在动物生长发育过程中,生长激素(growth hormone, GH)与胰岛素样生长因子(insulin-like growth factors, IGFs)组成的调控轴发挥了至关重要的作用[1];而细胞表面受体IGFR(insulin-like growth factor receptor)特异性结合IGFs,是GH-IGFs轴发挥作用的关键受体[2]。IGFR分为IGF1R(insulin like growth factor 1 receptor)和IGF2R (insulin like growth factor 2 receptor)2个亚型。IGF1R是由2个α亚基和2个β亚基通过3个二硫键结合而组成的跨膜四聚体,其中2个α亚基位于胞膜外,被半胱氨酸富集区分隔开,该富集区与IGFs的特异性结合相关[3-4]。目前认为,哺乳动物中IGF1R介导IGFs(IGF1和IGF2)信号促进机体多种组织及器官的发育。例如,IGF1R基因缺失会导致小鼠胚胎致死或者胚胎期肌肉发育迟缓[5],降低肝再生能力或肺部异常发育[6-7]。相反,在肌肉中IGF1R过表达则会引起肌肉肥大[5]。相应地,在小鼠成肌细胞系C2C12中也发现,miR-133a抑制IGF1R表达,从而延迟骨骼肌卫星细胞的激活和分化,减弱肌肉的再生能力[8]。核转录因子NF-κB也可以通过间接性地减少IGF1R表达,抑制成肌细胞的增殖[9]。不同于IGF1R的跨膜四聚体结构,IGF2R是跨膜二聚体,其膜外结构域由147个氨基酸残基组成的15个重复构成,其中一个重复介导与IGF2结合[10]。IGF2R通过清除游离的IGF2,参与器官和组织的发育[11],如协同IGF2参与心肌细胞的凋亡[12]。小鼠缺失IGF2R基因会导致胚胎过度发育或围产期死亡[13]。此外,IGF2R通过调控Gαq信号诱导胚胎期绵羊心肌细胞肥大[14]。以上研究表明,IGF1R和IGF2R在机体组织的生长发育过程中发挥了重要的调控作用,鉴于此,探明IGF1R和IGF2R在家畜动物重要组织生长发育中的表达模式极为重要。例如在猪肌肉组织发育过程中,IGF1R和IGF2R的表达水平随胚胎发育逐渐增加,出生后IGF1R表达显著降低,而IGF2R在骤减之后上升[15]。
综上,IGF1R、IGF2R与动物组织尤其是肌肉组织的生长发育密切相关,但其作用机制尚未完全明确,并且两者在山羊肌肉组织发育和肌细胞增殖分化中的表达模式亦未见报道。本试验以我国培育的第一个肉用山羊品种南江黄羊为研究对象,利用实时荧光定量PCR检测了IGF1R和IGF2R基因在山羊胎儿期和出生后早期5个组织(包括背最长肌和臂三头肌以及心、肝、肺)中的表达水平,并进一步比较了体外培养的山羊骨骼肌卫星细胞增殖和分化过程中二者的表达变化,以期为阐释IGF1R和IGF2R在动物肌肉生长发育中的分子机制奠定基础。
1 材料与方法 1.1 试验材料 1.1.1 试验动物及组织试验动物南江黄羊来自四川省南江黄羊原种场。采集妊娠期第45、60和105天(分别记为E45、E60和E105)胎儿的背最长肌(longissimus dorsi,LD)组织,以及出生后第3、30、60、90和120天(记为B3、B30、B60、B90和B120)羔羊的心、肝、肺、背最长肌和臂三头肌(triceps brachii,TB)样品,每个时期均采集3只母羔。所有样品采集后速冻于液氮保存备用。试验操作严格按照四川农业大学实验动物操作规范与福利管理委员会制定的“实验动物操作规范”进行(川农大校发〔2014〕18号)。
1.1.2 山羊骨骼肌卫星细胞(SMSCs)的分离培养山羊SMSCs来自畜禽遗传资源发掘与创新利用四川省重点实验室。为获得足够数量和活力较强的肌卫星细胞,以初生的南江黄羊背最长肌为材料分离培养SMSCs。经过0.2%链酶蛋白酶消化后,利用percoll液密度梯度离心法纯化细胞并用免疫荧光鉴定细胞获得纯度高于95%的SMSCs[16]。
1.2 试验方法 1.2.1 总RNA提取和cDNA合成采用TRIZOL®Reagent(Invitrogen,美国)进行总RNA的提取,核酸蛋白检测仪(Bio-Rad,美国)测定RNA浓度,经琼脂糖凝胶电泳鉴定后使用SYBR®PrimeScriptTM RT-PCR Kit(TaKaRa,中国大连)进行cDNA的合成,置于-20 ℃冰箱内保存备用。
1.2.2 引物合成依据NCBI数据库中山羊相应基因的序列,采用Primer Premer 6.0设计定量引物(表 1),送至上海生工生物工程有限公司合成。利用Blast检索和PCR产物测序验证引物的特异性。
|
|
表 1 荧光定量PCR引物 Table 1 Primer sequences used for real-time fluorescence quantitative PCR |
荧光定量PCR反应体系为25 μL:12.5 μL SYBR® Green real-time PCR Master Mixture,上下游引物(10 μmol·L-1)各0.5 μL,cDNA模板1 μL,ddH2O 10.5 μL。反应程序:95 ℃ 3 min;95 ℃ 5 s,退火温度30 s,72 ℃ 10 s,40个循环;以0.5 ℃·s-1的速度从65 ℃缓慢递增到95 ℃。每个样品3次重复,反应在Bio-Rad CFX96荧光定量PCR仪上进行。
1.2.4 SMSCs培养和HE染色用含10%胎牛血清(Gibco,美国)的生长培养基在37 ℃、5% CO2条件下培养SMSCs;当细胞密度达到80%~90%时利用含2%孕马血清(Gibco,美国)的培养基诱导成肌分化。观察并收集增殖阶段(24、48和72 h)和分化阶段(0、1、3、5、7、9和11 d)的细胞用于定量PCR检测。此外,分化阶段的细胞进行HE染色分析。主要操作流程:PBS缓冲液清洗细胞后,用预冷的4%多聚甲醛溶液固定细胞30 min;PBS缓冲液清洗细胞后苏木精染液(Solarbio, 北京)染色5 min,0.5%盐酸乙醇分化液分化30 s;去掉多余苏木精染液后使用伊红染液(Solarbio, 北京)染色30 s,70%乙醇洗涤2次,在相差显微镜下对细胞进行观察拍照。
