图 6 3个特定基因在填饲鹅和对照鹅肝中的表达量 Fig. 6 Expression of 3 specified genes in the livers of overfed and control geese |
NAFLD在人、家禽和家畜等动物中是一种比较常见的代谢性疾病[22]。对于人与啮齿类等哺乳动物NAFLD的大量研究表明,胰岛素抵抗在脂肪肝形成中起关键作用,ERS是胰岛素抵抗形成的机制之一[5, 23]。然而,最近发现,就鹅肥肝而言情形并非如此,即ERS水平在鹅肥肝中不升反降[24-25]。这意味着ERS可能在鹅肥肝形成中发挥某种独特作用。由于ERS可以通过一些信号途径与免疫/炎症的信号途径(如NFκB/IKK、JNK/AP1信号途径)[11-12]和氧化应激信号途径[13-14]相连接,因此,ERS可能参与免疫/炎症和氧化应激信号的调控。有趣的是,鹅肥肝中的许多炎症/免疫因子的表达也是不升反降,与哺乳动物NAFLD中的情形相反[26-28]。因此推测,ERS可能在鹅肥肝炎症/免疫相关基因的表达调控中发挥作用。本研究通过测定ERS的标记基因Grp78在填饲不同阶段不同组织(肝、脂肪和肌肉组织,这些组织与能量代谢密切相关)中的表达量,分析Grp78基因过表达所影响的信号通路和基因,以及检测脂肪肝形成相关因子(高葡萄糖、脂肪酸和胰岛素)对Grp78基因表达的影响,对该推测进行验证,并为进一步研究Grp78基因在鹅肥肝形成中的作用奠定基础。
数据显示,在整个填饲期,除了胸肌中Grp78基因的表达相对于对照组无显著差异外,肝和腹脂中的表达均受填饲影响(在肝中呈下降趋势,而腹脂中呈上升趋势)。这表明ERS可能通过直接影响肝功能参与鹅肥肝的形成,或通过影响脂肪组织的功能间接参与鹅肥肝的形成。3种组织中Grp78的表达模式不一样,各组织中的表观遗传学机制可能起到了决定性的作用,但这还有待今后加以验证。再者,通过对转染了Grp78基因过表达载体或空载体的鹅原代肝细胞转录组分析,本研究一共筛选到61个差异表达基因(46个基因在过表达后上调),其中有19个富集于免疫/炎症通路上,而且大多集中在“IL-17 signaling pathway”、“Toll-like receptor signaling pathway”、“TNFα signaling pathway”、“NF-kappa B signaling pathway”通路。这些结果表明,ERS的确影响鹅肝细胞中免疫/炎症相关基因的表达。此外,在ERS影响的差异表达基因中,还有一些归于信号分子与互作类、癌症类、传染疾病类、内分泌与代谢病类、细胞生长和凋亡类、内分泌系统类等,其中的代谢病和细胞凋亡是已知与ERS相关的类别[29-31]。这大大拓展了对ERS/Grp78生物学功能的认识。
通过对Tnfsf10、Map3k7cl和Ccl20这3个与免疫/炎症相关基因的验证及其在鹅肥肝中表达的测定比较,不仅部分验证了转录组分析的结果,而且获得了支持Grp78/ERS调控鹅肥肝中Tnfsf10和Map3k7cl基因表达的证据。至于Ccl20基因在Grp78基因过表达的鹅原代肝细胞表达抑制与Ccl20基因在Grp78基因低表达的鹅肥肝中表达抑制相冲突,其原因可能是活体中存在比单纯肝细胞中更复杂的调控因素。比如,在单纯肝细胞中主要是Grp78过表达引起的效应,但鹅肝中有其他激活了的转录因子调控Ccl20的表达。进一步研究Grp78影响Ccl20表达的机制以及其他调控Ccl20表达的机制将有助于阐明上述矛盾。
4 结论本研究揭示了Grp78/ERS新的生物学功能,即除了可影响已知的代谢病和细胞凋亡通路外,还影响信号转导、免疫相关通路,特别是“Toll-like receptor signaling pathway”、“TNFα signaling pathway”、“NF-kappa B signaling pathway”等通路。此外,本研究还发现,在鹅肥肝中Grp78的表达抑制与免疫相关基因Tnfsf10的表达抑制、Map3k7cl的表达增强相关联,而在鹅原代肝细胞中,Grp78过表达会引起Tnfsf10表达增加和Map3k7cl表达减少。这些发现表明,Grp78/ERS可对细胞的免疫/炎症状态进行调控,并借此参与鹅肥肝的形成。
| [1] | GENG T Y, XIA L L, LI F Y, et al. The role of endoplasmic reticulum stress and insulin resistance in the occurrence of goose fatty liver[J]. Biochem Biophys Res Commun, 2015, 465(1): 83–87. DOI: 10.1016/j.bbrc.2015.07.134 |
| [2] | GENG T Y, YANG B, LI F Y, et al. Identification of protective components that prevent the exacerbation of goose fatty liver:Characterization, expression and regulation of adiponectin receptors[J]. Comp Biochem Physiol B Biochem Mol Biol, 2016, 194-195: 32–38. DOI: 10.1016/j.cbpb.2016.01.006 |
| [3] | LIU L, ZHAO X, WANG Q, et al. Prosteatotic and protective components in a unique model of fatty liver:Gut microbiota and suppressed complement system[J]. Sci Rep, 2016, 6: 31763. DOI: 10.1038/srep31763 |
| [4] | OSMAN R H, SHAO D, LIU L, et al. Expression of mitochondria-related genes is elevated in overfeeding-induced goose fatty liver[J]. Comp Biochem Physiol B Biochem Mol Biol, 2016, 192: 30–37. DOI: 10.1016/j.cbpb.2015.11.006 |
