2. 宜春学院生命科学与资源环境学院, 肉牛羊疾病控制实验室, 宜春 336000;
3. 江西绿科农牧科技有限公司, 宜春 336000
2. Laboratory For Disease Control of Beef Cattle and Sheep, College of Life Science and Resources and Environment, Yichun University, Yichun 336000, China;
3. Jiangxi Lvke Agriculture and Animal Husbandry Technology Co. LTD, Yichun 336000, China
随着畜禽贸易的飞速发展,跨区县、省市的活禽家畜贸易市场逐渐发展、壮大,但随之而来的畜禽运输应激综合征不仅造成畜禽减重、肉品质下降,引起动物发病、死亡,给国民经济带来巨大的损失,更涉及到运输过程中的动物福利问题。研究证实,长时间运输会导致绵羊体重下降[1-2],运输后成年山羊和羔羊体内的中性粒细胞和淋巴细胞等数量发生变化,表明机体免疫机能受到了影响,甚至出现免疫抑制[3-4],增加了运输后疾病发生的概率。
热休克蛋白(HSP)能够应对各种生理和环境变化的应激反应,HSP27、HSP70、HSP90是目前研究最多的蛋白质,它们有调节蛋白质的折叠、运输、帮助蛋白质组装、抗凋亡的功能和特性[5]。有研究证实42 ℃的热应激对大鼠的心肌细胞有损伤作用,推测HSP70表达水平的下降和心肌细胞损伤程度加剧有关[6];鲍恩东等[7]在运输应激和正常的猪心组织中均检测到HSP70、HSP90和HSP27,发现运输6 h后这些蛋白质含量都显著下降,但血液中的肌酸激酶(CK)随之上升。可见,运输应激不仅影响动物血液的生化指标,还对心造成组织细胞层面的损伤,随着运输时间的延长,热休克蛋白的含量也随之变化,说明热休克蛋白与运输应激相关联。
有研究发现HSP70在反刍动物牦牛的肠道中广泛表达[8],也有学者研究运输应激对肉牛肝和脾中急性期蛋白的影响[9],但运输应激、热休克蛋白与山羊心损伤的关系还不清楚。本研究旨在从显微结构和超微结构变化阐明运输应激对山羊心的病理损伤作用,分析HSP27、HSP70、HSP90在心组织中的定位和运输前后表达量的变化,为进一步研究热休克蛋白在运输应激中的功能提供基础资料。
1 材料与方法 1.1 实验动物及设计12头健康且体重相近(13.89 kg±2.96 kg)的赣西公山羊购于宜春本地某养殖场,随机分为对照组、运输2 h组和运输6 h组,对照组不处理,运输应激组的实验山羊以35~45 km·h-1的速度进行公路运输,温度28~32 ℃,运输时间分别为06:00至08:00(2 h)和06:00至12:00(6 h),运输过程中禁食、禁水。试验处理后将山羊致死立即取心组织,一部分放入4%多聚甲醛溶液(PFA)和2.5%戊二醛固定液中,另一部分放入液氮速冻,之后放入-80 ℃冰箱中保存。
1.2 实验材料鼠源HSP70(ab5439)、HSP27(ab79868)、HSP90(ab13492)一抗和羊抗鼠IgG HRP酶标二抗(ab6789)均购自艾博抗上海贸易有限公司,小鼠SP免疫组织化学检测试剂盒(SP-9002)和DAB试剂盒购自北京中杉生物工程公司;苏木精和伊红染色液购自北京梦怡美生物科技有限公司;Mouse Anti-β-actin内参蛋白(BM0627)、WB彩色预染蛋白Marker(AR1113)和SDS-PAGE凝胶制备试剂盒(AR0138)购自博士德生物,组织细胞总蛋白抽提试剂盒(P1250)购自北京普利莱基因技术有限公司,超敏化学发光检测试剂盒(GS0720)购自US EVERBRIGHT INC公司。山羊热休克蛋白HSP70(STH180004-96T)、HSP27(STH180003-96T)和HSP90(STH180005-96T)ELISA试剂盒由北京爱普锐晟科技有限公司提供。
1.3 显微和超微病理学观察经4%PFA溶液固定48 h以上的心组织,先流水冲洗24 h以上,再脱水、透明、浸蜡和石蜡包埋制备组织切片,并对切片进行HE染色,在光学显微镜下观察心组织的病理变化,做好记录并拍照;经2.5%戊二醛固定后的组织用锇酸进行后固定,之后进行脱水、浸透、包埋、超薄切片和染色,最后在透射电镜下观察并拍照记录。