1.2.5 数据统计分析采用Bio-Rad IQTM 5软件获得qPCR测定的各基因Ct值,以β-actin为内参基因,利用2-ΔΔCt法计算基因表达量[17]。试验数据用SAS 9.0中GLM过程进行最小二乘分析,Duncan法进行多重比较。基因相对表达量统计分析模型:yijl=μ+Di+Tj+(DT)ij+εijl;其中,yijl代表基因相对表达量,μ代表总体均值,Di代表时期效应,Tj代表组织效应,(DT)ij代表时期与组织间互作效应,εijl为随机残差,相互独立,且服从N(0,σ2)。统计结果用“平均值±标准误”表示。P < 0.05表示差异显著,P < 0.01表示差异极显著。
2 结果 2.1 山羊胎儿期背最长肌中IGF1R和IGF2R的表达模式IGF1R和IGF2R基因在南江黄羊胎儿期背最长肌中的表达量结果显示(图 1),两者的表达变化皆为先上升后下降,即从E45开始升高,E60达到最高,随后下降。另外,二者在不同发育阶段的表达水平不完全一致,其中IGF1R的表达量为E60>E45>E105,IGF2R的表达量则为E60>E105>E45。比较IGF1R与IGF2R的表达量发现,IGF1R在E45和E60的表达高于IGF2R(P < 0.05,P>0.05),在E105的表达量极显著低于IGF2R(P < 0.01)。
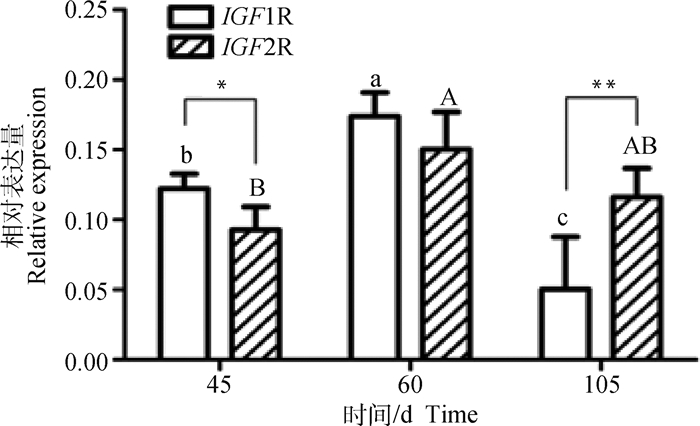
|
图中不同小写字母表示在不同时期IGF1R mRNA水平差异显著(P < 0.05),不同大写字母表示在不同时期IGF2R mRNA水平差异显著(P < 0.05)。同一阶段不同基因表达量之间,*. P < 0.05,**. P < 0.01,图 4、图 5同 The different uppercase letters represent significant difference for IGF1R mRNA levels between different stages (P < 0.05), while different lowercase letters represent significant difference for IGF2R mRNA levels (P < 0.05) between different stages.Between the expression of genes at the same stage, *. P < 0.05, **. P < 0.01, the same as figure 4 and figure 5 图 1 IGF1R和IGF2R在山羊胎儿不同时期背最长肌中的相对表达量 Fig. 1 Relative expression of IGF1R and IGF2R in LD at different stages of goat fetus |
进一步检测南江黄羊出生后5个时期(B3、B30、B60、B90和B120)的5个组织中IGF1R和IGF2R的表达情况(图 2),发现二者在出生后心、肝、肺、LD和TB中均有表达,且组织间表达模式基本不随日龄的增长而变化。其中,IGF1R在羔羊肺中表达量均显著高于其它组织(P < 0.05),在心、LD和TB中的表达量次之,肝中最低(除B3,P < 0.05,图 2A)。与此相反,IGF2R则在5个时期的肺中表达量最低,而在LD中表达量显著高于其它组织(P < 0.05),其余3种组织中表达水平中等(图 2B)。将两者在各组织中的表达量进行对比发现,IGF1R在山羊肺中的表达量约为IGF2R的2 800倍以上(表 2);而IGF2R在其余4个组织(心、肝、LD和TB)中的表达量均高于IGF1R,其中IGF2R在LD中表达量约为IGF1R的300~2 000倍。
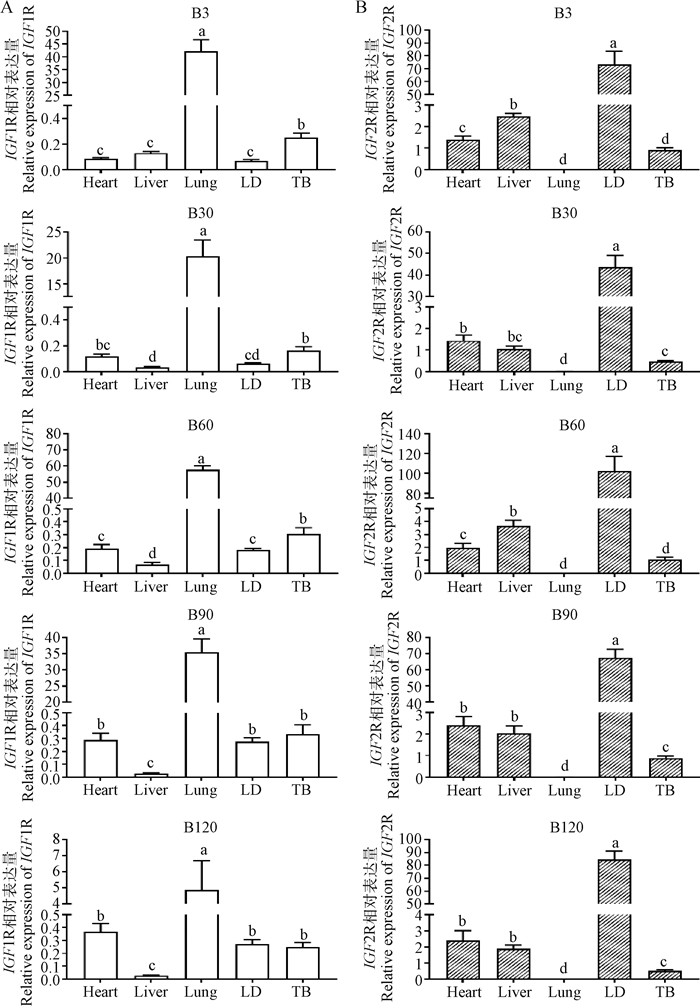
|
A.IGF1R在出生后不同时期的组织表达;B. IGF2R在出生后不同时期的组织表达。不同小写字母代表差异显著(P < 0.05)。 A.Tissue expression of IGF1R in different periods after birth; B. Tissue expression of IGF2R in different periods after birth. Different lowercase letters represent significant difference (P < 0.05) 图 2 IGF1R和IGF2R在山羊出生后不同时期不同组织中的相对表达量 Fig. 2 Relative expression of IGF1R and IGF2R in different tissues at different stages after birth of goats |
|
|
表 2 山羊出生后不同时期组织中IGF2R与IGF1R表达量比值 Table 2 Expression ratio of IGF2R to IGF1R in different tissues at different stages of postnatal goats |
随着出生后山羊日龄的增加,在心或肝中IGF1R和IGF2R呈现出相似的时序性表达模式,即在心中均呈现上升的趋势(图 3A),在肝中呈现“下降-上升-下降”的表达变化(图 3B)。然而在肺中二者的模式却完全相反(图 3C)。此外,在LD和TB两种骨骼肌组织中,IGF1R表达量呈现持续上升模式(B90前)(图 3D);而IGF2R则呈现出与肝组织中相似的“下降-上升-下降”表达趋势(图 3E)。进一步分析出生前后共8个时期LD中的表达数据,发现IGF1R和IGF2R均在E60表达丰度最高,随后下降并于B30达到最低,此后IGF1R表达量呈上调趋势,IGF2R则略微上调至B60后持续下调(图 3F)。
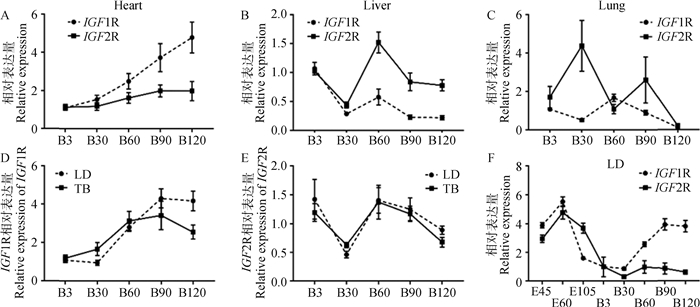
|