| [5] | GENG T, HU W, BROADWATER M H, et al. Fatty acids differentially regulate insulin resistance through endoplasm reticulum stress-mediated induction of tribbles homologue 3:a potential link between dietary fat composition and the pathophysiological outcomes of obesity[J]. Diabetologia, 2013, 56(9): 2078–2087. DOI: 10.1007/s00125-013-2973-2 |
| [6] | LEE A S. The glucose-regulated proteins:stress induction and clinical applications[J]. Trends Biochem Sci, 2001, 26(8): 504–510. DOI: 10.1016/S0968-0004(01)01908-9 |
| [7] | TABAS I, RON D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress[J]. Nat Cell Biol, 2011, 13(3): 184–190. DOI: 10.1038/ncb0311-184 |
| [8] | RAO R V, ELLERBY H M, BREDESEN D E. Coupling endoplasmic reticulum stress to the cell death program[J]. Cell Death Differentiation, 2004, 11(4): 372–380. DOI: 10.1038/sj.cdd.4401378 |
| [9] | SIDRAUSKI C, WALTER P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response[J]. Cell, 1997, 90(6): 1031–1039. DOI: 10.1016/S0092-8674(00)80369-4 |
| [10] | RON D, WALTER P. Signal integration in the endoplasmic reticulum unfolded protein response[J]. Nat Rev Mol Cell Biol, 2007, 8(7): 519–529. DOI: 10.1038/nrm2199 |
| [11] | DENG J, LU P D, ZHANG Y H, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2[J]. Mol Cell Biol, 2004, 24(23): 10161–10168. DOI: 10.1128/MCB.24.23.10161-10168.2004 |
| [12] | HU P, HAN Z, COUVILLON A D, et al. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-αB activation and down-regulation of TRAF2 expression[J]. Mol Cell Biol, 2006, 26(8): 3071–3084. DOI: 10.1128/MCB.26.8.3071-3084.2006 |
| [13] | CULLINAN S B, DIEHL J A. Coordination of ER and oxidative stress signaling:the PERK/Nrf2 signaling pathway[J]. Int J Biochem Cell Biol, 2006, 38(3): 317–332. DOI: 10.1016/j.biocel.2005.09.018 |
| [14] | GOTOH T, MORI M. Nitric oxide and endoplasmic reticulum stress[J]. Arterioscler Thromb Vasc Biol, 2006, 26(7): 1439–1446. DOI: 10.1161/01.ATV.0000223900.67024.15 |
| [15] | JI C, KAPLOWITZ N, LAU M Y, et al. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice[J]. Hepatology, 2011, 54(1): 229–239. DOI: 10.1002/hep.v54.1 |
| [16] | TEODORO-MORRISON T, SCHUIKI I, ZHANG L, et al. GRP78 overproduction in pancreatic beta cells protects against high-fat-diet-induced diabetes in mice[J]. Diabetologia, 2013, 56(5): 1057–1067. DOI: 10.1007/s00125-013-2855-7 |
| [17] | PALLEPATI P, AVERILL-BATES D A. Mild thermotolerance induced at 40℃ protects HeLa cells against activation of death receptor-mediated apoptosis by hydrogen peroxide[J]. Free Radic Biol Med, 2011, 50(6): 667–679. DOI: 10.1016/j.freeradbiomed.2010.11.022 |
| [18] | SAGE A T, HOLTBY-OTTENHOF S, SHI Y Y, et al. Metabolic syndrome and acute hyperglycemia are associated with endoplasmic reticulum stress in human mononuclear cells[J]. Obesity, 2012, 20(4): 748–755. DOI: 10.1038/oby.2011.144 |
