2. 四川农业大学生命科学院, 雅安 625014
2. College of Life Science, Sichuan Agricultural University, Ya'an 625014, China
白色念珠菌(Candida albicans)是一种常见的条件致病菌,常存在于口腔、肠道、上呼吸道及阴道,是目前研究最多的致病酵母之一[1]。
群体感应(quorum sensing,QS)最早在细菌中发现[2],是指微生物自发产生并释放的群体感应分子(quorum sensing molecule,QSM),感知微生物的浓度变化并调节其自身的一种群体行为。群体感应依赖微生物密度并调控微生物体中耐药和毒力相关基因的表达[3-6]。近年来,陆续发现在真菌中也存在着群体感应。不同属的真菌群体感应分子可能不同,如白色念珠菌产生的群体感应分子为法尼醇和酪醇[3-4], 酿酒酵母(Saccharomyces cerevisiae)产生的群体感应分子为苯乙醇和色醇[5],构巢曲霉(Aspergilus nidulans)产生的群体感应分子为氧化酯类等[6]。
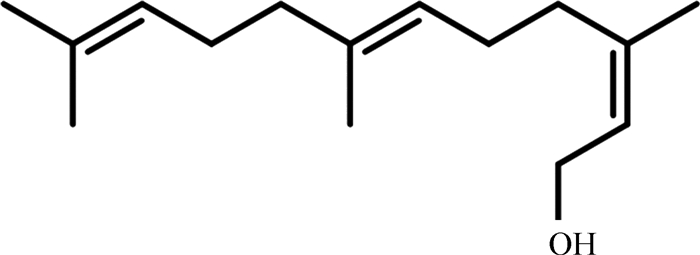
法尼醇,其分子式为C15H26O(图 1);其性状为无色液体,几乎不溶于水,可溶于有机溶剂;其来源为真菌麦角甾醇合成前体焦磷酸法尼酯的脱磷酸化[7-8],是麦角甾醇合成途径中的关键衍生物[9]。麦角甾醇是真菌细胞膜上的重要组成部分,对确保细胞活力,细胞膜的流动性、完整性及细胞物质运输等具有重要作用[8]。因此,法尼醇普遍存在于所有真菌中,而在不同属真菌中可能扮演着不同的角色。如在烟曲霉菌(Aspergilus fumigatus)影响其细胞壁和细胞骨架的完整性[10];在假丝酵母属(Candida spp)中诱导细胞凋亡[11];在皮肤癣菌抑制其生长[12];在变色栓菌(Trametes versicolor)促进其胞外多糖的生物合成和活性而促进菌丝的生长[13];在白色念珠菌作为群体感应分子影响其毒力和耐药性等[3]。
|
图 1 法尼醇分子式 Figure 1 Molecular formula of farnesol |
此外,真菌特有的群体感应系统不仅仅只作用于真菌内部,还与真菌-细菌的共存有一定的相关性[14]。白色念珠菌的群体感应分子法尼醇在与细菌共存时可以提高细菌对抗生素的敏感性, 如可抑制假单胞菌喹诺酮信号的产生并提高环丙沙星的疗效[15-16];提高金黄色葡萄球菌对多黏菌素B的敏感性[17];与萘夫西林和万古霉素协同抑制表皮葡萄球菌生物膜形成[18];增强β内酰胺对类鼻疽杆菌的功效等[19]。在临床上,真菌-细菌共感染比其二者单独感染的疾病更加棘手。因此,深入研究真菌群体感应,不仅旨在寻找真菌治疗的药物新靶点,还旨在解决真菌-细菌共感染的难题,具有十分重要的意义。
1 法尼醇对白色念珠菌的作用法尼醇是白色念珠菌的群体感应分子,在一定浓度时对白色念珠菌的几个方面都有显著影响,主要影响白色念珠菌耐药性、毒力因子和菌体凋亡。其影响方式如表 1所示。
|
|
表 1 法尼醇对白色念珠菌的作用 Table 1 The effect of farnesol on Candida albicans |
药物外排泵是存在于细菌或真菌细胞膜上的一类蛋白质,负责将进入细胞的药物转运出细胞外。常见的药物外排泵转运蛋白有ATP能量依赖型的结合转运蛋白(ATP-binding cassette, ABC)和易化载体超家族蛋白(major facilitator superfamily, MFS)。在酵母菌中存在的ABC类型主要为MDR、MRP和PDR,在PDR结合转运蛋白亚家族中成员Cdr1p和Cdr2p是白色念珠菌中多种药物的转运子, 尤其与唑类药物耐药密切相关[20]。在白色念珠菌上CDR1编码外排蛋白是主要的药物外排机制,因为Cdr1p不仅充当各种底物的转运子,还参与细胞膜磷脂双分子层中磷脂酰乙醇胺、磷脂酰丝氨酸与磷脂酰胆碱的转运和分布[20]。目前已有研究证明法尼醇是白色念珠菌药物外排蛋白Cdr1p和Cdr2p的特殊调控子[21]。数据显示在CDR1p过表达的细胞中法尼醇竞争性抑制荧光染料罗达明6G的外排[21]。在敲除了CDR1的白色念珠菌的突变株中法尼醇毒性减少,而在CDR1过表达的菌株中法尼醇的毒性增强[9]。