A.IGF1R和IGF2R在心组织的发育性表达;B. IGF1R和IGF2R在肝组织的发育性表达;C. IGF1R和IGF2R在肺组织的发育性表达;D. IGF1R在出生后背最长肌和臂三头肌组织的发育性表达;E. IGF2R在出生后背最长肌和臂三头肌组织的发育性表达;F. IGF1R和IGF2R在背最长肌组织的发育性表达 A.Developmental expression of IGF1R and IGF2R in heart; B. Developmental expression of IGF1R and IGF2R in liver; C. Developmental expression of IGF1R and IGF2R in lung; D. Developmental expression of IGF1R in postnatal longissimus dorsi and triceps brachii muscle; E. Developmental expression of IGF2R in postnatal longissimus dorsi and triceps brachii muscle; F. Developmental expression of IGF1R and IGF2R in longissimus dorsi muscle 图 3 山羊各组织中IGF1R和IGF2R的时序表达模式 Fig. 3 Temporal expression patterns of IGF1R and IGF2R in tissues of goats |
为了探究IGF1R和IGF2R在山羊骨骼肌发育中的潜在作用,本研究检测了它们在SMSCs增殖期3个时间点(24、48和72 h)的表达量。如图 4A显示,随着SMSCs培养生长时间的延长,细胞密度逐渐增加,培养72 h时细胞密度已达到70%~80%。表达量检测结果发现(图 4B),SMSCs增殖过程中,IGF1R和IGF2R均呈现下降-上升的表达模式。在生长24和72 h的SMSCs中IGF1R的表达量均显著高于48 h时(P < 0.05),而在24和72 h之间则无显著差异(P>0.05)。在生长24 h的SMSCs中,IGF2R的表达量显著高于48 h的表达(P < 0.05)。另外,IGF1R在增殖细胞中的表达量均极显著高于IGF2R基因(P < 0.01)。
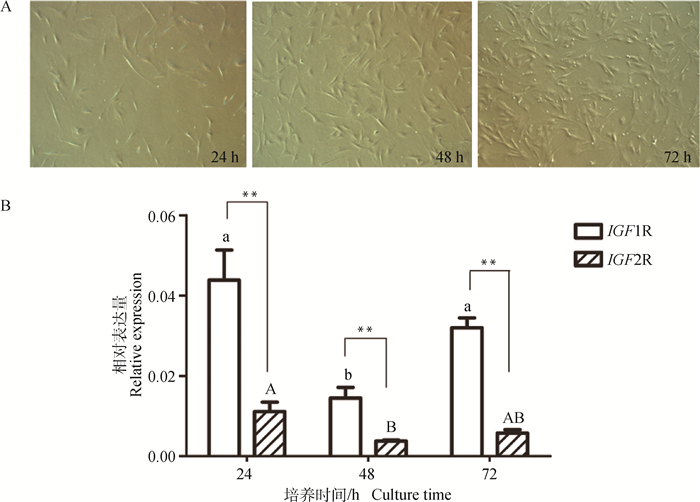
|
A.山羊SMSCs增殖阶段的形态变化(40×);B. SMSCs增殖过程中IGF1R和IGF2R的相对表达量 A. Morphological change of goat SMSCs at different proliferation stages(40×); B. Relative expression of IGF1R and IGF2R during SMSCs proliferation 图 4 山羊SMSCs增殖过程的形态变化及IGF1R和IGF2R的表达 Fig. 4 Morphological change of goat SMSCs during proliferation and expression of IGF1R and IGF2R |
本研究进一步比较了山羊SMSCs分化过程中IGF1R和IGF2R的表达差异,以分化标志基因MyoD和MyoG作为SMSCs正常分化的Marker。结果显示,诱导SMSCs分化5 d,细胞明显融合形成肌管(图 5A)。分化标志基因MyoD及MyoG均在分化初期(0~3 d)显著上升(P < 0.05),而后缓慢下降(图 5B);其中MyoD在分化0 d的表达量极显著高于MyoG(P < 0.01),其它时期则均极显著低于MyoG(P < 0.01),这些结果表明,山羊SMSCs处于正常的分化状态。在SMSCs分化阶段,IGF1R与IGF2R呈现相似的表达模式(图 5C):在分化早期(0~3 d),两者均处于较低水平,但IGF1R表达量显著高于IGF2R(P < 0.05);分化5 d两者的表达量均显著上调(P < 0.05),随后逐渐下降。
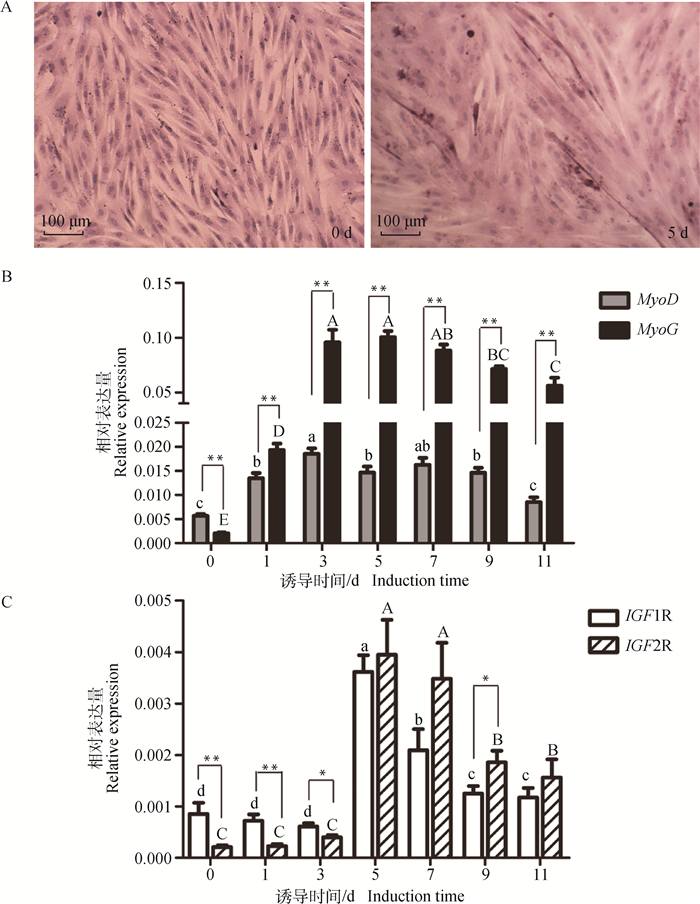
|
A.山羊SMSCs分化0和5 d的细胞形态;B. SMSCs分化过程中MyoD和MyoG基因的表达模式;C. SMSCs分化过程中IGF1R和IGF2R基因的表达模式。不同小写字母表示不同诱导时间MyoD或IGF1R表达量差异显著(P < 0.05),不同大写字母表示不同诱导时间MyoG或IGF2R表达量差异显著(P < 0.05) A. Morphological change of goat SMSCs at day 0 and 5 after differentiation induction; B. Expression patterns of MyoD and MyoG during SMSCs differentiation; C. Expression patterns of IGF1R and IGF2R during SMSCs differentiation. The different lowercase letters represent significant difference for MyoD or IGF1R expression between different induction times (P < 0.05), while different uppercase letters represent significant difference for MyoG or IGF2R expression between different induction times (P < 0.05) 图 5 MyoD、MyoG、IGF1R和IGF2R在山羊SMSCs分化过程中的表达模式 Fig. 5 Expression profiles of MyoD, MyoG, IGF1R and IGF2R during goat skeletal muscle satellite cells differentiation |
