大肠湿热证系指湿热蕴结大肠,下焦气机壅滞,致使大肠传导失职,所表现出来的一系列以泄泻下痢为主的证候。在兽医临床中常见多发,给畜牧业经济造成一定损失。郁金散为治疗大肠湿热证的经典中兽药方剂,具有涩肠止泻、利湿导滞、清热解毒、调血行气、止痛等功效[1]。
有研究表明,湿热证本质与机体免疫、氧化与抗氧化等功能密切相关[2]。湿热证是内外湿邪共同作用引起的急性外感热病,湿热病邪侵犯机体与正气相争,出现“湿阻”“热蕴”的病理变化,这与现代医学中病原微生物感染引起机体免疫功能紊乱的机制相似[3]。湿热证的外感病邪与病毒、细菌等的感染有直接关系,病原微生物感染则会引起机体氧化应激现象[4]。机体抗氧化系统与免疫系统密不可分,免疫细胞处于氧化与抗氧化平衡中维持细胞正常功能。大肠湿热证会使机体处于氧化应激状态,过量ROS会阻滞Ca2+内流,导致淋巴细胞不能正常进行有丝分裂,最终导致淋巴细胞出现凋亡;同时过量ROS氧化血清白蛋白,进而诱导自身抗体合成[5],从而加剧免疫功能的紊乱。
本课题组以前的研究表明:郁金散可通过抗腹泻、抗炎、降血脂、缓解血瘀、修复肠黏膜以及调节和保护多脏器等多方面的综合作用治疗大肠湿热证[6],另外可通过调控机体胃肠激素水平而调节胃肠道运动功能[7]。因此,本研究在此基础上,通过各组大鼠血清免疫球蛋白的检测及相关免疫器官的系统观察,以及分析血清及肝抗氧化因子的变化,以从体液免疫、抗氧化功能等方面阐明郁金散对大肠湿热证的治疗作用机制,为开发治疗大肠湿热证型腹泻药物奠定基础。
1 材料与方法 1.1 主要仪器和试剂旋转蒸发仪(上海亚荣仪器厂),酶标仪(美国BIO-RAD公司),切片机(Leica公司),OlympusDP-71显微照相系统,白酒(北京红星二锅头,56°),IgA、IgG、IgM、一氧化氮(NO)、脂质过氧化物(LPO)、丙二醛(MDA)、谷胱甘肽(GSH)试剂盒(北京奇松生物科技有限公司)。
1.2 大肠杆菌悬液制备菌种为产毒性大肠杆菌O101,购于中国兽医药品监察所,菌号CVCC231。菌株保存在-80 ℃条件下备用。临用前室温下解冻,接种至营养肉汤中37 ℃培养18 h,然后接种于营养琼脂培养基培养24 h,挑取单菌落于营养肉汤中37 ℃振荡培养至所需浓度,置4 ℃下保存备用。
1.3 郁金散制备根据《中兽医学》配方组成[1]:郁金30 g、白芍15 g、黄芩30 g、诃子15 g、黄柏30 g、黄连30 g、大黄60 g、栀子30 g,进行郁金散制备:按比例称取药物于10倍水中浸泡30 min,武火煮沸后改用文火煎煮30 min,4层纱布过滤;药渣再以6倍水重复煎煮30 min;合并两次滤液,减压浓缩,定容至1.04 g·mL-1,置4 ℃下保存备用。依据《中国兽药典》计算大鼠临床等效给药剂量为0.52 g·mL-1,设置郁金散高、中、低剂量分别为10.4、5.2、2.6 g·kg-1。以上中药均购自甘肃西域阳光大药房,经甘肃农业大学魏彦明教授鉴定。
1.4 动物分组及造模60只体重180~220 g的Wistar大鼠,清洁级,雌雄各半,购于兰州大学医学院实验动物中心,动物生产合格证号:SCXK(甘)2013-0002。自然环境,常规饲养,适应环境1周后,随机分6组,每组10只:分别为正常对照组、模型组、自愈组、郁金散高、中、低剂量组。按照实验室建立的方法[6-7]进行大肠湿热证模型复制,分3个阶段,(1)高糖高脂阶段:模型组,自愈组,郁金散高、中、低剂量组大鼠造模全程自由饮用300 g·L-1蜂蜜水,单日禁食,双日饲喂充足饲料并灌服猪油2 mL·100 g-1,共10 d;(2)高温高湿阶段:除对照组大鼠,其余大鼠每天灌喂56°白酒(北京红星二锅头)1 mL·100 g-1,置于高温仓(温度31~35 ℃,相对湿度93%±2%)8 h·d-1,共5 d;(3)攻毒阶段:经高温高湿阶段的大鼠腹腔注射浓度为1.69×109 CFU·mL-1的产毒性大肠杆菌菌液0.2 mL·100 g-1,24 h后,再以0.1 mL·100 g-1的剂量注射一次,自然环境饲养1 d,模型建立成功。