1.1.2 法尼醇对生物膜厚度和耐药基因的转录水平的调控真菌生物膜是指由真菌自身分泌并包裹在其表面富含多糖的细胞外多聚基质(extra cellular maxtrax, ECM)[22]。这种ECM具有抵抗外来物理化学因素侵害的能力。通常,真菌生物膜越成熟,真菌细胞的耐药性越强[23]。
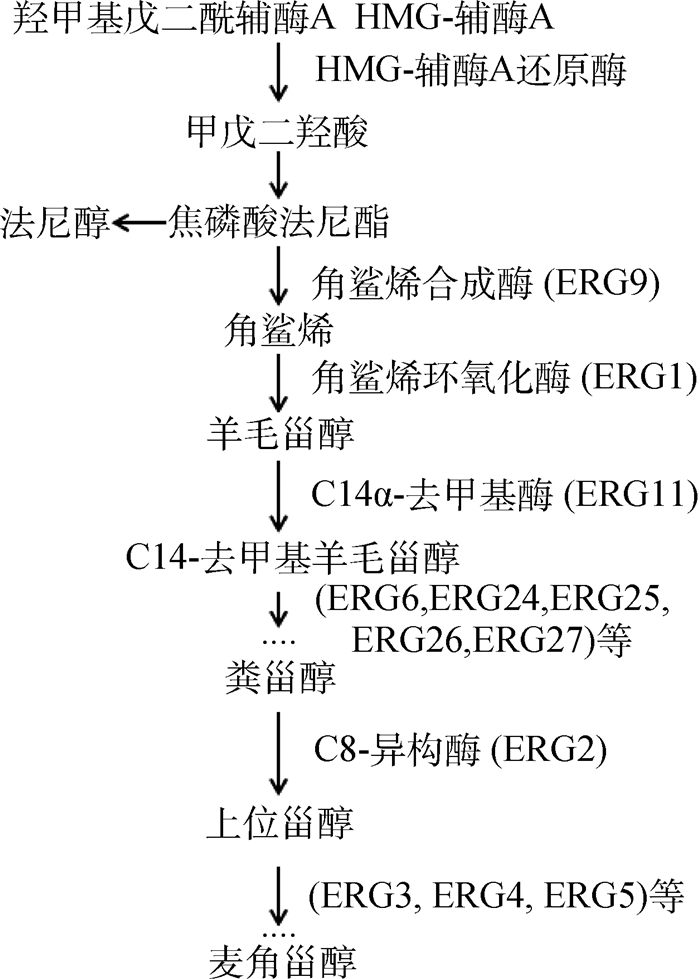
麦角甾醇是真菌细胞膜和细胞壁上的重要组成部分[24],其合成途径中的关键酶是大多数抗真菌药的作用靶点。抗真菌药与靶酶结合后抑制麦角甾醇的合成,使细胞质溢出于胞外, 从而导致真菌细胞死亡[1, 24]。因此,麦角甾醇(ergosterol, ERG)合成途径相关基因(ERG)的表达上调或突变也是导致真菌耐药的原因[1]。ERG系列基因参与麦角甾醇合成示意图见图 2。ERG基因分为必需基因(ERG1、ERG9、ERG7、ERG11、ERG24、ERG25、ERG26和ERG27)和非必需基因[8, 25], 当必需基因所表达的功能被阻断时真菌死亡,而非必需基因表达的功能被阻断时不会影响真菌的正常生长[25]。如烯丙胺类药物的靶酶为麦角甾醇合成途径中由ERG1基因所编码的角鲨烯环氧化酶,该类药物抑制角鲨烯环氧化酶催化角鲨烯为羊毛甾醇,进而抑制麦角甾醇的合成[26];又如唑类药物作用于ERG11基因所编码的C14α-去甲基酶,抑制C14α-去甲基酶催化羊毛甾醇为C14-去甲基羊毛甾醇,进而抑制麦角甾醇合成[26]。当ERG基因上调或突变时,抗真菌药物减弱或完全失去与麦角甾醇合成途径中相关靶酶的亲和性, 从而导致真菌耐药的形成。
|
图 2 麦角甾醇合成途径与ERG基因的关系 Figure 2 The relationship between ergosterol biosynthetic pathway and ERG genes |
有研究证明法尼醇不仅可减少白色念珠菌生物膜的形成,还可使一些ERG基因表达下调[27]。在对数生长期的白色念珠菌中添加生物合成的法尼醇,可以显著降低其生物膜厚度,同时使其麦角甾醇合成途径相关基因ERG11、ERG25、ERG6、ERG3、ERG1、ERG9和ERG20等转录水平降低,从而导致白色念珠菌的黏附力和对氟康唑的耐药性降低[7, 27]。
1.2 群体感应分子法尼醇降低白色念珠菌的毒力 1.2.1 天门冬酰胺蛋白酶基因表达下调天门冬酰胺蛋白酶(secreted aspartyl proteinases, saps)基因主要参与黏膜和系统性感染、黏附力、组织损伤和免疫逃避[28-29]。在对数生长期的白色念珠菌中添加法尼醇,分泌天门冬酰胺蛋白酶的基因saps2、saps4、saps5和saps6均表达下调[30], 导致白色念珠菌的毒力降低。同时还改变细胞壁的超微结构,使细胞壁与细胞之间出现明显的裂痕[30]。因此法尼醇通过下调saps基因的表达,使白色念珠菌的毒力降低。
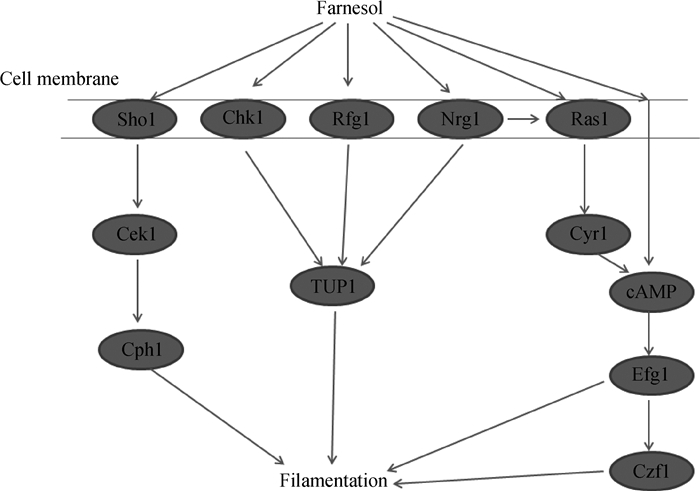
1.2.2 抑制白色念珠菌酵母相向菌丝相的转换白色念珠菌主要存在菌丝相和酵母相两种形态,两者可以互相转换以适应不同环境[31]。这种形态的可塑性成为其毒力因素的一隅[32],而法尼醇反应则促使菌丝相向酵母相转化。因为菌丝相的毒力远远强于酵母相,所以法尼醇通过此反应降低了白色念珠菌的毒力,白色念珠菌由酵母相向菌丝相发展,有以下几条调控通路。法尼醇参与白色念珠菌菌丝形成通路见图 3。
|
图 3 法尼醇影响白色念珠菌菌丝形成的部分通路 Figure 3 Some pathways of farnesol influence C. albicans filamentation |