动物生长激素GH结合其受体刺激肝产生IGF1和IGF2,以自分泌、旁分泌和内分泌的形式参与动物器官组织的生长发育[18]。其中,IGF1介导GH促进动物出生后的生长,而IGF2主要促进胎盘和胎儿的发育[19-20]。IGF1具有很强的促进细胞增殖和分化并抑制细胞凋亡的作用[21],如IGF1基因P2启动子甲基化增加可导致新生儿身高减少[22]。目前认为,GH的作用主要通过IGF1介导;IGF1R是IGF1和IGF2发挥功能的关键受体[23-25]。例如,敲除IGF1R基因,小鼠出生体重约为野生型的45%,并在不久后死亡[7];干扰小鼠成骨细胞中的IGF1R基因导致骨重降低[26];脂肪组织中IGF1R失活增加脂肪重量[27]。本研究发现,南江黄羊羔羊出生后早期发育阶段,IGF1R基因高度富集于肺组织中,但在肺中几乎检测不到IGF2R mRNA表达,并且IGF1R和IGF2R在肺发育早期表达趋势相反。对高邮鸭和金定鸭的研究发现,IGF1R在肝中各阶段的表达量均高于肌肉组织,说明该基因的表达存在明显的组织差异[28]。肺是动物呼吸及造血的部位,而且是成熟较晚的器官,如大尾寒羊肺正常结构形成于胎儿期120~140 d[29],羔羊早期生长阶段也是肺发育的关键时期[30]。大量的研究也证实,IGF1R的表达与肺癌密切相关[31-33],并且IGF1-IGF1R轴的遗传变异可能是肺癌的主要决定因子[34]。此外有研究表明,IGF1R在维持机体组织生长和发育中起主要作用[35-36],因此,可以推测IGF1R在南江黄羊肺生长和功能发挥中具有重要作用,而IGF2R对肺的影响极小。肝是产生IGF1和IGF2尤其是IGF1的主要器官[18]。据报道,山羊肝组织中IGF1表达水平高,而IGF2低,并在B60时表达量达到最高水平[37]。与此相反,在山羊肝中IGF1R为低丰度表达,IGF2R为中等丰度表达。随着羔羊的生长,IGF1R和IGF2R呈现出“下降-上升-下降”的表达变化趋势,这与绵羊[38]和大鼠[39]肝组织中的研究结果类似,也与山羊中IGFs表达模式完全一致[37]。同时,相较于正常组织,人肝癌组织(hepatocellular carcinoma,HCC)中IGF1下调表达,而IGF1R上调表达[18]。这些结果表明,一方面肝产生的IGF1主要以内分泌的方式比如运输到肺发挥功能,另一方面,IGF1在肝中的作用可能主要通过IGF2R来介导,同时IGF1R的低水平表达是维持肝正常功能的关键。
IGFs及其受体在肌肉发育和再生中具有关键作用。IGF2在肌肉组织中高表达并且是肌肉的一个数量性状位点(quantitative trait locus, QTL)。在成肌分化过程中,MyoD结合在一个IGF2增强子上促进其表达[40],IGF1R及其下游信号分子Akt也被活化,而干扰IGF2后抑制了肌肉特异性结构蛋白表达和肌细胞的融合[41]。反之,IGF1R通过Akt信号调控MyoD的激活和促进肌肉分化,也能诱导MyoG表达来刺激肌原细胞终末分化[42]。本研究发现,虽然出生后南江黄羊背最长肌与臂三头肌组织中,IGF1R与IGF2R的表达模式略有不同,但总的来看,IGF1R和IGF2R在山羊胎儿期富集,出生早期骤减,与猪、牛和大鼠中的研究结果存在相似性[39, 43-44]。同样地,出生后山羊肌肉组织中IGF1R与IGF1、IGF2R与IGF2表达模式完全一致[37],也证实了动物骨骼肌中IGF1主要与IGF1R结合,IGF2R结合IGF2。此外,背最长肌主要由IIb型慢速酵解型肌纤维组成,臂三头肌则大多由Ⅰ型快速氧化型肌纤维组成[45-46],表明在哺乳动物肌肉组织中IGF1R与IGF2R协同表达,没有明显的肌肉类型特异性。肌卫星细胞对于骨骼肌的生长发育及损伤后修复均具有重要作用[47]。在体外培养的山羊骨骼肌卫星细胞中发现,IGF1R和IGF2R皆在增殖阶段和分化后期(5~11 d)高表达;在增殖期IGF1R mRNA水平显著高于IGF2R,分化后期IGF2R mRNA丰度更高。IGF1主要在肌生成的早期促进细胞向成肌分化,IGF2则促进后期成肌分化的完成[48]。而且,自分泌的IGF2可以通过刺激IGF1R进而维持肌细胞的活力[49-51],表明IGF1R和IGF2R协同IGFs共同作用于肌细胞的增殖分化以及肌细胞的内平衡,这与在肌肉组织中两者协同表达的结果一致。
一直以来,IGF1R的作用及其机制得到广泛深入研究。IGF2R对于小鼠胎儿正常生长发育至关重要[15]。本研究发现,除了肺组织,IGF2R在南江黄羊心、肝、背最长肌和臂三头肌中的表达均高于IGF1R。妊娠阶段母牛限饲影响后代肌肉组织中IGF2R mRNA水平,但IGF1R表达变化不大[52-53]。埃及水牛IGF2R编码区突变(A266C)显著增加肌肉中IGF2 mRNA水平以及犊牛日增重[54]。这些结果充分暗示,IGF2R在动物个体发育尤其是肌肉组织中具有广泛的作用,其具体作用机制尚待进一步研究。
4 结论本研究获得了IGF1R和IGF2R在南江黄羊胎儿期和出生后部分组织以及骨骼肌卫星细胞中的表达模式,发现IGF1R在肺中高度富集,但在肝中表达水平极低;相反,IGF2R在肺中表达极低,而在肝呈现中等丰度的表达。同时,在动物肌肉组织和肌细胞中IGF1R与IGF2R mRNA均处于较高水平,并且随个体发育呈现类似的表达模式。初步揭示IGF1R在动物肺中的高表达与肝中的低表达对器官的生长和功能维持具有关键作用,IGF1R与IGF2R协同促进肌肉组织发育和肌细胞的增殖分化。
| [1] | FLORINI J R, EWTON D Z, COOLICAN S A. Growth hormone and the insulin-like growth factor system in myogenesis[J]. Endocr Rev, 1996, 17(5): 481–517. |
| [2] | DUAN C M, REN H X, GAO S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins:roles in skeletal muscle growth and differentiation[J]. Gen Comp Endocrinol, 2010, 167(3): 344–351. DOI: 10.1016/j.ygcen.2010.04.009 |
| [3] | HASTY P, BRADLEY A, MORRIS J H, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene[J]. Nature, 1993, 364(6437): 501–506. DOI: 10.1038/364501a0 |