1.4 免疫组织化学先在烘片机上烘片40 min,再将切片依次经二甲苯、酒精和微波抗原修复,按照小鼠SP免疫组织化学试剂盒要求操作,HSP27、HSP70、HSP90抗体按照1:400比例进行稀释,设置阴性对照(用PBS代替一抗),一抗4 ℃过夜。DAB显色,苏木精染色,经分化、返蓝、脱水、透明、封片后立即观察、记录结果并拍照。棕色代表强表达,黄色代表中等表达,淡黄色代表弱表达,不着色代表不表达。
1.5 Western blot按照组织细胞总蛋白抽提试剂盒步骤,取少量的心组织提取总蛋白,用BCA法测定蛋白浓度并调节对照组和实验组的总蛋白浓度一致。根据试剂盒操作说明制胶,加样后先90 V后120 V进行SDS-PAGE凝胶电泳2 h,取凝胶孵PVDF膜300 mA湿转50 min,取膜用TBST漂洗后5%脱脂奶粉封闭2 h,将膜剪成适当大小后用TBST漂洗,放入垂直混合仪4 ℃条件下在一抗(HSP27、HSP70、HSP90和内参蛋白的稀释比例分别为1:1 500、1:1 000、1:1 000和1:1 000)溶液中孵育18 h,之后TBST漂洗3次,每次10 min,二抗(HSP27、HSP70、HSP90和内参蛋白的稀释比例分别为1:8 000、1:20 000、1:2 000和1:1 000)孵育2 h,TBST漂洗3次。最后将PVDF膜按照超敏化学发光试剂盒的说明显色,用AI600化学发光成像仪进行拍照、记录。
1.6 ELISA分析参照ELISA试剂盒说明书进行操作,应用双抗体夹心法测定山羊心中3种热休克蛋白水平。将50 μL标准品加入酶标包被板标准孔,将40 μL样品稀释液加入待测样品孔,然后再加入10 μL待测样品,封板,37 ℃条件下孵育30 min,甩干、洗板后加入50 μL酶标试剂,封板,37 ℃条件下孵育30 min,甩干、洗板后依次加入显色剂A、B各50 μL,充分混匀37 ℃避光孵育10 min,终止反应后应用美国Awareness Chromate 4300酶标读数仪在450 nm波长下测定吸光度(OD值),以OD值为纵坐标、标准物浓度为横坐标绘制标准曲线,通过标准曲线计算浓度。
1.7 统计分析用Image Pro Plus 6.0软件对采集的图像进行密度值分析,将目的条带的密度值与相对应的内参蛋白密度值的比值记为测试值。用SPSS18.0软件进行数据分析,单因素方差分析后进行LSD多重比较。结果记为x±s,P < 0.01表示差异极显著,P < 0.05表示差异显著,P>0.05表示差异不显著。
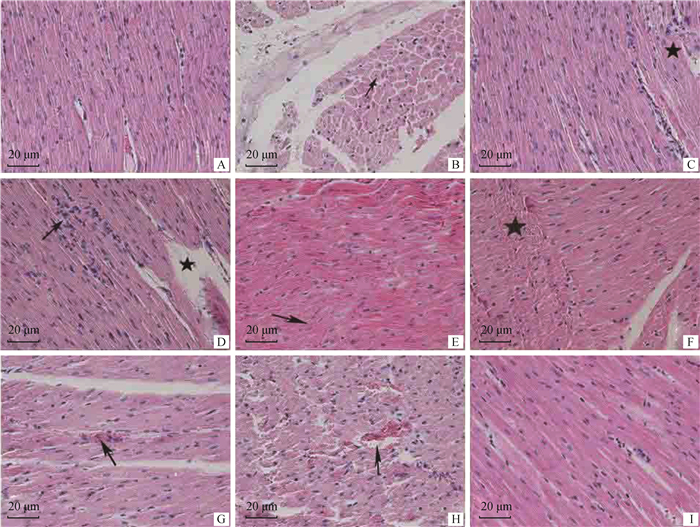
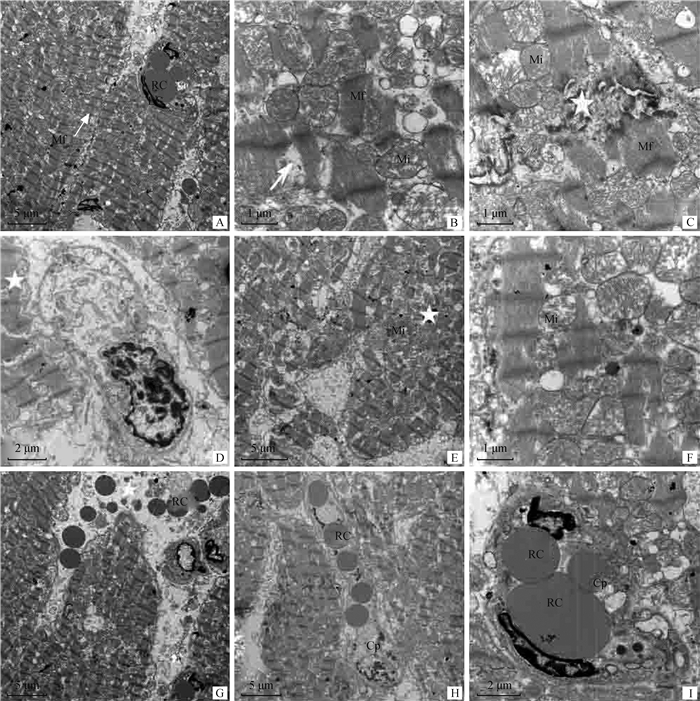
2 结果 2.1 运输应激山羊心的病理变化与正常组相比较,运输应激组山羊的心肌纤维表现为程度不一的弥漫性变性、充血出血,部分心肌纤维受损,如图 1所示。运输应激处理2 h组山羊心肌纤维轻度肿胀,间隙增宽(图 1A),心肌纤维细胞细胞质轻度浑浊(图 1B)。间质毛细血管和小动脉扩张充血(图 1C)。部分心肌纤维受损、断裂,有炎性细胞存在(图 1D)。运输应激处理6 h组山羊心肌纤维肿胀明显,心肌纤维的横纹模糊不清甚至消失(图 1E),部分心肌纤维坏死(图 1F),间质内小血管充血严重(图 1G、H)。电镜结果如图 2所示,运输应激处理组山羊心肌部分肌原纤维断裂、萎缩、排列不整齐(图 2A、B),细胞间连接处的闰盘不规则,部分出现分离情况(图 2C)。心肌细胞细胞膜呈锯齿状突起,膜下可见髓鞘样结构和明显的水肿(图 2D);细胞质内线粒体数量增多,外形肿胀,线粒体嵴排列紊乱甚至断裂(图 2E、F)。心肌纤维轻度水肿,间隙增大,有较多的红细胞分布(图 2G),毛细血管扩张,管腔内充满红细胞(图 2H、I)。
|
A~D.运输应激2 h组:图A示心肌纤维轻微肿胀;图B箭头所示心肌细胞轻微颗粒变性;图C星号所示间质少量出血;图D箭头所示炎性细胞浸润,星号所示心肌纤维断裂。E~H.运输应激6 h组:图E箭头所示心肌纤维肿胀明显,横纹模糊;图F星号所示心肌纤维坏死,炎性细胞浸润;图G箭头所示小血管扩张,管腔内充满红细胞;图H箭头所示心肌间质血管充满红细胞。I.