造模完成后,处死模型组大鼠取样。郁金散高、中、低剂量组分别以10.4、5.2、2.6 g·(kg·d)-1剂量灌胃方药,共5 d;自愈组灌服等量生理盐水。试验期间,正常对照组处于自然环境,喂普通饲料,灌胃、腹腔注射等量生理盐水。试验结束后处死所有大鼠取样。
1.5 样本采集大鼠处死前禁食12 h,腹腔注射1%的戊巴比妥钠进行麻醉(30 mg·kg-1),腹主动脉采血收集于真空采血管中,室温静置2~4 h,3 000 r·min-1离心10 min,取上清液,-20 ℃保存。采血完毕,迅速剖取脾、胸腺,用生理盐水洗净,放入10%的福尔马林(即4%甲醛)溶液中固定,用来制作病理切片。迅速剖取肝,用预冷生理盐水洗净,称取0.5 g左右,加生理盐水1:9进行匀浆,4 ℃ 3 500 r·min-1离心15 min,再以2 500 r·min-1离心10 min,分别吸取上清液,-20 ℃保存备用。
1.6 血清免疫球蛋白的检测按ELISA试剂盒说明书方法检测各组血清免疫球蛋白IgA、IgG、IgM水平。
1.7 病理组织切片观察将脾和胸腺组织用10%福尔马林溶液固定15 d,漂洗组织中的固定液,梯度乙醇溶液脱水,二甲苯透明,进行石蜡包埋,切片(4 μm),常规HE染色,用显微照相系统观察并采集照片。
1.8 血清及肝抗氧化指标的检测按ELISA试剂盒说明书方法检测血清及肝组织匀浆上清液LPO、MDA、GSH、NO水平。
1.9 数据统计数据均用“平均数±标准差(x±s)”表示,采用SPSS22.0以单因素方差分析(ANOV)法进行统计分析,P < 0.01为差异极显著,P<0.05为差异显著。
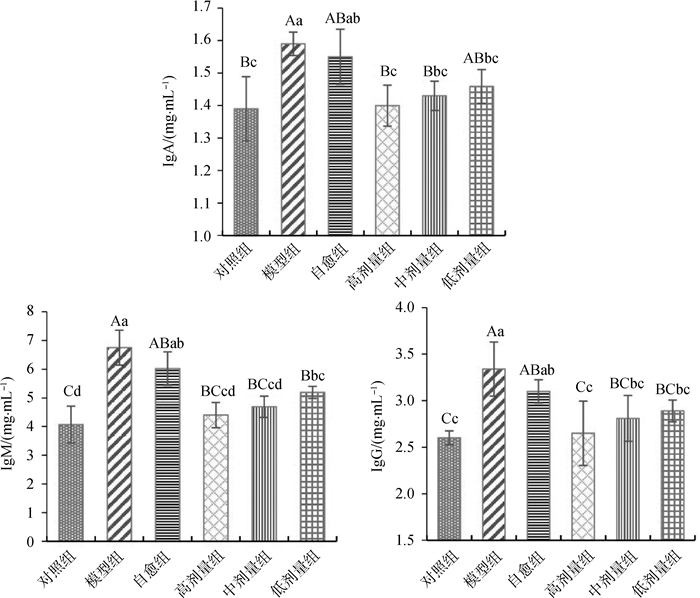
2 结果 2.1 血清免疫球蛋白含量变化由图 1可知:与正常对照组相比,模型组中IgA、IgM和IgG含量极显著升高(P < 0.01);自愈组中IgA含量显著升高(P < 0.05),IgM和IgG含量极显著升高(P < 0.01)。与自愈组相比,郁金散高剂量组中IgA和IgM的含量显著降低(P < 0.05),IgG含量极显著降低(P < 0.01);郁金散中剂量组中IgM含量显著降低(P < 0.05),IgA和IgG的含量降低,但差异不显著(P > 0.05)。结果表明,大肠湿热证模型大鼠血清IgA、IgM和IgG含量升高,郁金散治疗后其含量下降,其中高剂量治疗效果最好。
|
标有不同字母表示差异极显著(大写字母,P < 0.01)或差异显著(小写字母,P < 0.05),标有相同字母表示差异不显著(P > 0.05) Values with different capital letter superscripts mean significant difference (P < 0.01), and with different small letter superscripts mean significant difference (P < 0.05), with the same letter superscripts mean no significant difference (P > 0.05) 图 1 血清中IgA、IgM和IgG的含量变化 Figure 1 Change of content of IgA, IgM and IgG in serum |
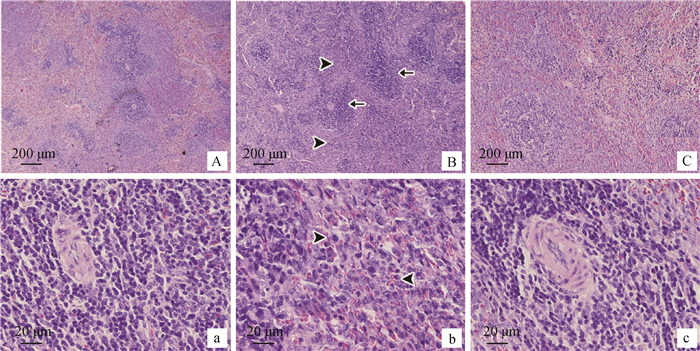