| [19] | LUO S Z, MAO C H, LEE B, et al. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development[J]. Mol Cell Biol, 2006, 26(15): 5688–5697. DOI: 10.1128/MCB.00779-06 |
| [20] | KONG L N, ZHANG D X, JI C L, et al. Association analysis between SNPs in the 5'-flanking region of the chicken GRP78 gene, thermotolerance parameters, and tissue mRNA expression[J]. Genet Mol Res, 2015, 14(2): 6110–6123. DOI: 10.4238/2015.June.8.9 |
| [21] | OSMAN R H, LIU L, XIA L L, et al. Fads1 and 2 are promoted to meet instant need for long-chain polyunsaturated fatty acids in goose fatty liver[J]. Mol Cell Biochem, 2016, 418(1-2): 103–117. DOI: 10.1007/s11010-016-2737-7 |
| [22] | EKSTEDT M, HAGSTRÖM H, NASR P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up[J]. Hepatology, 2015, 61(5): 1547–1554. DOI: 10.1002/hep.27368 |
| [23] | DU K Y, HERZIG S, KULKARNI R N, et al. TRB3:a tribbles homolog that inhibits Akt/PKB activation by insulin in liver[J]. Science, 2003, 300(5625): 1574–1577. DOI: 10.1126/science.1079817 |
| [24] | GENG T Y, XING Z, XIA L L, et al. Supplementing dietary sugar promotes endoplasmic reticulum stress-independent insulin resistance and fatty liver in goose[J]. Biochem Biophys Res Commun, 2016, 476(4): 665–669. DOI: 10.1016/j.bbrc.2016.05.149 |
| [25] | LAI S J, LI Y, KUANG Y, et al. PKCδ silencing alleviates saturated fatty acid induced ER stress by enhancing SERCA activity[J]. Biosci Rep, 2017, 37(6): BSR20170869. DOI: 10.1042/BSR20170869 |
| [26] | DING S T, LIU B H, KO Y H. Cloning and expression of porcine adiponectin and adiponectin receptor 1 and 2 genes in pigs[J]. J Anim Sci, 2004, 82(11): 3162–3174. DOI: 10.2527/2004.82113162x |
| [27] | WANG Y J, WANG X L, LAU W B, et al. Adiponectin Inhibits TNF-α-induced vascular inflammatory response via caveolin-mediated ceramidase recruitment and activation[J]. Circ Res, 2014, 114(5): 792–805. DOI: 10.1161/CIRCRESAHA.114.302439 |
| [28] | MACEYKA M, SPIEGEL S. Sphingolipid metabolites in inflammatory disease[J]. Nature, 2014, 510(7503): 58–67. DOI: 10.1038/nature13475 |
| [29] | LI T, JIANG S, LU C, et al. Snapshots:Endoplasmic reticulum stress in lipid metabolism and cardiovascular disease[J]. Curr Issues Mol Biol, 2018, 28: 14–28. |
| [30] | JING L, JIA X W. Lycium barbarum polysaccharide arbitrates palmitate-induced apoptosis in MC3T3-E1 cells through decreasing the activation of ERS-mediated apoptosis pathway[J]. Mol Med Rep, 2018, 17(2): 2415–2421. |
| [31] | WANG L L, HU R C, DAI A G, et al. Bevacizumab induces A549 cell apoptosis through the mechanism of endoplasmic reticulum stress in vitro[J]. Int J Clin Exp Pathol, 2015, 8(5): 5291–5299. |