正常心肌组织 A-D. The group of transport stress for 2 h: A. Myocardial fiber was slightly swollen; B. Arrow showed slight granular degeneration of myocardial cells; C. Asterisk showed slight interstitial hemorrhage; D. Arrow showed inflammatory cell infiltration, asterisk showed myocardial fiber rupture. E-H. The group of transport stress for 6 h: E. Myocardial fiber was obvious swollen and blurred horizontal lines; F. Asterisk showed myocardial fiber necrosis and inflammatory cell infiltration; G. Arrow showed vessels were dilated and filled with red blood cells; H. Arrow showed myocardial stromais filled with red blood cells. I. Normal cardiac tissue 图 1 山羊心的显微结构(HE染色) Fig. 1 The microscopic structure of the goat heart (HE staining) |
|
Cp.毛细血管;RC.红细胞;EC.内皮细胞;Mi.线粒体;Mf.肌原纤维;N.细胞核。图A箭头所示Z线较为清楚;图B箭头所示肌原纤维断裂溶解;图C星号所示肌原纤维断裂缺失,闰盘结构模糊、连续性中断;图D星号所示心肌细胞的细胞膜呈锯齿状;图E星号所示局部线粒体数量增多;图F线粒体大小不一,有的线粒体嵴排列紊乱;图G间质中有红细胞分布,星号所示间质水肿;图H、I毛细血管管腔内充满了红细胞 Cp. Capillary; RC. Red cell; EC. Endothelial cell; Mi. Mitochondria; Mf. Myofibril; N. Nucleus. A. Arrow showed the Z-line clearly; B. Arrow showed myofibril rupture and dissolution; C. Asterisk showed the myofibril fracture and loss, with blurry structure and interrupted continuity of the leap disc; D. Asterisk showed myocardial cell membrane was serrated; E. Asterisk showed increased mitochondria; F. Mitochondria of different sizes, some mitochondrial ridge disorder; G. Red blood cells were distributed in the stroma, and the asterisk indicated interstitial edema; H, I. Capillary lumen filled with red blood cells 图 2 运输组山羊心的超微结构(醋酸铀-枸橼酸铅染色) Fig. 2 Ultrastructure of goat heart in transport group (uranium-lead citrate staining) |
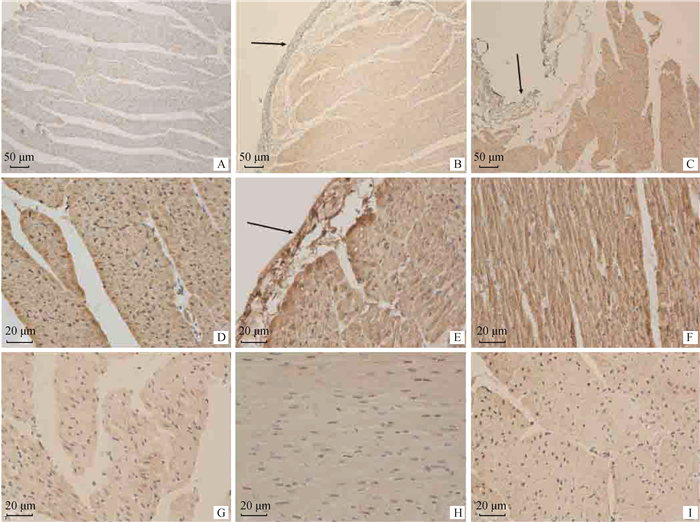
如图 3所示,免疫组织化学结果表明,HSP70阳性信号主要出现在心肌纤维的细胞质中,在不同区域的心肌细胞中分布不均,从靠近心内膜一侧心肌细胞中的强表达逐渐向后递减,直至不表达,细胞受损区较完整区表达强;对照组中HSP70弱表达,运输应激2和6 h组中等表达。HSP27在心肌细胞中普遍表达、广泛分布,主要集中在心肌细胞的细胞核周围和小血管的内皮细胞中,HSP27阳性颗粒在心肌细胞的细胞质中分布不均,在不同区域也分布不均,受损区表达强,靠近肌束边缘、血管周围的心肌细胞在细胞膜周围表达强;对照组HSP27呈中等表达,运输应激2和6 h组呈强表达。HSP90在心肌纤维中表达弱,特别是在对照组中,HSP90蛋白仅表达在部分区域的心肌纤维细胞质和细胞核中,血管内皮有弱表达,且在细胞质中分布不均,在对照组中,仅靠近心内膜一侧的心肌纤维有弱表达,其他区域不表达;运输应激2和6 h组中,HSP90表达范围更大,表达更强。