显微镜下观察,正常对照组,脾红、白髓分界清楚,组织形态正常,动脉周围淋巴鞘无异常变化,而红髓中可见含铁血黄素的巨噬细胞,表明脾免疫功能正常(图 2A、a)。大肠湿热证模型组,红、白髓界限模糊,白髓比例上升;红细胞数目增多,没有含铁血黄素的巨噬细胞;白髓中淋巴细胞数目减少,可见坏死的淋巴细胞;红髓中有大量淋巴细胞弥漫性增生和浸润(图 2B、b)。治疗组白髓与红髓分界比较清楚;未见含铁血黄素的巨噬细胞,但形态正常;动脉周围淋巴鞘的形态较正常组变化不明显,而淋巴细胞数目相较于正常对照组少(图 2C、c)。
|
A、a.正常对照组;B、b.大肠湿热证模型组;C、c.郁金散高剂量治疗组。箭头表示白髓;三角箭头表示红髓 A, a. Normal control group; B, b. LIDHS model group; C, c.High dose of Yujin Powder groups. The arrows indicate white pulps; The triangular arrow indicate red pulps 图 2 脾病理组织学观察(HE染色) Figure 2 Spleen histopathological observation (HE staining) |
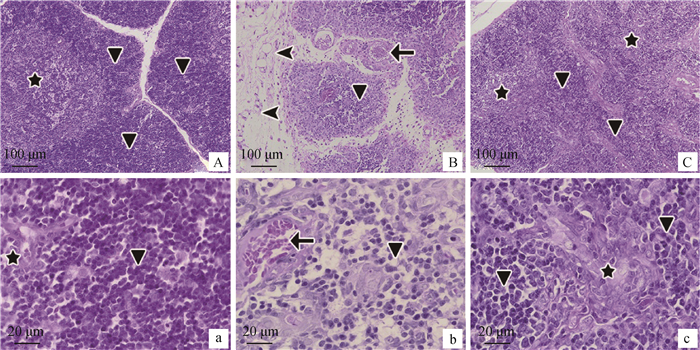
显微镜观察发现,正常对照组,皮质区淋巴细胞密集(图 3a),髓质区淋巴细胞稀松(图 3A),形态完整,结构正常。大肠湿热证模型组,未被脂肪代替的胸腺组织,皮质、髓质交界模糊,淋巴细胞数量减少,小叶内皮质网状细胞增生,胸腺小体数量减少,小血管充血,皮质部被脂肪代替的胸腺组织,可以看到有大量的脂肪细胞(图 3B、b)。郁金散高剂量治疗组,皮质区淋巴细胞密集,被膜较厚(图 3C),髓质区淋巴细胞少而稀疏,形态完整,结构趋于正常(图 3c)。
|
A、a.正常对照组;B、b.大肠湿热证模型组;C、c.郁金散高剂量治疗组。五角星代表髓质;三角形表示皮质;箭头指充血血管;三角箭头指脂肪细胞 A, a. Normal control group; B, b. LIDHS model group; C, c.High dose of Yujin Powder groups. The pentagram represents the medulla and the triangle represents the cortex; Arrows indicate vascular congestion; The triangular arrow refers to fat cells 图 3 胸腺病理组织学观察(HE染色) Figure 3 Thymus histopathological observation (HE staining) |
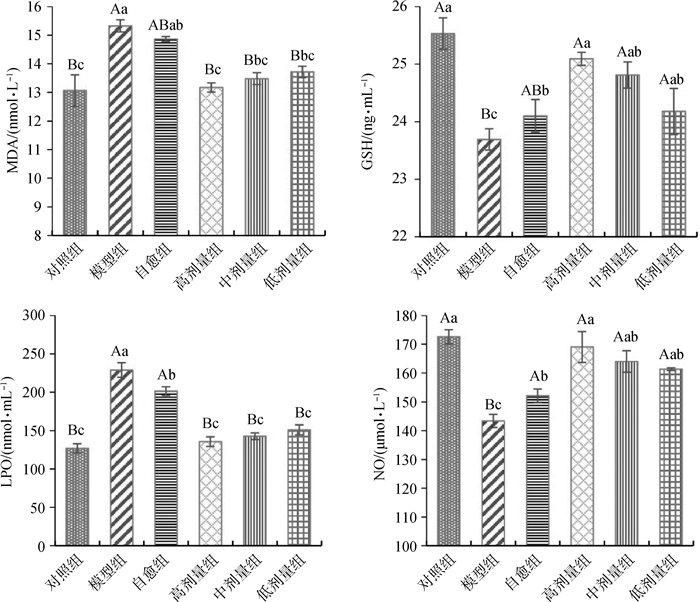
由图 4可知:与正常对照组相比,模型组MDA、LPO含量极显著升高(P < 0.01),GSH和NO含量极显著降低(P < 0.01);自愈组MDA含量显著升高(P < 0.05),LPO含量极显著升高(P < 0.01),GSH和NO含量显著降低(P < 0.05)。与自愈组相比,高剂量组MDA显著降低(P < 0.05),LPO含量极显著降低(P < 0.01),GSH和NO含量显著升高(P < 0.05);中剂量组LPO含量极显著降低(P < 0.01),MDA的含量降低,但差异不显著(P > 0.05),GSH和NO含量升高,但差异不显著(P > 0.05);低剂量组LPO含量极显著降低(P < 0.01),MDA含量降低但差异不显著(P > 0.05),GSH和NO含量升高但差异不显著(P > 0.05)。说明郁金散能够降低大肠湿热证机体血清中的MDA和LPO,升高血清中GSH和NO,且高剂量郁金散的效果最好。