首先,在通路Ras1-Cyr1p-PKA-Efg1[33]中,起始需要一个短暂存在的转录抑制因子Nrg1激活cAMP-PKA通路,小G蛋白被激活后激活腺苷酸环化酶Cyr1(adenylate cyclase)产生cAMP,cAMP迅速促进PKA调控的转录因子Efg1的激活,在此通路的更下游与Efg1互作并调控转录因子Czf1,二者均为调控菌丝形成的转录因子且共同促进白色念珠菌酵母相向菌丝相的转化[34-35], 同时也调控大量与白色念珠菌毒力相关基因的表达[36]。
有研究证明法尼醇可以直接作用于Cyr1的活性位点而抑制cAMP的产生[37],通过调控cAMP依赖的PKA信号通路来改变白色念珠菌的表型[38-39]。Lu等[40]还发现通路Ras1-Cyr1p-PKA-Efg1的启动需要消除Nrg1的抑制作用,而法尼醇可以直接作用于Nrg1并抑制Nrg1的降解。换言之,法尼醇可抑制Nrg1的降解而阻止菌丝的形成启动,但是不能消除已经存在的菌丝。此外,与cAMP/PKA通路有关的还有组氨酸激酶Chk1,Chk1参与真菌细胞的菌丝形成、细胞壁完整性和细胞分化,且在功能上和RAM (regulation of Ace2 and morphogenesis)通路有所重叠[41]。Saputo等[42]研究发现Chk1的表达需要RAM通路的限速酶Cbk1的存在。Chk1是法尼醇抑制白色念珠菌菌丝形成过程中的重要蛋白质[43-44],有研究发现敲除了Chk1的白色念珠菌不会被法尼醇抑制菌丝形成[43]。这表明Chk1和Cbk1两个基因都对法尼醇抑制菌丝形成起着重要作用,但法尼醇对其的作用机制目前还不甚清楚。
法尼醇与DNA结合蛋白Nrg1和Rfg1关联的TUP1作为一个转录调节因子抑制hypha-specific基因的表达。用法尼醇处理对数生长期白色念珠菌,发现TUP1 mRNA和TUP1蛋白水平有一个小而持续的增加,同时与TUP1调控hypha-specific基因相关的两个基因HWP1 和 RBT1表达有很大的下调[45-46],证明法尼醇可通过促进菌丝抑制基因TUP1的表达抑制菌丝形成。
再者,法尼醇还可通过抑制Cek1的激活抑制白色念珠菌的形态形成和菌丝生长。MAPK也是真核细胞中存在的一种信号转导机制,主要参与调控真菌的化学胁迫、适应环境和增殖等。Cek1是白色念珠菌中存在的四种MAPK之一,它的激活由膜蛋白Sho1所调控,同时也受法尼醇的影响。Cek1-MAPK在白色念珠菌中参与其形态形成和菌丝生长,敲除Cek1可以导致白色念珠菌菌丝形成减少和在小鼠感染模型上毒力的减小[47]。Román等[48]在对数生长期白色念珠菌中添加法尼醇,Western blot分析发现Cek1的活化显著被抑制,而未添加法尼醇的白色念珠菌在达到稳定生长期后活化的Cek1消失。该试验证明了法尼醇是Cek1激活的抑制因子,通过抑制Cek1激活而抑制白色念珠菌酵母相向菌丝相的转化。
最后,法尼醇在白色念珠菌通过调控特定信号和转录组件抑制菌丝生长。其在起始步骤抑制翻译,靶点为真核的启动因子eIF2B(eukaryotic initiation factor 2B)。法尼醇影响mRNA和小核糖体亚基之间的相互作用,影响真菌细胞内的翻译途径而限制真菌细胞生长和菌丝形成[49]。
1.2.3 法尼醇诱导白色念珠菌和白色念珠菌宿主机体细胞的凋亡当法尼醇达到一定浓度时可以引起白色念珠菌的凋亡。有研究证明法尼醇可以在白色念珠菌细胞内与谷胱甘肽结合成为结合态然后被Cdr1p排出细胞外,导致细胞内谷胱甘肽耗尽,细胞氧化应激死亡,同时抗氧化应激的主要酶SOD基因显著表达上调[9]。还有研究证明法尼醇与抗真菌药物协同作用于白色念珠菌时白色念珠菌细胞内活性氧ROS积累,ROS激活半胱天冬酶(细胞凋亡蛋白酶)而导致白色念珠菌的早期凋亡。而这种情况都可以通过添加抗氧化剂来扭转[21, 50]。此外Ras1-cAMP信号通路下游的转录因子Czf1也介导白色念珠菌的凋亡[35]。