| [4] | ULLRICH A, GRAY A, TAM A W, et al. Insulin-like growth factor Ⅰ receptor primary structure:comparison with insulin receptor suggests structural determinants that define functional specificity[J]. EMBO J, 1986, 5(10): 2503–2512. DOI: 10.1002/embj.1986.5.issue-10 |
| [5] | LIU J P, BAKER J, PERKINS A S, et al. Mice carrying null mutations of the genes encoding insulin-like growth factor Ⅰ (Igf-1) and type 1 IGF receptor (Igf1r)[J]. Cell, 1993, 75(1): 59–72. |
| [6] | EPAUD R, AUBEY F, XU J, et al. Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis[J]. PLoS One, 2012, 7(11): e48071. DOI: 10.1371/journal.pone.0048071 |
| [7] | BAKER J, LIU J P, ROBERTSON E J, et al. Role of insulin-like growth factors in embryonic and postnatal growth[J]. Cell, 1993, 75(1): 73–82. |
| [8] | HUANG M B, XU H, XIE S J, et al. Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis[J]. PLoS One, 2011, 6(12): e29173. DOI: 10.1371/journal.pone.0029173 |
| [9] | WEI W, ZHANG W Y, BAI J B, et al. The NF-κB-modulated microRNAs miR-195 and miR-497 inhibit myoblast proliferation by targeting Igf1r, Insr and cyclin genes[J]. J Cell Sci, 2016, 129(1): 39–50. DOI: 10.1242/jcs.174235 |
| [10] | MORGAN D O, EDMAN J C, STANDRING D N, et al. Insulin-like growth factor Ⅱ receptor as a multifunctional binding protein[J]. Nature, 1987, 329(6137): 301–307. DOI: 10.1038/329301a0 |
| [11] | NIELSEN F C. The molecular and cellular biology of insulin-like growth factor Ⅱ[J]. Prog Growth Factor Res, 1992, 4(3): 257–290. DOI: 10.1016/0955-2235(92)90023-B |
| [12] | CHU C H, HUANG C Y, LU M C, et al. Enhancement of AG1024-induced H9c2 cardiomyoblast cell apoptosis via the interaction of IGF2R with Galpha proteins and its downstream PKA and PLC-beta modulators by IGF-Ⅱ[J]. Chin J Physiol, 2009, 52(1): 31–37. |
| [13] | LAU M M, STEWART C E, LIU Z, et al. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality[J]. Genes Dev, 1994, 8(24): 2953–2963. DOI: 10.1101/gad.8.24.2953 |
| [14] | WANG K C, BROOKS D A, BOTTING K J, et al. IGF-2R-mediated signaling results in hypertrophy of cultured cardiomyocytes from fetal sheep[J]. Biol Reprod, 2012, 86(6): 183. |
| [15] | WANG K C, TOSH D N, ZHANG S, et al. IGF-2R-Gαq signaling and cardiac hypertrophy in the low-birth-weight lamb[J]. Am J Physiol Regul Integr Comp Physiol, 2015, 308(7): R627–R635. DOI: 10.1152/ajpregu.00346.2014 |
| [16] | LI D D, ZHAN S Y, WANG Y L, et al. Role of microRNA-101a in the regulation of goat skeletal muscle satellite cell proliferation and differentiation[J]. Gene, 2015, 572(2): 198–204. DOI: 10.1016/j.gene.2015.07.010 |
| [17] | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt Method[J]. Methods, 2001, 25(4): 402–408. DOI: 10.1006/meth.2001.1262 |