|
标有不同字母表示差异极显著(大写字母,P < 0.01)或差异显著(小写字母,P < 0.05),标有相同字母表示差异不显著(P > 0.05) Values with different capital letter superscripts mean significant difference (P < 0.01), and with different small letter superscripts mean significant difference (P < 0.05), with the same letter superscripts mean no significant difference (P > 0.05) 图 4 血清中MDA、GSH、LPO、NO的含量 Figure 4 Content of MDA, GSH, LPO, NO in serum |
由图 5可知:与正常对照组相比,模型组中MDA和LPO含量极显著升高(P < 0.01),NO含量极显著降低(P < 0.01),GSH含量极显著降低(P < 0.01);自愈组MDA含量极显著升高(P < 0.01),LPO含量显著升高(P < 0.05),NO和GSH含量显著降低(P < 0.05)。与自愈组相比,高剂量组MDA含量极显著降低(P < 0.01),LPO显著降低(P < 0.05),NO极显著升高(P < 0.01),GSH显著升高(P < 0.05);中剂量组LPO含量显著降低(P < 0.05),NO和GSH含量显著升高(P < 0.05);低剂量组MDA、LPO、NO和GSH均差异不显著。说明郁金散能升高大肠湿热证大鼠肝中的NO和GSH含量,降低MDA和LPO含量,且高剂量效果最好。
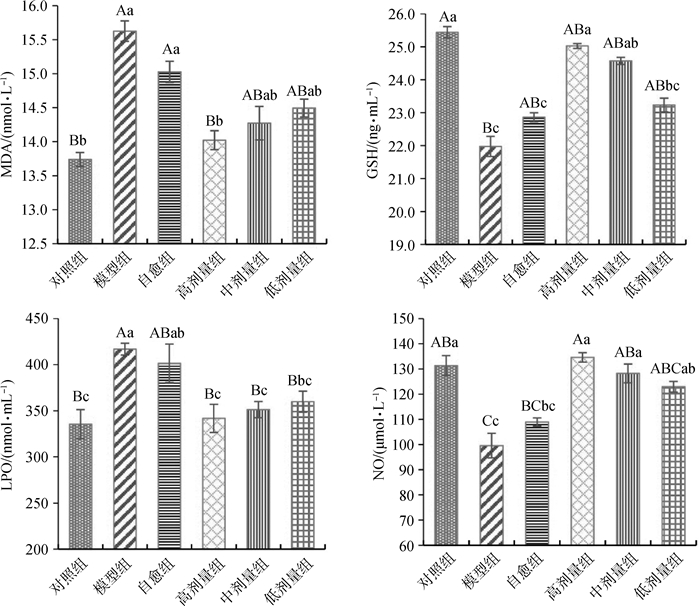
|
标有不同字母表示差异极显著(大写字母,P < 0.01)或差异显著(小写字母,P < 0.05),标有相同字母表示差异不显著(P > 0.05) Values with different capital letter superscripts mean significant difference (P < 0.01), and with different small letter superscripts mean significant difference (P < 0.05), with the same letter superscripts mean no significant difference (P > 0.05) 图 5 肝中MDA、GSH、LPO、NO的含量 Figure 5 Content of MDA, GSH, LPO, NO in liver |
本研究中发现大肠湿热证大鼠血清IgM、IgA和IgG的含量显著升高,提示免疫系统功能变化在湿热证转化转归中起重要作用。中医辨证属湿热证的患者存在着免疫功能异常现象[8]。陈江华等[9]对湿热证患者体液免疫状况的临床研究发现,湿热证患者血清IgA、IgM、IgG、C3水平较正常人显著升高。白爱平等[10]、佟丽等[11]研究发现,湿热证机体免疫球蛋白IgG、IgM、IgA、C3、C4补体水平显著升高。这与本研究结果一致。体液免疫增强,可能与湿热证为多种急性热病,机体对致病微生物等的急性炎症反应,免疫系统对病原微生物的错误识别引起的自身免疫反应[12]等有关。经郁金散治疗后,湿热证大鼠IgM、IgA和IgG含量显著降低。说明郁金散可以有效缓解大肠湿热证引起的体液免疫亢进,从而调节机体免疫功能。
