家禽具有特殊的光受体通路,光照是家禽生长发育过程中重要的环境因素。波长是光照的三大要素之一,对家禽的生产性能和生理行为可产生重要影响[1]。研究表明在蓝绿光条件下饲养的肉鸡增重效果显著高于红、白光下饲养的[2-5],且肉的品质有所提高[6]。但不同发育阶段的肉鸡对光的敏感性也不一样,绿光在肉鸡出壳早期能促进骨骼肌的快速生长,而蓝光则在肉鸡生长后期对骨骼肌有显著的促生长作用[7-8]。对相关机制的研究发现,单色光通过影响骨骼肌卫星细胞的活性和数量来影响肌肉的生长发育[9]。但动物肌纤维的数量主要与胚胎期时肌肉的卫星细胞数量有关[10],动物出生后肌纤维数量基本保持恒定。因此有学者建议,在肉鸡饲养过程中将单色光的照射时间由出壳后提前至孵化期,结果发现在肉鸡胚胎期给予单色绿光照射能增强代谢,促进鸡胚骨骼肌卫星细胞的增殖与分化,并能促进胚胎发育,改善出壳后的饲料转化率[11-14]。
在胚胎发育过程中,有氧代谢会产生多种活性氧自由基。此时机体尚未建立起完整有效的免疫系统,易受到各种氧化产物的损害,使骨骼肌蛋白质分解代谢增加,合成代谢减少;同时其分解代谢率大于合成代谢率,不利于骨骼肌的生长发育[15]。机体的抗氧化能力主要体现在抗氧化酶活性高低上。正常情况下,体内抗氧化酶可清除氧化过程中活性氧自由基等代谢产物,从而对机体起到动态保护作用[16]。如果机体抗氧化能力下降,过量的自由基会使不饱和脂肪酸发生脂质过氧化,严重危及细胞膜及胞内大分子蛋白和核酸,对机体造成损伤,使禽类产品肉品质降低[17-18]。为此本文拟研究孵化期单色光照射对鸡胚骨骼肌抗氧化功能的影响,以期有助于阐明孵化期单色光刺激影响鸡胚骨骼肌卫星细胞增殖的机制。
1 材料与方法 1.1 试验动物和光照强度将225枚AA(Arber Acres)肉鸡受精蛋随机分为5组,分别置于黑暗、白光(400~760 nm)、红光(660 nm)、绿光(560 nm)和蓝光(480 nm)下孵化21 d(n=45)。孵化温度维持在37 ℃,湿度为50%~60%。光源为专用LED灯(Light-Emitting Diode)。使用照度计(MS6610,上海隆拓仪器设备有限公司)标定检验光照强度。蛋壳上的光照强度为15 lx ± 0.3 lx,光照周期(L:D)为24:0。
1.2 样品采集与检测在15胚龄(E15)、18胚龄(E18)和21胚龄(E21)时取鸡胚胸大肌和腓肠肌。将肌肉组织与0.75%生理盐水配比(m:V=1:9)制备匀浆,于低温冷冻离心机中离心20 min (3 000 r·min-1),将上清液置于-80 ℃冷冻保存备用[19]。采用抗氧化酶活性检测试剂盒检测肌肉组织中超氧化物歧化酶(SOD)(S0101)、谷胱甘肽过氧化物酶(GSH-Px)(S0056)、过氧化物脱氢酶(CAT)(S0051)、总抗氧化能力(T-AOC)(S0116)及丙二醛(MDA)(S0131)含量,各检测试剂盒均购于南京建成生物工程研究所。参照试剂盒说明书配制相关试剂,按照操作步骤添加药品。于相应的光波长下检测吸光度(OD值),最终根据公式计算得出蛋白含量和各氧化酶活性。上述试验在各样本中重复3次。
1.3 数据分析试验数据采用SPSS 18.0统计软件进行单因素方差分析(ANONA)和LSD多重比较,结果表示为平均数±标准误(x±sx),P < 0.05为差异显著,P < 0.01为差异极显著。为了比较不同光处理的影响,用不同光色处理组间的百分比差值来显示。例如比较白光组与黑暗组,计算公式为:(白光组数值-黑暗组数值)/黑暗组数值×100%。
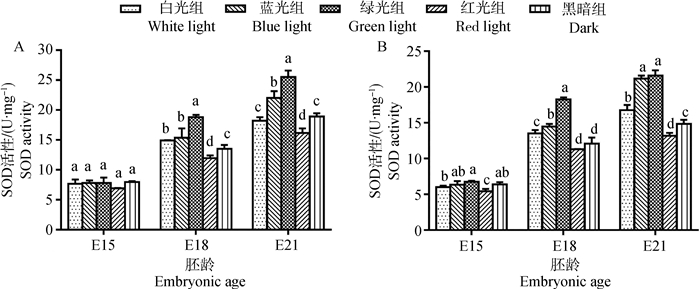
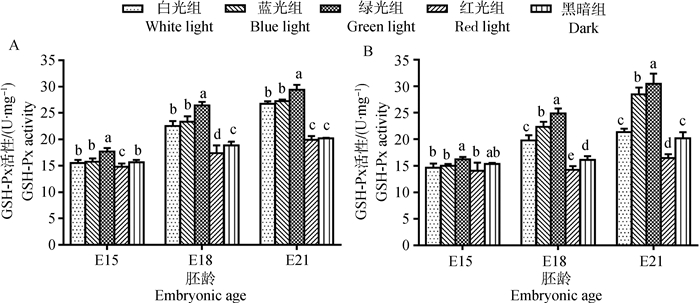
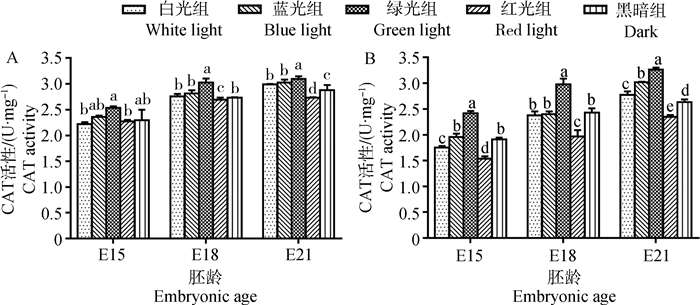