| [18] | KASPRZAK A, ADAMEK A. The insulin-like growth factor (IGF) signaling axis and hepatitis C virus-associated carcinogenesis (review)[J]. Int J Oncol, 2012, 41(6): 1919–1931. DOI: 10.3892/ijo.2012.1666 |
| [19] | VARY T C. IGF-Ⅰ stimulates protein synthesis in skeletal muscle through multiple signaling pathways during sepsis[J]. Am J Physiol Regul Integr Comp Physiol, 2006, 290(2): R313–R321. DOI: 10.1152/ajpregu.00333.2005 |
| [20] | GERRARD D E, OKAMURA C S, RANALLETTA M A M, et al. Developmental expression and location of IGF-Ⅰ and IGF-Ⅱ mRNA and protein in skeletal muscle[J]. J Anim Sci, 1998, 76(4): 1004–1011. DOI: 10.2527/1998.7641004x |
| [21] | FURSTENBERGER G, SENN H J. Insulin-like growth factors and cancer[J]. Lancet Oncol, 2002, 3(5): 298–302. DOI: 10.1016/S1470-2045(02)00731-3 |
| [22] | LE STUNFF C, CASTELL A L, TODD N, et al. Fetal growth is associated with CpG methylation in the P2 promoter of the IGF1 gene[J]. Clin Epigenetics, 2018, 10: 57. DOI: 10.1186/s13148-018-0489-9 |
| [23] | DUPONT J, LE ROITH D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells:new insights into their synergistic effects[J]. Mol Pathol, 2001, 54(3): 149–154. DOI: 10.1136/mp.54.3.149 |
| [24] | NARASIMHAN S D, YEN K, TISSENBAUM H A. Converging pathways in lifespan regulation[J]. Curr Biol, 2009, 19(15): R657–R666. DOI: 10.1016/j.cub.2009.06.013 |
| [25] | SCHIAFFINO S, MAMMUCARI C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway:insights from genetic models[J]. Skelet Muscle, 2011, 1: 4. DOI: 10.1186/2044-5040-1-4 |
| [26] | ZHANG M, XUAN S H, BOUXSEIN M L, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization[J]. J Biol Chem, 2002, 277(46): 44005–44012. DOI: 10.1074/jbc.M208265200 |
| [27] | KLÖTING N, KOCH L, WUNDERLICH T, et al. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth[J]. Diabetes, 2008, 57(8): 2074–2082. DOI: 10.2337/db07-1538 |
| [28] |
束婧婷, 朱文奇, 单艳菊, 等. 胰岛素样生长因子系统基因在不同鸭种发育早期肝和肌肉中的表达[J]. 畜牧兽医学报, 2014, 45(2): 183–190.
SHU J T, ZHU W Q, SHAN Y J, et al. Expressionof insulin-like growth factor system genes in liver and breast muscle tissue during early development of different duck breeds[J]. Acta Veterinaria et Zootechnica Sinica, 2014, 45(2): 183–190. (in Chinese) |
| [29] |
李守军, 范秀军, 葛利江, 等. 发育中后期羊胎儿肺脏显微与超微结构的研究[J]. 中国兽医杂志, 2006, 42(7): 20–22.
LI S J, FAN X J, GE L J, et al. Micro and ultra-structural changes of sheep fetal lungs during the mid and late phases of development[J]. Chinese Journal of Veterinary Medicine, 2006, 42(7): 20–22. DOI: 10.3969/j.issn.0529-6005.2006.07.007 (in Chinese) |
| [30] |
柴建民, 刁其玉, 屠焰, 等. 早期断奶时间对湖羊羔羊组织器官发育、屠宰性能和肉品质的影响[J]. 动物营养学报, 2014, 26(7): 1838–1847.