脾是免疫应答、进而产生抗体分泌细胞的关键免疫器官,其形态结构的变化可反映免疫应答调节的状态[13]。胸腺是中枢免疫器官,T、B细胞发育的重要场所之一[14]。本研究大肠湿热证模型组大鼠脾和胸腺组织结构出现紊乱,脾白髓与红髓界限模糊,淋巴细胞数目减少,可见坏死的淋巴细胞。胸腺组织皮质、髓质交界模糊,淋巴细胞数量减少,胸腺小体数量减少。这些结果表明大肠湿热证可引起大鼠脾和胸腺组织结构紊乱,淋巴细胞的异常凋亡,从而导致机体免疫功能异常。郁金散高剂量组,脾红、白髓交界较为清晰,淋巴细胞数目增多,但低于正常组;胸腺皮质区淋巴细胞密集,被膜较厚。提示经郁金散治疗后,各器官形态结构趋于正常。这些结果表明郁金散可有效缓解大肠湿热证引起的免疫器官的功能异常。
郁金散影响机体免疫功能的可能原因:郁金散组方中君药郁金对体液免疫具有抑制作用[15]。臣药中黄连可抑制免疫细胞凋亡,减轻免疫器官萎缩[16];黄芩化学成分黄芩苷、黄芩素具有免疫调节作用;黄柏能够抑制小鼠迟发型超敏反应,从而抑制细胞免疫,减轻炎症损伤[17]。佐药中大黄可显著提高免疫低下小鼠淋巴细胞的增殖能力[18];白芍总苷具有多途径抑制自身免疫反应的作用[19];诃子同样具有免疫调节作用[20]。因此,郁金散可通过降低大肠湿热证机体血液中免疫球蛋白的含量,调节体液免疫,同时通过调节免疫器官功能达到治疗大肠湿热证的目的。
3.2 郁金散对大肠湿热证大鼠抗氧化相关因子的调节本研究结果显示,大肠湿热证大鼠血清和肝中MDA和LPO的含量显著性升高,GSH和NO含量下降。徐萌等[21]研究发现,湿热证动物模型由于病原微生物的刺激而发生不同程度的氧化应激,致使机体LPO和MDA水平升高。佟丽等[11]、张霓[22]研究表明湿热证大鼠模型MDA含量上升。这与本研究结果一致。MDA是脂质过氧化的最终产物,其水平可反应组织过氧化损伤的程度;LPO浓度的改变可直接反应机体脂质过氧化的强度。本研究MDA、LPO含量增高,提示湿热证机体脂质过氧化强度过高,出现明显的过氧化损伤。刘德传等[23]研究表明,湿热证模型大鼠GSH-Px活性明显降低,NO含量显著下降,这与本试验结果相符。GSH可清除体内自由基和ROS,防止肝细胞损伤[24]。NO是一种重要的非酶抗氧化剂,正常水平会起到清除氧自由基的作用,且NO在高温损伤过程中发挥重要的调节作用[25-26]。GSH水平降低提示机体自由基清除障碍,抗氧化能力减弱,NO含量降低则加剧高温损伤。对中医湿热证证候的应用研究表明,湿热证患者和模型动物机体氧化反应增强,抗氧化功能减弱,细胞膜完整性受损,引起坏死,从而导致大量病理产物堆积[27],这是湿热证形成的关键。因此,大肠湿热证模型大鼠机体中存在严重的氧化和抗氧化系统失衡。
经郁金散治疗后,MDA、LPO、GSH和NO的含量均明显回调,且郁金散高剂量组效果最明显,各指标含量趋于正常。药理研究表明,郁金散组方中黄连、黄柏、栀子具有清除自由基、脂质过氧化的作用[17, 28-29];黄芩可减少自由基产生及体内脂质过氧化,增强细胞膜的保护作用[30];白芍的活性成分五没食子酰基葡萄糖有抗氧化活性[31];诃子提取物可显著增加模型鼠体内GSH的含量,降低LPO的含量[32];大黄中的大黄酚可增强抗氧化酶的活性,减少过氧化脂质[33]。表明郁金散具有调节机体氧化和抗氧化功能,增强清除自由基的能力,减轻机体过氧化损伤,提高机体免疫力,从而起到治疗大肠湿热证的作用。
4 结论大肠湿热证机体存在免疫功能异常和氧化/抗氧化失衡现象,郁金散可通过调控大肠湿热证机体的免疫功能和抗氧化/氧化平衡而治疗大肠湿热证。为研究郁金散治疗大肠证的机制以及开发治疗湿热证疾病的药物奠定了理论基础。
| [1] |
胡元亮.