2 结果 2.1 单色光对鸡胚骨骼肌抗氧化酶活性的影响E15时,白光组和黑暗组的胸大肌与腓肠肌中SOD活性差异不显著(图 1)。在E18时,白光组胸大肌和腓肠肌SOD活性均显著高于黑暗组(10.80%~11.92%,P < 0.05)。到E21时,白光组腓肠肌SOD活性仍极显著高于黑暗组(13.02%,P < 0.01,此处P值依据具体值判断,图中统一在0.05水平标记,下同),而白光组胸大肌的SOD活性略低于黑暗组,但差异不显著(P > 0.05)。胸大肌和腓肠肌中GSH-Px活性的变化与SOD相似(图 2)。在E15时,白光组与黑暗组间差异不显著(P > 0.05);但E18和E21时,白光组的GSH-Px活性均高于黑暗组(5.67%~32.79%)。对于CAT,比较白光组与黑暗组在E15与E18时的胸大肌中活性,两组间差异不显著(P > 0.05,图 3);但E21时,则无论胸大肌或腓肠肌中CAT活性在白光组均显著高于黑暗组(3.82%~5.45%,P < 0.05)。
|
A.胸大肌;B.腓肠肌;不同字母表示同胚龄不同光色间差异显著(P < 0.05) A. Pectoralis major; B. Gastrocnemius; Different letters indicated significant difference among different light colors at the same embryonic age (P < 0.05) 图 1 单色光对不同日龄鸡胚胸大肌和腓肠肌SOD活性的影响 Figure 1 Effects of various monochromatic lights on the change of SOD activity in pectoralis major and gastrocnemius of broiler embryos |
|
A.胸大肌;B.腓肠肌;不同字母表示同胚龄不同光色间差异显著(P < 0.05) A. Pectoralis major; B. Gastrocnemius; Different letters indicated significant difference among different light colors at the same embryonic age (P < 0.05) 图 2 单色光对不同日龄鸡胚胸大肌和腓肠肌GSH-Px活性的影响 Figure 2 Effects of various monochromatic lights on the change of GSH-Px activity in pectoralis major and gastrocnemius of broiler embryos |
|
A.胸大肌;B.腓肠肌;不同字母表示同胚龄不同光色间差异显著(P < 0.05) A. Pectoralis major; B. Gastrocnemius; Different letters indicated significant difference among different light colors at the same embryonic age (P < 0.05) 图 3 单色光对不同日龄鸡胚胸大肌和腓肠肌CAT活性的影响 Figure 3 Effects of various monochromatic lights on the change of CAT activity in pectoralis major and gastrocnemius of broiler embryos |
比较不同光处理组,E15的胸大肌中SOD活性差异不显著(P > 0.05)。在E18,绿光组的胸大肌和腓肠肌中SOD活性最高,极显著高于其他光处理组和黑暗组(22.53%~61.89%,P < 0.01);相反,红光组肌肉中SOD活性最低,甚至比黑暗组低6.75%~11.34%,且在胸大肌中差异显著(P < 0.05)。而蓝光组肌肉中SOD活性次之,略高于白光组(2.90%~6.93%)。到E21,肌肉中SOD活性在绿光组最高、蓝光和白光组次之、红光组最低的变化趋势更为明显(图 1)。