法尼醇还可诱导白色念珠菌宿主机体细胞的凋亡。首先,在个体水平的研究表明,法尼醇对机体免疫系统有促炎作用,可通过影响先天免疫细胞而影响疾病的发展进程[51-53]。Navarathna等[54-55]通过口服及腹腔注射的方式给予法尼醇到人工感染白色念珠菌的小鼠体内,明显提高了小鼠的死亡率,并且使小鼠干扰素IFN-γ和白细胞介素IL-12的产生受到抑制,巨噬细胞产生的IL-12 p40、p70减少,Th2细胞产生的IL-5增加。其次,在细胞水平的研究表明,法尼醇诱导了白色念珠菌宿主机体内巨噬细胞的氧化应激反应[53]。此外,在分子水平的研究表明,法尼醇可使白色念珠菌宿主机体细胞蛋白显著改变。其中参与新陈代谢、糖酵解、蛋白质合成、线粒体电子传递和呼吸链的蛋白表达明显下调[11];而参与蛋白质折叠、氧化应激、肌动蛋白细胞骨架重组和细胞凋亡的蛋白表达明显上调[11, 56]。Scheper等[57]用人口腔鳞状细胞癌细胞验证了法尼醇与哺乳动物细胞凋亡呈正相关,且凋亡途径仍然为机体细胞内大量活性氧的积累,活性氧激活半胱天冬酶而使半胱天冬酶的表达上调。这也印证了法尼醇是通过活性氧的大量产生而导致哺乳动物细胞的凋亡[53]。
综上所述,法尼醇是使白色念珠菌细胞或白色念珠菌宿主细胞产生大量的活性氧而造成二者的早期凋亡的原因。
2 白色念珠菌对法尼醇的耐受性法尼醇在20~50 μmol·L-1浓度时就可以对其他真菌产生作用,表现为完全的生长抑制或促发菌体凋亡[58]。如25 μmol·L-1的法尼醇就可完全抑制酿酒酵母的生长[9];300 μmol·L-1的法尼醇可诱导禾谷镰刀菌(Fusarium graminearum)凋亡等[59]。而在30 μmol·L-1时只能轻微下调白色念珠菌的ERG9而上调ERG20和ERG11基因,在200 μmol·L-1时明显下调ERG9、ERG20和ERG11基因[60-61], 在300 μmol·L-1时也仅仅只能导致白色念珠菌ERG基因的下调和生物膜形成减少而不能导致白色念珠菌的凋亡[27],这表明白色念珠菌对法尼醇的耐受力明显强于其他真菌。
目前知晓的白色念珠菌对法尼醇耐受的几种原因如下。首先,白色念珠菌对法尼醇的耐受性与白色念珠菌的磷脂成分的变化相关。有研究发现敲除合成磷脂酰丝氨酸和磷脂酰乙醇胺的酶的基因可以提高白色念珠菌对法尼醇的耐受性[62]。其次,与白色念珠菌基因EED1相关,EED1基因对菌丝的延展和维持至关重要,敲除EED1基因可导致白色念珠菌对法尼醇敏感性显著增加[58, 63-64]。再者,白色念珠菌的细胞膜上可能存在法尼醇转运子将法尼醇转运出细胞外,虽然这种转运机制目前还不清楚,但这可能是白色念珠菌对法尼醇表现非凡耐受的关键原因。目前已有研究证明白色念珠菌细胞内的法尼醇浓度可低于细胞外[63],这表明白色念珠菌对法尼醇的耐受是依赖能量的[65],白色念珠菌细胞膜上可能存在法尼醇受体和转运蛋白[66]。综上,也许可以解释为什么白色念珠菌对法尼醇表现明显的耐受,为白色念珠菌群体感应的研究打开了新的大门。
3 小结与展望白色念珠菌与其群体感应分子法尼醇的相互作用,主要表现为法尼醇对白色念珠菌麦角甾醇合成途径相关基因ERG的表达调控和对白色念珠菌生物膜形成的抑制,降低白色念珠菌的耐药性;表现为法尼醇抑制白色念珠菌酵母相向菌丝相形态的转化,降低白色念珠菌的毒力;也表现为白色念珠菌对法尼醇的高耐受。正是因为法尼醇在白色念珠菌的这些作用,证明了法尼醇是潜在的抗真菌分子。所以,将法尼醇作为抗真菌药物进行开发具有十分广阔的前景。在体外的研究表明法尼醇协同酪醇、氟康唑对白色念珠菌有累加的抗菌活性[61, 67]。虽然在小鼠体内的研究表明法尼醇与氟康唑联用对于氟康唑敏感的白色念珠菌治疗无增效作用,但是其联用对于氟康唑耐药的白色念珠菌治疗有明显的增效作用[68],说明法尼醇在小鼠体内也可明显降低白色念珠菌的耐药性。目前,法尼醇在造价上并不高昂,不论是用于科研还是用于药物开发,都是非常合适的原材料,值得人们对它的作用和临床疗效进行深度的挖掘。
| [1] |
王彬, 魏曼, 方华, 等. 念珠菌耐药机制研究新进展[J]. 中国病原生物学杂志, 2014, 9(5): 473–477.