|
A、B、C为HSP70在山羊心的表达,D、E、F为HSP27在山羊心的表达,G、H、I为HSP90在山羊心的表达;A、D、G为对照组,B、E、H为运输应激2 h组,C、F、I为运输应激6 h组。箭头所示为心内膜 A, B and C were the expression of HSP70 in goat heart. D, E and F were the expression of HSP27 in goat heart. G, H and I were the expression of HSP90 in goat heart. A, D and G were the control groups; B, E and H were the groups of transport stress for 2 h; C, F and I were the groups of transport stress for 6 h. The arrows showed the endocardium 图 3 山羊心中HSP70、HSP27和HSP90三种热休克蛋白的表达(免疫组织化学) Fig. 3 Expression of HSP70, HSP27 and HSP90 proteins in goat heart (immunohistochemistry) |
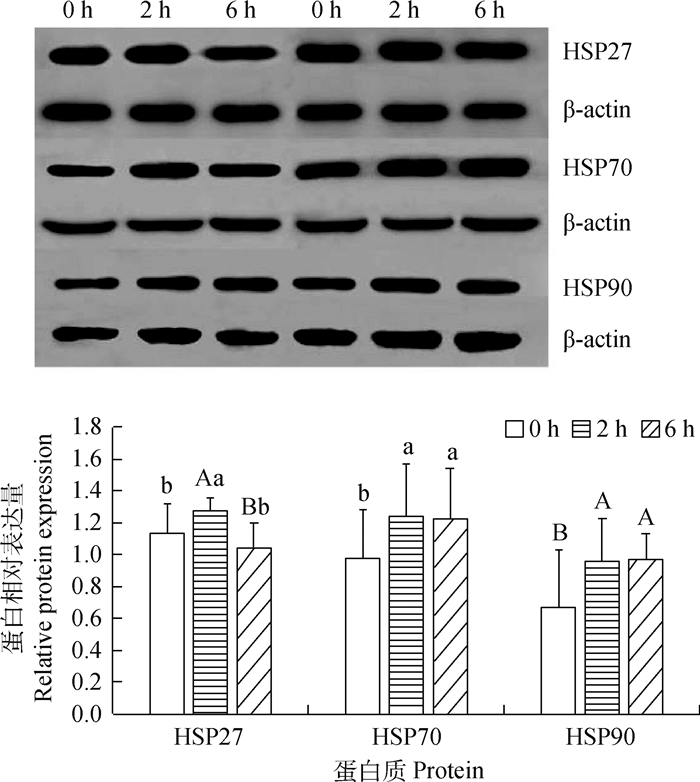
Western blot试验结果显示(图 4),与对照组相比,运输应激2 h组山羊心HSP27蛋白表达量显著升高(P < 0.05),运输应激6 h组HSP27蛋白表达量下降,差异不显著(P>0.05),但运输应激6 h组HSP27蛋白表达量比运输应激2 h组极显著降低(P < 0.01)。与对照组相比,运输应激2和6 h组HSP70蛋白表达量显著升高(P < 0.05),而HSP90蛋白表达量极显著升高(P < 0.01),运输应激2和6 h组之间HSP70和HSP90蛋白表达量都无显著差异(P>0.05)。
|
同一蛋白三个组间大写字母标记不同的组之间差异极显著(P < 0.01),不同小写字母表示差异显著(P < 0.05),相同字母表示差异不显著(P>0.05) The differences among the three groups with the same protein marked with different capital letters are extremely significant (P < 0.01). Different lowercase letters indicate significant differences (P < 0.05), and the same letters indicate insignificant differences (P>0.05) 图 4 运输应激2 h组、6 h组与对照组(0 h)山羊心HSP27、HSP70和HSP90三种热休克蛋白相对表达量 Fig. 4 Relative expression levels of HSP27, HSP70 and HSP90 proteins in the heart of goats in the 2 h and 6 h transport stress group and the control group (0 h) were compared |
表 1所示为对照组和运输应激组山羊心中HSP27、HSP70和HSP90三种蛋白的含量,ELISA试验结果表明运输应激2 h组与对照组相比HSP27含量明显升高(P < 0.05),运输应激6 h组与运输应激2 h组相比HSP27含量极显著下降(P < 0.01),与对照组无明显差异(P>0.05)。与对照组相比,运输应激2 h组(P < 0.05)和6 h组(P < 0.01)HSP70和HSP90两种蛋白含量均显著或极显著升高,运输应激2 h组和6 h组之间两种蛋白含量均无明显变化(P>0.05)。
|
|