E15时,绿光组骨骼肌中的GSH-Px活性极显著高于其他光处理组(5.71%~19.33%,P < 0.01);而红光组在胸大肌中GSH-Px活性显著低于其他组(7.67%~34.05%,P < 0.05),其他处理组的组间差异不显著(P > 0.05)。在E18,绿光组骨骼肌中GSH-Px活性最高,极显著高于其他组(11.22%~74.71%,P < 0.01);相反,红光组肌肉中GSH-Px活性最低,且极显著低于其他光处理组(7.67%~42.76%,P < 0.01)。蓝光组GSH-Px活性次于绿光组,略高于白光组(3.68%~12.93%)。E21时,与SOD的变化规律相似,肌肉中GSH-Px活性在绿光组最高、蓝光和白光组次之、红光组最低(图 2)。
由图 3可知,对CAT来说,E15时胸大肌中各处理组间的差异未表现出明显规律。E18时,绿光组骨骼肌中CAT活性极显著高于其他处理组(7.65%~51.27%,P < 0.01),而红光组骨骼肌中CAT活性低于其他各组,且差异极显著(1.39%~33.89%,P < 0.01)。到E21,骨骼肌中CAT活性在绿光组最高、红光组最低的规律不变;同时蓝光组在肌肉中的CAT活性次于绿光组,略高于白光组(1.14%~8.83%)。
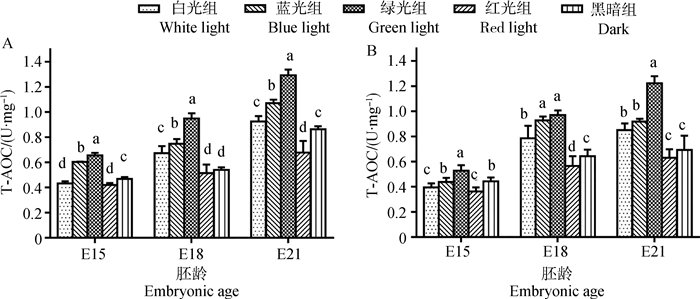
2.2 单色光对鸡胚骨骼肌总抗氧化能力T-AOC水平的影响在E18和E21,白光组骨骼肌总抗氧化能力T-AOC均高于黑暗组(7.20%~24.64%,图 4),且差异显著(P < 0.05)。但是,E15时骨骼肌总抗氧化能力T-AOC在白光组低于黑暗组,且差异显著(7.21%~10.80%,P < 0.05)。
|
A.胸大肌;B.腓肠肌;不同字母表示同胚龄不同光色间差异显著(P < 0.05) A. Pectoralis major; B. Gastrocnemius; Different letters indicated significant difference among different light colors at the same embryonic age (P < 0.05) 图 4 单色光对不同日龄鸡胚胸大肌和腓肠肌T-AOC的影响 Figure 4 Effects of various monochromatic lights on the change of T-AOC in pectoralis major and gastrocnemius of broiler embryos |
胸大肌中,E15时绿光组与蓝光组的T-AOC显著高于其他组(P < 0.05,图 4),其中绿光组与其他组差异极显著(P < 0.01),而腓肠肌中绿光组T-AOC最高(高于其他处理组51.31%~72.14%);红光组肌肉中T-AOC均为最低(低于其他处理组4.68%~45.86%)。到E18,T-AOC在绿光最高、蓝光组次之、红光组最低的规律更为明显。E21时,延续E18的变化趋势,绿光组肌肉中T-AOC极显著高于其他光处理组及黑暗组(20.77%~94.28%,P < 0.01),蓝光组略高于白光组(7.78%~15.76%),红光组骨骼肌中T-AOC最低(低于其他处理组9.25%~48.53%)。
2.3 单色光对鸡胚骨骼肌MDA含量的影响各组骨骼肌中MDA含量的变化与T-AOC的变化相反(图 5)。在E18和E21时,白光组肌肉中MDA含量均低于黑暗组(1.75%~10.32%),E15时白光组胸大肌中MDA含量显著高于黑暗组(P < 0.05),MDA含量在腓肠肌中也高于黑暗组,但差异不显著(P > 0.05)。
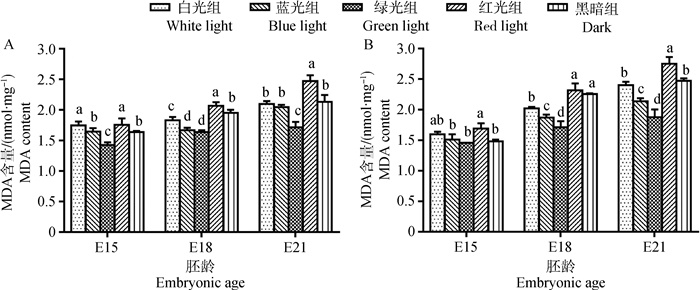
|