中兽医学[M]. 北京: 科学出版社, 2013.
HU Y L. Traditional chinese veterinary medicine[M]. Beijing: Science Press, 2013. (in Chinese) |
| [2] |
周燕萍, 胡作为. 温病湿热证本质研究新进展[J]. 湖北中医药大学学报, 2004, 6(1): 49–50.
ZHOU Y P, HU Z W. Research progress of the nature of epidemic febrile disease syndrome of damp-heat[J]. Journal of Hubei College of Chinese Medicine, 2004, 6(1): 49–50. DOI: 10.3969/j.issn.1008-987X.2004.01.022 (in Chinese) |
| [3] |
阮静.从自由基水平、免疫功能探讨岭南上呼道病毒感染湿热证的形成机理[D].广州: 广州中医药大学, 2008.
RUAN J. Investigation of formation mechanism of damp-heat syndrome of southern viral upper respiratory tract infection from free radical and immunological function[D]. Guangzhou: Guangzhou University of Chinese Medicine, 2008. (in Chinese) http://cdmd.cnki.com.cn/article/cdmd-10572-2008100330.htm |
| [4] |
黄丽卿, 罗丽萍, 张亚茹, 等. 屎肠球菌NCIMB11181对大肠杆菌O78感染肉鸡生产性能、肠道微生物和血液抗氧化功能的影响[J]. 中国家禽, 2017, 39(11): 17–22.
HUANG L Q, LUO L P, ZHANG Y R, et al. Effects of dietary enterococcus faecium NCIMB11181 on growth performance, intestinal microflora and serum antioxidant capacity in broilers challenged with Escherichia coli O78[J]. China Poultry, 2017, 39(11): 17–22. (in Chinese) |
| [5] | DE LA FUENTE M. Effects of antioxidants on immune system ageing[J]. Eur J Clin Nutr, 2002, 56(S3): S5–S8. DOI: 10.1038/sj.ejcn.1601476 |
| [6] | YAO W L, YANG C X, WEN Y Q, et al. Treatment effects and mechanisms of yujin powder on rat model of large intestine dampness-heat syndrome[J]. J Ethnopharmacol, 2017, 202: 265–280. DOI: 10.1016/j.jep.2017.03.030 |
| [7] |
文艳巧, 姚万玲, 杨朝雪, 等. 郁金散对大肠湿热证模型大鼠血清及肠道组织胃肠激素的影响[J]. 畜牧兽医学报, 2017, 48(6): 1140–1149.
WEN Y Q, YAO W L, YANG C X, et al. Effects of Yujin powder on gastrointestinal hormone in serum and intestinal tissue of large intestine dampness-heat syndrome rat model[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(6): 1140–1149. (in Chinese) |
| [8] |
吴湘华, 孙翠凤. 自拟清肠排毒汤对急性溃疡性结肠炎(大肠湿热证)免疫功能和炎症因子的影响[J]. 中国中医急症, 2016, 25(10): 1942–1944.
WU X H, SUN C F. Effect of qingchangpaidutang on immune function and inflammatory factors of acute ulcerative colitis (large intestine dampness-heat syndrome)[J]. Journal of Emergency in Traditional Chinese Medicine, 2016, 25(10): 1942–1944. DOI: 10.3969/j.issn.1004-745X.2016.10.035 (in Chinese) |
| [9] |
陈江华, 佟丽, 吴仕九, 等. 湿热证病人体液免疫状态观察[J]. 中国中医急症, 1998, 7(1): 6–7.