表 1 山羊心HSP27、HSP70和HSP90表达水平(x±s) Table 1 The expression levels of HSP27, HSP70 and HSP90 in the heart of goats(x±s) |
运输应激造成山羊肌肉代谢发生变化,装卸、噪声、高温、生活环境突变还会造成动物情绪上的应激压力[10]。运输给羊羔造成严重的应激反应,随着运输时间的延长,情绪压力反应减缓,但与脱水、饥饿有关的其他参数随着时间的推移增加[11]。研究发现12 h运输应激造成肉牛体重明显下降、体温升高和代谢增强[12],改变了血液循环中的各种生理和炎症指标以及类固醇激素含量[13]。运输应激还会引起雏鸡的体重下降,造成心损伤,长时间运输对雏鸡造成严重的负面影响[14]。本研究发现,运输应激对山羊心的组织结构造成破坏,引起心肌纤维急性变性,间质出现炎性细胞浸润和出血现象,运输应激6 h组较2 h组病变更加明显,超微观察发现除了心肌纤维断裂、水肿,线粒体病变也十分严重。以上山羊心的显微和超微结构病理变化极易发生因心血管损伤而引起的心血管疾病,导致运输应激动物的突然发病甚至急性死亡,这与Cheng等[15]在热应激大鼠上的试验结果是一致的,有研究也证实了热应激会造成小鼠的细胞损伤和凋亡[16]。在心的调节保护方面,线粒体发挥着重要作用[17],而本试验电镜结果显示线粒体出现较严重的病变,将影响线粒体的正常生理功能。此外,运输应激组炎性细胞浸润提示动物机体动用免疫系统清除受损的心肌细胞和毛细血管内皮细胞,将进一步加剧心结构的不完整性,在动物机体抵抗力低的情况下易诱发应激后心血管疾病的发生。
研究证明,热休克蛋白可以明显减缓肺间质水肿和炎性蛋白的渗出,保护心肌细胞免受心房颤动等急性和慢性应激,明显降低患有呼吸窘迫症动物在48 h内的死亡率[18-19]。由于机械或氧化应激降低了细胞内蛋白质的稳定性,引发的如心肌梗死和心力衰竭等心血管疾病促进了心肌细胞内错误蛋白质的折叠,而HSP70能感知氧化损伤,促进错误折叠或未折叠蛋白质的修复,作为分子伴侣,在细胞内外发挥着多重保护和免疫调节功能[20-22]。本研究表明HSP70主要在心肌纤维的细胞质中表达,与对照组相比,运输应激2和6 h组HSP70蛋白表达量显著升高,有研究结果也表明HSP70在心肌细胞的细胞质中表达[6-7],唐姝等[22]发现添加纯化的迷迭香提取物可以诱导肉鸡热应激后心HSP70表达水平的升高,改善心肌纤维的损伤,缓解肉鸡的热应激。在心肌缺血再灌注过程中,诱导HSP70表达可以调节细胞内蛋白质,抑制促炎信号通路从而发挥抗炎作用,有利于减少活性氧堆积和抑制细胞凋亡,增强对心肌缺血再灌注损伤的耐受[23]。运输后山羊心HSP70蛋白表达量显著升高,表明HSP70在运输应激中发挥保护作用,减缓心肌纤维的损伤。
HSP27是普遍存在的小热休克蛋白,参与动脉粥样硬化、充血性心力衰竭等多种心血管疾病过程[24],在Ⅰ型糖尿病大鼠中,高血糖作为一种温和应激,总HSP27不受影响,但磷酸化HSP27表达升高,增强了患者对心肌缺血再灌注损伤的抵抗力[25]。HSP27被证明能够提高细胞存活率[26],并在药物引起的应激反应中发挥积极作用[27]。猪运输应激试验结果表明HSP27在不同运输时间(0、1、2、4 h)猪心中呈现不同的变化趋势[28],大鼠热应激试验发现HSP27在心肌细胞中的表达量随着时间推移增多或减少[29-30]。在本研究中,HSP27在心肌细胞的细胞质和细胞核中普遍表达,运输应激2 h组蛋白表达量较对照组显著升高,运输6 h组较2 h组极显著降低,表明在应激过程中HSP27并没有一致的变化趋势,与动物品种、应激的强度和时间等因素有关,同时受HSP27消耗与生成之间动态变化的影响。有研究证明心肌缺血诱导HSP27释放,在细胞外介导心肌和冠状动脉内皮细胞的炎症反应[31]。总之,HSP27的动态变化与运输应激有关,对心肌细胞发挥一定的调节作用。
近年来HSP90抑制剂治疗疾病成为研究的热点,有研究论证了应用HSP90的药物抑制剂能够抑制其在胃肠道癌症中的致瘤作用[32],但是也有研究证明阿司匹林促进热应激时HSP90的表达,降低了细胞凋亡的数量并缓解鸡的氧化应激[33],作为分子伴侣帮助稳定蛋白质构象和清除错误折叠蛋白质[34]。有学者研究发现,在非适应性心肌肥厚中,抑制HSP90的表达反而导致左心室的功能障碍[35],HSP90介导阿片类药物对心的保护作用[17],以上这些结果提示诱导HSP90高表达对心血管疾病有着积极的影响。与HSP27和HSP70在心的表达相比,本试验结果显示HSP90阳性颗粒主要表达在心肌纤维细胞质和细胞核中,有研究证明HSP90在心肌细胞核中也有强烈表达[36],与对照组比较,运输组的蛋白表达量都显著上升,运输后HSP90阳性反应物集中在细胞核和细胞质中,说明HSP90对运输应激山羊心发挥着一定的保护作用。