WANG B, WEI M, FANG H, et al. Advances in the study of the mechanism of Candida's drug resistance[J]. Journal of Pathogen Biology, 2014, 9(5): 473–477. (in Chinese) |
| [2] | LATIFI A, WINSON M K, FOGLINO M, et al. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1[J]. Mol Microbiol, 1995, 17(2): 333–343. DOI: 10.1111/mmi.1995.17.issue-2 |
| [3] | CHEN H, FUJITA M, FENG Q H, et al. Tyrosol is a quorum-sensing molecule in Candida albicans[J]. Proc Natl Acad Sci U S A, 2004, 101(14): 5048–5052. DOI: 10.1073/pnas.0401416101 |
| [4] | HORNBY J M, JENSEN E C, LISEC A D, et al. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol[J]. Appl Environ Microbiol, 2001, 67(7): 2982–2992. DOI: 10.1128/AEM.67.7.2982-2992.2001 |
| [5] | CHEN H, FINK G R. Feedback control of morphogenesis in fungi by aromatic alcohols[J]. Gene Dev, 2006, 20(9): 1150–1161. DOI: 10.1101/gad.1411806 |
| [6] | AFFELDT K J, BRODHAGEN M, KELLER N P. Aspergillus oxylipin signaling and quorum sensing pathways depend on G protein-coupled receptors[J]. Toxins (Basel), 2012, 4(9): 695–717. DOI: 10.3390/toxins4090695 |
| [7] | YU L H, WEI X, MA M, et al. Possible inhibitory molecular mechanism of farnesol on the development of fluconazole resistance in Candida albicans biofilm[J]. Antimicrob Agents Chemother, 2012, 56(2): 770–775. DOI: 10.1128/AAC.05290-11 |
| [8] |
曹龙辉, 李晓珺, 赵文红, 等. 麦角甾醇的研究进展[J]. 中国酿造, 2014, 33(4): 9–12.
CAO L H, LI X J, ZHAO W H, et al. Research progress on ergosterol[J]. China Brewing, 2014, 33(4): 9–12. DOI: 10.3969/j.issn.0254-5071.2014.04.003 (in Chinese) |
| [9] | ZHU J S, KROM B P, SANGLARD D, et al. Farnesol-induced apoptosis in Candida albicans is mediated by cdr1-p extrusion and depletion of intracellular glutathione[J]. PLoS One, 2011, 6(12): e28830. DOI: 10.1371/journal.pone.0028830 |
| [10] | DICHTL K, EBEL F, DIRR F, et al. Farnesol misplaces tip-localized Rho proteins and inhibits cell wall integrity signalling in Aspergillus fumigatus[J]. Mol Microbiol, 2010, 76(5): 1191–1204. DOI: 10.1111/mmi.2010.76.issue-5 |
| [11] | SHIRTLIFF M E, KROM B P, MEIJERING R A M, et al. farnesol-induced apoptosis in Candida albicans[J]. Antimicrob Agents Chemother, 2009, 53(6): 2392–2401. DOI: 10.1128/AAC.01551-08 |
| [12] | BRASCH J, HORTER F, FRITSCH D, et al. Acyclic sesquiterpenes released by Candida albicans inhibit growth of dermatophytes[J]. Med Mycol, 2014, 52(1): 46–55. |
| [13] | WANG K F, SUI K Y, GUO C, et al. Quorum sensing molecule-farnesol increased the production and biological activities of extracellular polysaccharide from Trametes versicolor[J]. Int J Biol Macromol, 2017, 104: 377–383. DOI: 10.1016/j.ijbiomac.2017.06.053 |