A.胸大肌;B.腓肠肌;不同字母表示同胚龄不同光色间差异显著(P < 0.05) A. Pectoralis major; B. Gastrocnemius; Different letters indicated significant difference among different light colors at the same embryonic age (P < 0.05) 图 5 单色光对不同日龄鸡胚胸大肌和腓肠肌MDA含量的影响 Figure 5 Effects of various monochromatic lights on the change of MDA content in pectoralis major and gastrocnemius of broiler embryos |
图 5表明,在E15时,绿光组胸大肌MDA含量最低,极显著低于其他处理组(1.87%~18.67%,P < 0.01)。胚胎发育至E18时,红光组骨骼肌MDA含量最高,其次是黑暗组;绿光组含量最低,在腓肠肌中与其他光处理组及黑暗组差异显著(1.73%~26.63%,P < 0.05);蓝光组骨骼肌MDA含量低于白光组,差异显著(7.63%~9.02%,P < 0.05)。E21时,红光组骨骼肌中MDA含量极显著高于其他处理组(11.38%~46.75%,P < 0.01);相反,绿光组肌肉MDA含量为最低(12.11%~31.86%),蓝光组MDA含量为次低,在胸大肌中与白光组差异不显著(P > 0.05)。
与白光组相比,在E18与E21时,绿光对鸡胚骨骼肌中抗氧化酶活性有极显著的增强作用(P < 0.01),蓝光可使抗氧化酶活性显著升高(P < 0.05),红光则会显著降低抗氧化酶活性(P < 0.05)。对总抗氧化能力T-AOC来说,绿光对T-AOC的增强作用极为显著(P < 0.01),蓝光对鸡胚骨骼肌T-AOC有增强作用,但不显著(P < 0.05);红光会使其显著降低(P < 0.05)。绿光组与蓝光组骨骼肌MDA含量均低于白光组,其中绿光组比蓝光组作用更明显;红光组与白光组相比MDA含量则极显著上升(P < 0.01)。
3 讨论检测了不同光色条件下肉鸡胚胎发育后期胸大肌、腓肠肌中抗氧化酶(SOD、GSH-Px、CAT)的活性、总抗氧化能力(T-AOC)和脂质过氧化产物丙二醛(MDA)含量的变化,分析了孵化期单色光照射对肉鸡骨骼肌抗氧化功能的影响。本文的结果表明在给予白光照射之后,骨骼肌中抗氧化酶含量升高,总抗氧化能力增强,而氧化产物积累量减少;肌肉的抗氧化能力与黑暗条件下相比有了一定程度的增强,尤其E18与E21胚龄时更明显。有研究提出,白光照射下胚胎发育的最后时期肝的抗氧化能力下降,弱于黑暗组。其中E15与E18两处理组抗氧化能力较为接近,E21时白光组MDA含量则显著高于黑暗组[20]。这种差异可能与检测的组织器官不同有关。我们进一步比较了不同波长的单色光对肉鸡胚胎骨骼肌抗氧化功能的影响。结果显示,与白光相比,绿光能显著提高鸡胚发育后期骨骼肌抗氧化酶含量增多,总抗氧化能力增强,氧化产物积累量减少;而红光则抑制抗氧化酶活性,使总抗氧化能力减弱,积累的氧化产物不能及时清除。前人的研究也发现孵化期单色绿光照射能够提高鸡胚小肠的抗氧化功能[19, 21-22]。另外,对刚出壳肉鸡的研究也表明绿光和蓝光可显著提高胸肌与腿肌中SOD和GSH-Px活性以及T-AOC (P < 0.05),显著减少MDA含量(P < 0.05)[23]。本实验室前期研究发现,鸡胚孵化期给予单色光照射,绿光可提高骨骼肌SOD与GSH-Px的含量,使T-AOC升高,降低了MDA;而红光则降低了SOD和GSH-Px,提高了MDA含量[24],这与我们本次对鸡胚的研究结果一致。由此可见,不同单色光对鸡胚肌肉的抗氧化性能有影响,绿光可显著提高抗氧化能力,红光则会抑制抗氧化能力。单色光影响鸡胚骨骼肌的抗氧化能力,可能与影响褪黑激素分泌有关,因为有报道认为单色绿光能刺激鸡胚松果体分泌褪黑激素,而红光则降低鸡胚血浆褪黑激素水平[21-22]。