4 结论运输应激2和6 h均对山羊心造成了病理损伤;与对照组相比,运输后山羊心HSP70和HSP90蛋白表达量显著增加,运输2 h组山羊心HSP27蛋白表达量显著升高,而6 h组表达量下降。本研究结果将为进一步探讨HSP27、HSP70和HSP90在运输应激中所起作用及研制缓解运输应激的方法和产品提供基础资料。
| [1] | FISHER A D, NIEMEYER D O, LEA J M, et al. The effects of 12, 30, or 48 hours of road transport on the physiological and behavioral responses of sheep[J]. J Anim Sci, 2010, 88(6): 2144–2152. DOI: 10.2527/jas.2008-1674 |
| [2] | ZHONG R Z, LIU H W, ZHOU D W, et al. The effects of road transportation on physiological responses and meat quality in sheep differing in age[J]. J Anim Sci, 2011, 89(11): 3742–3751. DOI: 10.2527/jas.2010-3693 |
| [3] | MIRANDA-DE LA LAMA G C, RODRÍGUEZ-PALOMARES M, CRUZ-MONTERROSA R G, et al. Long-distance transport of hair lambs: effect of location in pot-belly trailers on thermo-physiology, welfare and meat quality[J]. Trop Anim Health Prod, 2018, 50(2): 327–336. DOI: 10.1007/s11250-017-1435-0 |
| [4] | MINKA N S, AYO J O. Modulating effect of ascorbic acid on transport-induced immunosuppression in goats[J]. ISRN Vet Sci, 2011, 2011: 749753. |
| [5] | WANG X X, CHEN M J, ZHOU J, et al. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy (Review)[J]. Int J Oncol, 2014, 45(1): 18–30. DOI: 10.3892/ijo.2014.2399 |
| [6] | CHEN H B, ADAM A, CHENG Y F, et al. Localization and expression of heat shock protein 70 with rat myocardial cell damage induced by heat stress in vitro and in vivo[J]. Mol Med Rep, 2015, 11(3): 2276–2284. DOI: 10.3892/mmr.2014.2986 |
| [7] | BAO E D, SULTAN K R, NOWAK B, et al. Expression and distribution of heat shock proteins in the heart of transported pigs[J]. Cell Stress Chaperon, 2008, 13(4): 459–466. DOI: 10.1007/s12192-008-0042-4 |
| [8] |
廖博, 崔燕, 杨雪, 等. HSP70在牦牛肠道中的分布与变化规律[J]. 畜牧兽医学报, 2018, 49(8): 1735–1742.