| [14] | DIXON E F, HALL R A. Noisy neighbourhoods: quorum sensing in fungal-polymicrobial infections[J]. Cell Microbiol, 2015, 17(10): 1431–1441. DOI: 10.1111/cmi.12490 |
| [15] | CUGINI C, CALFEE M W, FARROW III J M, et al. Farnesol, a common sesquiterpene, inhibits PQS production in Pseudomonas aeruginosa[J]. Mol Microbiol, 2007, 65(4): 896–906. DOI: 10.1111/mmi.2007.65.issue-4 |
| [16] | BANDARA H M H N, HERPIN M J, KOLACNY D Jr, et al. Incorporation of farnesol significantly increases the efficacy of liposomal ciprofloxacin against Pseudomonas aeruginosa biofilms in vitro[J]. Mol Pharmaceutics, 2016, 13(8): 2760–2770. DOI: 10.1021/acs.molpharmaceut.6b00360 |
| [17] | BREHM-STECHER B F, JOHNSON E A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone[J]. Antimicrob Agents Chemother, 2003, 47(10): 3357–3360. DOI: 10.1128/AAC.47.10.3357-3360.2003 |
| [18] | GOMES F, CERCA N, AZEREDO J, et al. Farnesol as antibiotics adjuvant in Staphylococcus epidermidis control in vitro[J]. Am J Med Sci, 2011, 341(3): 191–195. DOI: 10.1097/MAJ.0b013e3181fcf138 |
| [19] | CASTELO-BRANCO D S C M, RIELLO G B, VASCONCELOS D C, et al. Farnesol increases the susceptibility of Burkholderia pseudomallei biofilm to antimicrobials used to treat melioidosis[J]. J Appl Microbiol, 2016, 120(3): 600–606. DOI: 10.1111/jam.2016.120.issue-3 |
| [20] | WHITE T C, MARR K A, BOWDEN R A, et al. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance[J]. Clin Microbiol Rev, 1998, 11(2): 382–402. DOI: 10.1128/CMR.11.2.382 |
| [21] | SHARMA M, PRASAD R. The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans[J]. Antimicrob Agents Chemother, 2011, 55(10): 4834–4843. DOI: 10.1128/AAC.00344-11 |
| [22] | RAMAGE G, ROBERTSON S N, WILLIAMS C, et al. Strength in numbers:antifungal strategies against fungal biofilms[J]. Int J Antimicrob Agents, 2014, 43(2): 114–120. DOI: 10.1016/j.ijantimicag.2013.10.023 |
| [23] |
叶陈伟. 抗真菌药物耐药机制的研究进展综述[J]. 世界最新医学信息文摘, 2016, 16(68): 19–20.
YE C W. Research progress on the mechanisms of antifungal drug resistance[J]. World Latest Medicine Information, 2016, 16(68): 19–20. (in Chinese) |
| [24] |
张坚磊. 深部真菌耐药性的研究进展[J]. 国外医学临床生物化学与检验学分册, 2005, 26(9): 609–611, 615.
ZHANG J L. Advances in studies on drug-resistance of fungi[J]. Clinical Biochemistry and Laboratory Medicine, Foreign Medical Sciences, 2005, 26(9): 609–611, 615. (in Chinese) |
| [25] |
林晓珊, 江宏文, 张毅. 酵母麦角固醇生物合成及其基因调控的研究[J]. 生物学杂志, 2010, 27(6): 83–86.
LIN X S, JIANG H W, ZHANG Y. Study on biosynthesis of ergosterol in yeast and its gene regulation[J]. Journal of Biology, 2010, 27(6): 83–86. DOI: 10.3969/j.issn.1008-9632.2010.06.083 (in Chinese) |
| [26] |
叶丽娟, 王辂, 朱辉. 抗真菌药物作用机制及真菌耐药机制的研究进展[J]. 国外医药抗生素分册, 2006, 27(5): 221–227.