CHAI J M, DIAO Q Y, TU Y, et al. Effects of early weaning time on tissue and organ development, slaughter performance and meat quality of Hu lambs[J]. Chinese Journal of Animal Nutrition, 2014, 26(7): 1838–1847. DOI: 10.3969/j.issn.1006-267x.2014.07.016 (in Chinese) |
| [31] | NURWIDYA F, ANDARINI S, TAKAHASHI F, et al. Implications of insulin-like growth factor 1 receptor activation in lung cancer[J]. Malays J Med Sci, 2016, 23(3): 9–21. |
| [32] | ZHAO F Y, HAN J, CHEN X W, et al. miR-223 enhances the sensitivity of non-small cell lung cancer cells to erlotinib by targeting the insulin-like growth factor-1 receptor[J]. Int J Mol Med, 2016, 38(1): 183–191. DOI: 10.3892/ijmm.2016.2588 |
| [33] | MEYER K F, VERKAIK-SCHAKEL R N, TIMENS W, et al. The fetal programming effect of prenatal smoking on Igf1r and Igf1 methylation is organ-and sex-specific[J]. Epigenetics, 2017, 12(12): 1076–1091. DOI: 10.1080/15592294.2017.1403691 |
| [34] | HUANG X P, ZHOU W H, ZHANG Y F. Genetic variations in the IGF-IGFR-IGFBP axis confer susceptibility to lung and esophageal cancer[J]. Genet Mol Res, 2014, 13(1): 2107–2119. |
| [35] | LEROITH D, WERNER H, BEITNER-JOHNSON D, et al. Molecular and cellular aspects of the insulin-like growth factor Ⅰ receptor[J]. Endocr Rev, 1995, 16(2): 143–163. DOI: 10.1210/edrv-16-2-143 |
| [36] | BELFIORE A, MALAGUARNERA R, VELLA V, et al. Insulin receptor isoforms in physiology and disease:An updated view[J]. Endocr Rev, 2017, 38(5): 379–431. DOI: 10.1210/er.2017-00073 |
| [37] | WANG L J, LI L, WANG B, et al. Postnatal development of IGF-Ⅰ and IGF-Ⅱ genes in goat skeletal muscle and liver tissues[J]. J Anim Vet Adv, 2011, 10(20): 2745–2750. |
| [38] | ZHANG T T, ZHANG G M, JIN Y H, et al. Energy restriction affect liver development in Hu sheep ram lambs through Hippo signaling pathway[J]. Tissue Cell, 2017, 49(5): 603–611. DOI: 10.1016/j.tice.2017.08.004 |
| [39] | WERNER H, WOLOSCHAK M, ADAMO M, et al. Developmental regulation of the rat insulin-like growth factor Ⅰ receptor gene[J]. Proc Natl Acad Sci U S A, 1989, 86(19): 7451–7455. DOI: 10.1073/pnas.86.19.7451 |
| [40] | ALZHANOV D, ROTWEIN P. Characterizing a distal muscle enhancer in the mouse Igf2 locus[J]. Physiol Genomics, 2016, 48(2): 167–172. DOI: 10.1152/physiolgenomics.00095.2015 |
| [41] | WILSON E M, HSIEH M M, ROTWEIN P. Autocrine growth factor signaling by insulin-like growth factor-Ⅱ mediates MyoD-stimulated myocyte maturation[J]. J Biol Chem, 2003, 278(42): 41109–41113. DOI: 10.1074/jbc.C300299200 |
| [42] | SUN L, LIU L, YANG X J, et al. Akt binds prohibitin 2 and relieves its repression of MyoD and muscle differentiation[J]. J Cell Sci, 2004, 117: 3021–3029. DOI: 10.1242/jcs.01142 |