LIAO B, CUI Y, YANG X, et al. Distribution and change rule of HSP70 in yak intestine[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(8): 1735–1742. (in Chinese) |
| [9] |
袁建彬, 薛琳琳, 高春柳, 等. 不同运输时间运输应激对肉牛组织中急性期蛋白的影响[J]. 中国兽医学报, 2018, 38(9): 1771–1774, 1778.
YUAN J B, XUE L L, GAO C L, et al. Effect of transport stress on acute phase proteins in beef cattle at different transport time[J]. Chinese Journal of Veterinary Science, 2018, 38(9): 1771–1774, 1778. (in Chinese) |
| [10] | KANNAN G, KOUAKOU B, TERRILL T H, et al. Endocrine, blood metabolite, and meat quality changes in goats as influenced by short-term, preslaughter stress[J]. J Anim Sci, 2003, 81(6): 1499–1507. DOI: 10.2527/2003.8161499x |
| [11] | DE LA FUENTE J, SÁNCHEZ M, PÉREZ C, et al. Physiological response and carcass and meat quality of suckling lambs in relation to transport time and stocking density during transport by road[J]. Animal, 2010, 4(2): 250–258. DOI: 10.1017/S1751731109991108 |
| [12] |
芦春莲, 李妍, 曹玉凤, 等. 肉牛宰前运输应激对其血液理化指标及免疫机能的影响[J]. 中国兽医学报, 2016, 36(7): 1173–1177.
LU C L, LI Y, CAO Y F, et al. Effects of preslaughter transportation stress on blood physiological and biochemical indexes and immunity function of beef cattle[J]. Chinese Journal of Veterinary Science, 2016, 36(7): 1173–1177. (in Chinese) |
| [13] | SPORER K R B, WEBER P S D, BURTON J L, et al. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress[J]. J Anim Sci, 2008, 86(6): 1325–1334. DOI: 10.2527/jas.2007-0762 |
| [14] | LI Z Y, LIN J, SUN F, et al. Transport stress induces weight loss and heart injury in chicks:disruption of ionic homeostasis via modulating ion transporting ATPases[J]. Oncotarget, 2017, 8(15): 24142–24153. |
| [15] | CHENG Y F, SUN J R, CHEN H B, et al. Expression and location of HSP60 and HSP10 in the heart tissue of heat-stressed rats[J]. Exp Ther Med, 2016, 12(4): 2759–2765. DOI: 10.3892/etm.2016.3650 |
| [16] |
高琛, 隋君霞, 周颖璐, 等. 黄芩苷对热应激小鼠睾丸氧化损伤的保护作用[J]. 农业生物技术学报, 2017, 25(9): 1470–1477.