CHEN J H, TONG L, WU S J, et al. Observation on the state of humoral immunity in patients with damp-heat syndrome[J]. Journal of Emergency in Traditional Chinese Medicine, 1998, 7(1): 6–7. (in Chinese) |
| [10] |
白爱平, 欧阳钦, 余世南, 等. 活动性溃疡性结肠炎患者外周血血小板功能及免疫球蛋白水平的研究[J]. 四川医学, 2003, 24(6): 555–557.
BAI A P, OUYANG Q, YU S N, et al. Exploration of the function of plovtelet and the immune globulin concetation of active ulcenative colitis[J]. Sichuan Medical Journal, 2003, 24(6): 555–557. DOI: 10.3969/j.issn.1004-0501.2003.06.003 (in Chinese) |
| [11] |
佟丽, 吴仕九, 陈江华, 等. 清香散对温病湿热模型大鼠红细胞免疫功能及脂质过氧化作用的影响[J]. 中国中西医结合杂志, 2000(S1): 101–102.
TONG L, WU S J, CHEN J H, et al. The effect of Qingxiangsanon the immune function and lipid peroxidation of erythrocytes in the Wet-heat syndrome rat model[J]. Chinese Journal of Integrated Traditional and Western Medicine, 2000(S1): 101–102. (in Chinese) |
| [12] |
贲莹, 高长玉, 刘桂宇, 等. 吉兰-巴雷综合征急性期湿热证与体液免疫的关系[J]. 河北中医, 2007, 29(10): 881–882.
BEN Y, GAO C Y, LIU G Y, et al. Relation of syndrome of damp-heat of acute period of GBS with humoral immunity[J]. Hebei Journal of Traditional Chinese Medicine, 2007, 29(10): 881–882. DOI: 10.3969/j.issn.1002-2619.2007.10.006 (in Chinese) |
| [13] | BOGDÁNDI E N, BALOGH A, FELGYINSZKI N, et al. Effects of low-dose radiation on the immune system of mice after total-body irradiation[J]. Radiat Res, 2010, 174(4): 480–489. DOI: 10.1667/RR2160.1 |
| [14] | ANDERSON G, HARMAN B C, HARE K J, et al. Microenvironmental regulation of T cell development in the thymus[J]. Semin Immunol, 2000, 12(5): 457–464. DOI: 10.1006/smim.2000.0260 |
| [15] | CHUEH S C J, LAI M K, LIU I S, et al. Curcumin enhances the immunosuppressive activity of cyclosporine in rat cardiac allografts and in mixed lymphocyte reactions[J]. Transplant Proc, 2003, 35(4): 1603–1605. DOI: 10.1016/S0041-1345(03)00377-4 |
| [16] |
宋兵.黄连素对脑缺血再灌注小鼠损伤和免疫系统的影响[D].广州: 暨南大学, 2009.
SONG B. The effects of Bererine on the damage and immune system of middle cerebral artery occlusion-reperfusion injury mouse[D]. Guangzhou: Jinan University, 2009. (in Chinese) http://cdmd.cnki.com.cn/article/cdmd-10559-2009108778.htm |
| [17] |
孙森凤, 张颖颖, 褚万春. 黄柏药理作用的研究进展[J]. 山东化工, 2017, 46(14): 99–100.
SUN S F, ZHANG Y Y, CHU W C. Progress in research on pharmacological activities of phellodendronamu-rense rupr[J]. Shandong Chemical Industry, 2017, 46(14): 99–100. DOI: 10.3969/j.issn.1008-021X.2017.14.036 (in Chinese) |
| [18] |
邬博, 刘彦晶, 连丽. 大黄的药理作用研究进展[J]. 中国中医药现代远程教育, 2015, 13(20): 152–154.
WU B, LIU Y J, LIAN L. Research progress of pharmacological effects of rheum officinale[J]. Chinese Medicine Modern Distance Education of China, 2015, 13(20): 152–154. DOI: 10.3969/j.issn.1672-2779.2015.20.079 (in Chinese) |
| [19] |
张利. 白芍的药理作用及现代研究进展[J]. 中医临床研究, 2014(29): 25–26.
ZHANG L. Pharmacological effects and research advances of Baishao[J]. Clinical Journal of Chinese Medicine, 2014(29): 25–26. DOI: 10.3969/j.issn.1674-7860.2014.29.012 (in Chinese) |
| [20] | BELAPURKAR P, GOYAL P, TIWARI-BARUA P. Immunomodulatory effects of triphala and its individual constituents:a review[J]. Indian J Pharm Sci, 2014, 76(6): 467–475. |
| [21] |
徐萌, 高云飞, 吴仕九. 清香散对湿热证大鼠的抗氧化和肝细胞凋亡作用[J]. 中药材, 2001, 24(6): 420–421.