有研究发现,组织细胞抗氧化功能的变化可影响细胞的增殖与分化,骨骼肌中GSH-Px含量提高可加快骨骼肌的生长速度[25]。笔者实验室和前人的前期研究发现,单色光可影响鸡胚骨骼肌卫星细胞增殖、分化,促进肌肉生长。绿光处理下E17-E20的鸡胚肌肉重量与肌纤维粗细优于白光组、蓝光组、红光组与黑暗组[24, 26]。在孵化期给予绿光照射的肉鸡在P1、P4、P7和P10日龄中肌肉卫星细胞的增殖活性均优于其他光色组[26]。本文的试验结果发现不同单色光对鸡胚骨骼肌抗氧化功能影响不同,绿光能显著增强鸡胚骨骼肌抗氧化功能,红光则对鸡胚骨骼肌抗氧化功能有抑制作用。这与肌肉卫星细胞的变化趋势一致。由此推论,在孵化期间给予不同单色光照射影响鸡胚卫星细胞增殖分化和肌肉生长发育,可能与影响肌肉抗氧化功能有关。
4 结论孵化期间给予不同波长单色光照射,经对比,绿光可显著增强鸡胚发育后期骨骼肌中抗氧化酶(SOD、GSH-Px、CAT)的活性来提高抗氧化能力;而红光则会减弱SOD、GSH-Px、CAT活性而降低抗氧化能力。
| [1] | OLANREWAJU H A, THAXTON J P, DOZIER Ⅲ W A, et al. A review of lighting programs for broiler production[J]. Int J Poult Sci, 2006, 5(4): 301–308. DOI: 10.3923/ijps.2006.301.308 |
| [2] | WABECK C J, SKOGLUND W C. Influence of radiant energy from fluorescent light sources on growth, mortality, and feed conversion of broilers[J]. Poult Sci, 1974, 53(6): 2055–2059. DOI: 10.3382/ps.0532055 |
| [3] | ROZENBOIM I, BIRAN I, UNI Z, et al. The effect of monochromatic light on broiler growth and development[J]. Poult Sci, 1999, 78(1): 135–138. DOI: 10.1093/ps/78.1.135 |
| [4] | ROZENBOIM I, ROBINZON B, ROSENSTRAUCH A. Effect of light source and regimen on growing broilers[J]. Br Poult Sci, 1999, 40(4): 452–457. DOI: 10.1080/00071669987197 |
| [5] |
曹静, 陈耀星, 王子旭, 等. 单色光对肉鸡生长发育的影响[J]. 中国农业科学, 2007, 40(10): 2350–2354.
CAO J, CHEN Y X, WANG Z X, et al. Effect of monochromatic light on broiler growth[J]. Scientia Agricultura Sinica, 2007, 40(10): 2350–2354. DOI: 10.3321/j.issn:0578-1752.2007.10.032 (in Chinese) |
| [6] |
谢强, 冯佩诗, 李孟孟, 等. 光色影响家禽肌肉生长及其调控机制研究进展[J]. 中国家禽, 2017, 39(23): 42–46.
XIE Q, FENG P S, LI M M, et al. Research advances on function and regulation of monochromatic light on muscle growth in poultry[J]. China Poultry, 2017, 39(23): 42–46. (in Chinese) |
| [7] |
刘文杰, 陈耀星, 王子旭, 等. 单色光对肉鸡肌肉生长、肌纤维发育及血清睾酮水平的影响[J]. 畜牧兽医学报, 2008, 39(12): 1759–1764.