GAO C, SUI J X, ZHOU Y L, et al. Protective effect of Baicalin on oxidative damage of testes in heat stressed mice (Mus musculus)[J]. Journal of Agricultural Biotechnology, 2017, 25(9): 1470–1477. (in Chinese) |
| [17] | SMALL B A, LU Y, HSU A K, et al. Morphine reduces myocardial infarct size via heat shock protein 90 in rodents[J]. Biomed Res Int, 2015, 2015: 129612. |
| [18] | WEISS Y G, MALOYAN A, TAZELAAR J, et al. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome[J]. J Clin Invest, 2002, 110(6): 801–806. DOI: 10.1172/JCI0215888 |
| [19] | MIN T J, JO W M, SHIN S Y, et al. The protective effect of heat shock protein 70 (Hsp70) in atrial fibrillation in various cardiomyopathy conditions[J]. Heart Vessels, 2015, 30(3): 379–385. DOI: 10.1007/s00380-014-0521-8 |
| [20] | RANEK M J, STACHOWSKI M J, KIRK J A, et al. The role of heat shock proteins and co-chaperones in heart failure[J]. Philos T R Soc B, 2018, 373(1738): 20160530. DOI: 10.1098/rstb.2016.0530 |
| [21] | RADONS J. The human HSP70 family of chaperones:where do we stand?[J]. Cell Stress Chaperon, 2016, 21(3): 379–404. DOI: 10.1007/s12192-016-0676-6 |
| [22] | TANG S, YIN B, XU J, et al. Rosemary reduces heat stress by inducing CRYAB and HSP70 expression in broiler chickens[J]. Oxid Med Cell Longev, 2018, 2018: 7014126. |
| [23] | SONG Y J, ZHONG C B, WANG X B. Heat shock protein 70:a promising therapeutic target for myocardial ischemia-reperfusion injury[J]. J Cell Physiol, 2019, 234(2): 1190–1207. DOI: 10.1002/jcp.27110 |
| [24] | GHAYOUR-MOBARHAN M, SABER H, FERNS G A A. The potential role of heat shock protein27 in cardiovascular disease[J]. Clin Chim Acta, 2012, 413(1-2): 15–24. DOI: 10.1016/j.cca.2011.04.005 |
| [25] | CHEN H, WU X J, LU X Y, et al. Phosphorylated heat shock protein 27 is involved in enhanced heart tolerance to ischemia in short-term type 1 diabetic rats[J]. Acta Pharmacol Sin, 2005, 26(7): 806–812. DOI: 10.1111/j.1745-7254.2005.00113.x |
| [26] | SHARP F R, ZHAN X H, LIU D Z. Heat shock proteins in the brain:role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential[J]. Transl Stroke Res, 2013, 4(6): 685–692. DOI: 10.1007/s12975-013-0271-4 |
| [27] | DANIEL R C, STEFFI O, GARY C C, et al. Biological and clinical implications of heat shock protein 27, 000 (Hsp27):a review[J]. J Natl Cancer Inst, 1993, 85(19): 1558–1570. DOI: 10.1093/jnci/85.19.1558 |
| [28] | ZHANG M, XIN L X, BAO E D, et al. Variation in the expression of Hsp27, αB-crystallin mRNA and protein in heart and liver of pigs exposed to different transport times[J]. Res Vet Sci, 2011, 90(3): 432–438. |
| [29] | TANG S, CHEN H B, CHENG Y F, et al. Expression profiles of heat shock protein 27 and αB-crystallin and their effects on heat-stressed rat myocardial cells in vitro and in vivo[J]. Mol Med Rep, 2016, 13(2): 1633–1638. |
| [30] | TANG S, BURIRO R, LIU Z, et al. Localization and expression of Hsp27 and αB-Crystallin in rat primary myocardial cells during heat stress in vitro[J]. PLoS One, 2013, 8(7): e69066. DOI: 10.1371/journal.pone.0069066 |
| [31] | JIN C H, CLEVELAND J C, AO L H, et al. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4[J]. Mol Med, 2014, 20(1): 280–289. |
| [32] | BOROUMAND N, SAGHI H, AVAN A, et al. Therapeutic potency of heat-shock protein-90 pharmacological inhibitors in the treatment of gastrointestinal cancer, current status and perspectives[J]. J Pharm Pharmacol, 2018, 70(2): 151–158. DOI: 10.1111/jphp.12824 |
| [33] | ZHANG X H, WU H, TANG S, et al. Apoptosis in response to heat stress is positively associated with heat-shock protein 90 expression in chicken myocardial cells in vitro[J]. J Vet Sci, 2017, 18(2): 129–140. DOI: 10.4142/jvs.2017.18.2.129 |
| [34] | ZUEHLKE A D, MOSES M A, NECKERS L. Heat shock protein 90:its inhibition and function[J]. Philos T R Soc B, 2018, 373(1738): 20160527. DOI: 10.1098/rstb.2016.0527 |
| [35] | ESCHRICHT S, JARR K U, KUHN C, et al. Heat-shock-protein 90 protects from downregulation of HIF-1α in calcineurin-induced myocardial hypertrophy[J]. J Mol Cell Cardiol, 2015, 85: 117–126. DOI: 10.1016/j.yjmcc.2015.05.018 |
| [36] | YU J M, BAO E D, YAN J Y, et al. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers[J]. Cell Stress Chaperon, 2008, 13(3): 327–335. DOI: 10.1007/s12192-008-0031-7 |