| [43] | GHANIPOOR-SAMAMI M, JAVADMANESH A, BURNS B M, et al. Atlas of tissue-and developmental stage specific gene expression for the bovine insulin-like growth factor (IGF) system[J]. PLoS One, 2018, 13(7): e0200466. DOI: 10.1371/journal.pone.0200466 |
| [44] | WANG L J, ZHANG G M, LIN F, et al. Expression of the insulin-like growth factor system in skeletal muscle during embryonic and postnatal development in the first filial generation pigs from Erhualian and Yorkshire reciprocal crosses[J]. Gen Comp Endocrinol, 2011, 173(1): 56–62. DOI: 10.1016/j.ygcen.2011.04.025 |
| [45] | PEINADO B, LATORRE R, VÁQUEZ-AUTÍN J M, et al. Histochemical skeletal muscle fibre types in the sheep[J]. Anat Histol Embryol, 2004, 33(4): 236–243. DOI: 10.1111/ahe.2004.33.issue-4 |
| [46] | CHOI Y M, KIM B C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality[J]. Livestock Sci, 2009, 122(2-3): 105–118. DOI: 10.1016/j.livsci.2008.08.015 |
| [47] | ZAMMIT P S, PARTRIDGE T A, YABLONKA-REUVENI Z. The skeletal muscle satellite cell:the stem cell that came in from the cold[J]. J Histochem Cytochem, 2006, 54(11): 1177–1191. DOI: 10.1369/jhc.6R6995.2006 |
| [48] | ABOALOLA D, HAN V K M. Different effects of insulin-like growth factor-1 and insulin-like growth factor-2 on myogenic differentiation of human mesenchymal stem cells[J]. Stem Cells Int, 2017, 2017: 8286248. |
| [49] | LAWLOR M A, FENG X H, EVERDING D R, et al. Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways[J]. Mol Cell Biol, 2000, 20(9): 3256–3265. DOI: 10.1128/MCB.20.9.3256-3265.2000 |
| [50] | LAWLOR M A, ROTWEIN P. Coordinate control of muscle cell survival by distinct insulin-like growth factor activated signaling pathways[J]. J Cell Biol, 2000, 151(6): 1131–1140. DOI: 10.1083/jcb.151.6.1131 |
| [51] | LAWLOR M A, ROTWEIN P. Insulin-like growth factor-mediated muscle cell survival:central roles for Akt and cyclin-dependent kinase inhibitor p21[J]. Mol Cell Biol, 2000, 20(23): 8983–8995. DOI: 10.1128/MCB.20.23.8983-8995.2000 |
| [52] | MICKE G C, SULLIVAN T M, MCMILLEN Ⅰ C, et al. Protein intake during gestation affects postnatal bovine skeletal muscle growth and relative expression of IGF1, IGF1R, IGF2 and IGF2R[J]. Mol Cell Endocrinol, 2011, 332(1-2): 234–241. DOI: 10.1016/j.mce.2010.10.018 |
| [53] | PARADIS F, WOOD K M, SWANSON K C, et al. Maternal nutrient restriction in mid-to-late gestation influences fetal mRNA expression in muscle tissues in beef cattle[J]. BMC Genomics, 2017, 18(1): 632. DOI: 10.1186/s12864-017-4051-5 |
| [54] | EL-MAGD M A, ABO-AL-ELA H G, EL-NAHAS A, et al. Effects of a novel SNP of IGF2R gene on growth traits and expression rate of IGF2R and IGF2 genes in gluteus medius muscle of Egyptian buffalo[J]. Gene, 2014, 540(2): 133–139. DOI: 10.1016/j.gene.2014.02.059 |