XU M, GAO Y F, WU S J. The effect of "Qingxiangsan" on inhibiting oxidation and hepatic cells apoptosis in the wet-heat syndrome[J]. Journal of Chinese Medicinal Materials, 2001, 24(6): 420–421. DOI: 10.3321/j.issn:1001-4454.2001.06.018 (in Chinese) |
| [22] |
张霓. 王氏连朴饮加味对脾胃湿热证大鼠抗氧化功能的影响[J]. 河南中医, 2013, 33(4): 519–520.
ZHANG N. Effect of Jiawei Wangshi Lianpu Drink on antioxidation of rats with splenogastric damp-heat syndrome[J]. Henan Traditional Chinese Medicine, 2013, 33(4): 519–520. (in Chinese) |
| [23] |
刘德传, 吴仕九, 杨运高, 等. 微量元素、抗氧化剂与湿热证的相关性的研究[J]. 广东微量元素科学, 2001, 8(2): 30–32.
LIU D C, WU S J, YANG Y G, et al. Correlative analysis on trace elements, antioxidants and damp-heat disease[J]. Guangdong Trace Elements Science, 2001, 8(2): 30–32. DOI: 10.3969/j.issn.1006-446X.2001.02.007 (in Chinese) |
| [24] | MARTIN H L, TEISMANN P. Glutathione-a review on its role and significance in Parkinson's disease[J]. FASEB J, 2009, 23(10): 3263–3272. DOI: 10.1096/fj.08-125443 |
| [25] | BRVNE B, SANDAU K, VON KNETHEN A. Apoptotic cell death and nitric oxide:activating and antagonistic transducing pathways[J]. Biochemistry, 1998, 63(7): 817–825. |
| [26] | MANUKHINA E B, POKIDYSHEV D A, MALENIUK E B, et al. The protective effect of nitric oxide in heat shock[J]. Izv Akad Nauk Ser Biol, 1997(1): 54–58. |
| [27] |
黎壮伟.温病湿热证线粒体氧化损伤、能量代谢及中药干预机制研究[D].广州: 广州中医药大学, 2010.
LI Z W. Study on pathogenesis of oxidative damage and energy metabolism of mitochondria in damp-heat syndrome of seasonal febrile disease and effects of chinese herbs[D]. Guangzhou: Guangzhou University of Chinese Medicine, 2010. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10572-2010126213.htm |
| [28] | SCHINELLA G R, TOURNIER H A, PRIETO J M, et al. Antioxidant activity of anti-inflammatory plant extracts[J]. Life Sci, 2002, 70(9): 1023–1033. DOI: 10.1016/S0024-3205(01)01482-5 |
| [29] | CHEN Y, ZHANG H, LI Y X, et al. Crocin and geniposide profiles and radical scavenging activity of gardenia fruits (Gardenia jasminoides Ellis) from different cultivars and at the various stages of maturation[J]. Fitoterapia, 2010, 81(4): 269–273. DOI: 10.1016/j.fitote.2009.09.011 |
| [30] |
田硕, 洪涛, 张多, 等. 黄芩素的药理作用及分子机制的最新研究进展[J]. 黑龙江医药, 2015, 28(6): 1195–1199.
TIAN S, HONG T, ZHANG D, et al. Recent advances in pharmacological effects and molecular mechanisms of baicalein[J]. Heilongjiang Medicine Journal, 2015, 28(6): 1195–1199. (in Chinese) |
| [31] |
夏颖, 殷志爽, 石晨, 等. 白芍提取物及其有效成分抗氧化活性的研究[J]. 首都医科大学学报, 2013, 34(1): 120–125.
XIA Y, YIN Z S, SHI C, et al. Antioxidant activity of the crude extract and the active ingredient extracted from Radix Paeoniae Alba[J]. Journal of Capital Medical University, 2013, 34(1): 120–125. DOI: 10.3969/j.issn.1006-7795.2013.01.023 (in Chinese) |
| [32] | SAHA S, VERMA R J. In vitro and in silico study of antioxidant effect of Bergenia ciliata and Terminalia chebula against sodium oxalate induced oxidative stress[J]. Toxicol Environ Health Sci, 2015, 7(1): 50–57. DOI: 10.1007/s13530-015-0220-6 |
| [33] |
于建玉, 廖欣, 丁厚伟, 等. 中药大黄药理作用研究进展及其临床应用[J]. 中国现代药物应用, 2016, 10(11): 286–287.
YU J Y, LIAO X, DING H W, et al. Research progress of pharmacological action of rhubarb and its clinical application[J]. Chinese Journal of Modern Drug Application, 2016, 10(11): 286–287. (in Chinese) |