YE L J, WANG L, ZHU H. Research progress on antifungal mechanism and fungal resistance mechanism[J]. World Notes on Antibiotics, 2006, 27(5): 221–227. DOI: 10.3969/j.issn.1001-8751.2006.05.006 (in Chinese) |
| [27] | FERNANDES R A, MONTEIRO D R, ARIAS L S, et al. Biofilm formation by Candida albicans and Streptococcus mutans in the presence of farnesol:a quantitative evaluation[J]. J Bioadhesion Biofilm Res, 2016, 32(3): 329–338. DOI: 10.1080/08927014.2016.1144053 |
| [28] | SCHALLER M, BORELLI C, KORTING H C, et al. Hydrolytic enzymes as virulence factors of Candida albicans[J]. Mycoses, 2005, 48(6): 365–377. DOI: 10.1111/myc.2005.48.issue-6 |
| [29] | NAGLIK J R, CHALLACOMBE S J, HUBE B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis[J]. Microbiol Mol Biol Rev, 2003, 67(3): 400–428. DOI: 10.1128/MMBR.67.3.400-428.2003 |
| [30] | DÉCANIS N, TAZI N, CORREIA A, et al. Farnesol, a fungal quorum-sensing molecule triggers Candida albicans morphological changes by downregulating the expression of different secreted aspartyl proteinase genes[J]. Open Microbiol J, 2011, 5(1): 119–126. DOI: 10.2174/1874285801105010119 |
| [31] | BISWAS S, VAN DIJCK P, DATTA A, et al. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans[J]. Microbiol Mol Biol Rev, 2007, 71(2): 348–376. DOI: 10.1128/MMBR.00009-06 |
| [32] | NOBLE S M, FRENCH S, KOHN L A, et al. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity[J]. Nat Genet, 2010, 42(7): 590–598. DOI: 10.1038/ng.605 |
| [33] | ROCHA C R, SCHRÖPPEL K, HARCUS D, et al. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans[J]. Mol Biol Cell, 2001, 12(11): 3631–3643. DOI: 10.1091/mbc.12.11.3631 |
| [34] | FANG H M, WANG Y. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development[J]. Mol Microbiol, 2006, 61(2): 484–496. DOI: 10.1111/mmi.2006.61.issue-2 |
| [35] | LANGFORD M L, HARGARTEN J C, PATEFIELD K D, et al. Candida albicans Czf1 and Efg1 coordinate the response to farnesol during quorum sensing, white-opaque thermal dimorphism, and cell death[J]. Eukaryot Cell, 2013, 12(9): 1281–1292. DOI: 10.1128/EC.00311-12 |
| [36] | KUMAMOTO C A, VINCES M D. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence[J]. Cell Microbiol, 2005, 7(11): 1546–1554. DOI: 10.1111/j.1462-5822.2005.00616.x |
| [37] | HALL R A, TURNER K J, CHALOUPKA J, et al. The quorum-sensing molecules farnesol/homoserine lactone and dodecanol operate via distinct modes of action in Candida albicans[J]. Eukaryot Cell, 2011, 10(8): 1034–1042. DOI: 10.1128/EC.05060-11 |
| [38] | DAVIS-HANNA A, PIISPANEN A E, STATEVA L I, et al. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis[J]. Mol Microbiol, 2008, 67(1): 47–62. |
| [39] | LINDSAY A K, DEVEAU A, PIISPANEN A E, et al. Farnesol and cyclic AMP signaling effects on the hypha-to-yeast transition in Candida albicans[J]. Eukaryot Cell, 2012, 11(10): 1219–1225. DOI: 10.1128/EC.00144-12 |
| [40] | LU Y, SU C, WANG A, et al. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance[J]. PLoS Biology, 2011, 9(7). |
| [41] | LI D M, WILLIAMS D, LOWMAN D, et al. The Candida albicans histidine kinase Chk1p:signaling and cell wall mannan[J]. Fungal Genet Biol, 2009, 46(10): 731–741. DOI: 10.1016/j.fgb.2009.06.008 |
| [42] | SAPUTO S, NORMAN K L, MURANTE T, et al. Complex haploinsufficiency-based genetic analysis of the NDR/lats kinase Cbk1 provides insight into its multiple functions in Candida albicans[J]. Genetics, 2016, 203(3): 1217–1233. DOI: 10.1534/genetics.116.188029 |
| [43] | KRUPPA M, KROM B P, CHAUHAN N, et al. The two-component signal transduction protein Chk1P regulates quorum sensing in Candida albicans[J]. Eukaryot Cell, 2004, 3(4): 1062–1065. DOI: 10.1128/EC.3.4.1062-1065.2004 |
| [44] | MAKSIMOV V, WÄNESKOG M, RODRIGUEZ A, et al. Stress sensitivity of a fission yeast strain lacking histidine kinases is rescued by the ectopic expression of Chk1 from Candida albicans[J]. Curr Genet, 2017, 63(2): 343–357. DOI: 10.1007/s00294-016-0644-9 |
| [45] | KEBAARA B W, LANGFORD M L, NAVARATHNA D H M L P, et al. Candida albicans tup1 is involved in farnesol-mediated inhibition of filamentous-growth induction[J]. Eukaryot Cell, 2008, 7(6): 980–987. DOI: 10.1128/EC.00357-07 |
| [46] | DE GROOT P W J, HELLINGWERF K J, KLIS F M. Genome-wide identification of fungal GPI proteins[J]. Yeast, 2003, 20(9): 781–796. DOI: 10.1002/(ISSN)1097-0061 |
| [47] | WHITEWAY M. Transcriptional control of cell type and morphogenesis in Candida albicans[J]. Curr Opin Microbiol, 2000, 3(6): 582–588. DOI: 10.1016/S1369-5274(00)00144-2 |
| [48] | ROMÁN E, ALONSO-MONGE R, GONG Q H, et al. The Cek1 MAPK is a short-lived protein regulated by quorum sensing in the fungal pathogen Candida albicans[J]. FEMS Yeast Res, 2009, 9(6): 942–955. DOI: 10.1111/fyr.2009.9.issue-6 |
| [49] | EGBE N E, DORNELLES T O, PAGET C M, et al. Farnesol inhibits translation to limit growth and filamentation in C. albicans and S. cerevisiae[J]. Microb Cell, 2017, 4(9): 294–304. DOI: 10.15698/mic |
| [50] | DIŽOVÁ S, BUJDÁKOVÁ H. Properties and role of the quorum sensing molecule farnesol in relation to the yeast Candida albicans[J]. Pharmazie, 2017, 72(6): 307–312. |
| [51] | LEONHARDT I, SPIELBERG S, WEBER M, et al. The fungal quorum-sensing molecule farnesol activates innate immune cells but suppresses cellular adaptive immunity[J]. mBio, 2015, 6(2): e00143. |
| [52] | GHOSH S, HOWE N, VOLK K, et al. Candida albicans cell wall components and farnesol stimulate the expression of both inflammatory and regulatory cytokines in the murine RAW264. 7 macrophage cell line[J]. FEMS Immunol Med Microbiol, 2010, 60(1): 63–73. DOI: 10.1111/fim.2010.60.issue-1 |
| [53] | ABE S, TSUNASHIMA R, IIJIMA R, et al. Suppression of anti-Candida activity of macrophages by a quorum-sensing molecule, farnesol, through induction of oxidative stress[J]. Microbiol Immunol, 2009, 53(6): 323–330. DOI: 10.1111/mim.2009.53.issue-6 |
| [54] | NAVARATHNA D H M L P, HORNBY J M, KRISHNAN N, et al. Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans[J]. Infect Immun, 2007, 75(4): 1609–1618. DOI: 10.1128/IAI.01182-06 |
| [55] | NAVARATHNA D H M L P, NICKERSON K W, DUHAMEL G E, et al. Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model[J]. Infect Immune, 2007, 75(8): 4006–4011. DOI: 10.1128/IAI.00397-07 |
| [56] | CAO Y Y, CAO Y B, XU Z, et al. cDNA microarray analysis of differential gene expression in Candida albicans biofilm exposed to farnesol[J]. Antimicrob Agents Chemother, 2005, 49(2): 584–589. DOI: 10.1128/AAC.49.2.584-589.2005 |
| [57] | SCHEPER M A, SHIRTLIFF M E, MEILLER T F, et al. Farnesol, a fungal quorum-sensing molecule triggers apoptosis in human oral squamous carcinoma cells[J]. Neoplasia, 2008, 10(9): 954–963. DOI: 10.1593/neo.08444 |
| [58] | MARTIN R, MORAN G P, JACOBSEN I, et al. The Candida albicans-specific gene EED1 encodes a key regulator of hyphal extension[J]. PLoS One, 2011, 6(4): e18394. DOI: 10.1371/journal.pone.0018394 |
| [59] | SEMIGHINI C P, MURRAY N, HARRIS S D. Inhibition of Fusarium graminearum growth and development by farnesol[J]. FEMS Microbiol Lett, 2008, 279(2): 259–264. DOI: 10.1111/fml.2008.279.issue-2 |
| [60] | POLKE M, LEONHARDT I, KURZAI O, et al. Farnesol signalling in Candida albicans-more than just communication[J]. Crit Rev Microbiol, 2018, 44(2): 230–243. DOI: 10.1080/1040841X.2017.1337711 |
| [61] | DIŽOVÁ S, ČERNÁKOVÁ L, BUJDÁKOVÁ H, et al. The impact of farnesol in combination with fluconazole on Candida albicans biofilm:regulation of ERG20, ERG9, and ERG11 genes[J]. Folia Microbiol, 2018, 63(3): 363–371. DOI: 10.1007/s12223-017-0574-z |
| [62] | HASIM S, VAUGHN E N, DONOHOE D, et al. Influence of phosphatidylserine and phosphatidylethanolamine on farnesol tolerance in Candida albicans[J]. Yeast, 2018, 35(4): 343–351. DOI: 10.1002/yea.3297 |
| [63] | POLKE M, SPRENGER M, SCHERLACH K, et al. A functional link between hyphal maintenance and quorum sensing in Candida albicans[J]. Mol Microbiol, 2017, 103(4): 595–617. DOI: 10.1111/mmi.2017.103.issue-4 |
| [64] | POLKE M, JACOBSEN I D. Quorum sensing by farnesol revisited[J]. Curr Genet, 2017, 63(5): 791–797. DOI: 10.1007/s00294-017-0683-x |
| [65] | LANGFORD M L, HASIM S, NICKERSON K W, et al. Activity and toxicity of farnesol towards Candida albicans are dependent on growth conditions[J]. Antimicrob Agents Chemother, 2010, 54(2): 940–942. DOI: 10.1128/AAC.01214-09 |
| [66] | NICKERSON K W, ATKIN A L. Deciphering fungal dimorphism:farnesol's unanswered questions[J]. Mol Microbiol, 2017, 103(4): 567–575. DOI: 10.1111/mmi.2017.103.issue-4 |
| [67] | MONTEIRO D R, ARIAS L S, FERNANDES R A, et al. Antifungal activity of tyrosol and farnesol used in combination against Candida species in the planktonic state or forming biofilms[J]. J Appl Microbiol, 2017, 123(2): 392–400. DOI: 10.1111/jam.2017.123.issue-2 |
| [68] | BOZÓ A, DOMÁN D, MAJOROS L, et al. The in vitro and in vivo efficacy of fluconazole in combination with farnesol against Candida albicans isolates using a murine vulvovaginitis model[J]. J Microbiol, 2016, 54(11): 753–760. DOI: 10.1007/s12275-016-6298-y |