LIU W J, CHEN Y X, WANG Z X, et al. Effect of monochromatic light on the muscle growth and muscle fiber development and testosterone secretion in broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2008, 39(12): 1759–1764. DOI: 10.3321/j.issn:0366-6964.2008.12.021 (in Chinese) |
| [8] | CAO J S, LIU W J, WANG Z H, et al. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth[J]. J Appl Poult Res, 2008, 17(2): 211–218. DOI: 10.3382/japr.2007-00043 |
| [9] | LIU W J, WANG Z X, CHEN Y X. Effects of monochromatic light on developmental changes in satellite cell population of pectoral muscle in broilers during early posthatch period[J]. Anat Rec, 2010, 293(8): 1315–1324. DOI: 10.1002/ar.21174 |
| [10] | PICARD B, LEFAUCHEUR L, BERRI C, et al. Muscle fibre ontogenesis in farm animal species[J]. Reprod Nutr Dev, 2002, 42(5): 415–431. DOI: 10.1051/rnd:2002035 |
| [11] | HALEVY O, PIESTUN Y, ROZENBOIM I, et al. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks[J]. Am J Physiol Regul Integr Comp Physiol, 2006, 290(4): R1062–R1070. DOI: 10.1152/ajpregu.00378.2005 |
| [12] | TONG Q, MCGONNELL I M, DEMMERS T G M, et al. Effect of a photoperiodic green light programme during incubation on embryo development and hatch process[J]. Animal, 2018, 12(4): 765–773. DOI: 10.1017/S1751731117002117 |
| [13] | DISHON L, AVITAL-COHEN N, MALAMUD D, et al. In-ovo monochromatic green light photostimulation enhances embryonic somatotropic axis activity[J]. Poult Sci, 2017, 96(6): 1884–1890. DOI: 10.3382/ps/pew489 |
| [14] |
张林, 张海军, 武书庚, 等. 单色光间歇性刺激胚蛋对肉仔鸡胸肉生长及肉品质的影响[J]. 中国农业科学, 2012, 45(5): 951–957.
ZHANG L, ZHANG H J, WU S G, et al. Effect of intermittently monochromatic light Stimuli during the embryogenesis on breast muscular growth and meat quality in male broiler chicks[J]. Scientia Agricultura Sinica, 2012, 45(5): 951–957. DOI: 10.3864/j.issn.0578-1752.2012.05.016 (in Chinese) |
| [15] | DAVOLI R, BRAGLIA S. Molecular approaches in pig breeding to improve meat quality[J]. Brief Funct Genomic Proteomic, 2007, 6(4): 313–321. |
| [16] | HILSCHEROVA K, BLANKENSHIP A L, NIE M, et al. Oxidative stress in liver and brain of the hatchling chicken (Gallus domesticus) following in ovo injection with TCDD[J]. Comp Biochem Physiol Toxicol Pharmacol, 2003, 136(1): 29–45. DOI: 10.1016/S1532-0456(03)00167-4 |
| [17] | KONDAIAH N, ANJANEYULU A S R, RAO V K, et al. Effect of salt and phosphate on the quality of Buffalo and Goat meats[J]. Meat Sci, 1985, 15(3): 183–192. DOI: 10.1016/0309-1740(85)90036-1 |
| [18] | FEDOROV Y V, ROSENTHAL R S, OLWIN B B. Oncogenic Ras-induced proliferation requires autocrine fibroblast growth factor 2 signaling in skeletal muscle cells[J]. J Cell Biol, 2001, 152(6): 1301–1306. DOI: 10.1083/jcb.152.6.1301 |
| [19] |
李素琪, 陈耀星, 王子旭, 等. 单色光及松果体摘除对肉鸡肝抗氧化功能的影响[J]. 畜牧兽医学报, 2014, 45(11): 1883–1887.
LI S Q, CHEN Y X, WANG Z X, et al. Effect of Monochromatic light and pinealectomy on antioxidant function of liver in broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2014, 45(11): 1883–1887. (in Chinese) |
| [20] | WANG T J, DONG Y L, WANG Z X, et al. Secretion pathway of liver IGF-1 via JAK2/STAT3 in chick embryo under the monochromatic light[J]. Growth Factors, 2016, 34(1-2): 51–63. DOI: 10.3109/08977194.2016.1170679 |
| [21] | WANG T J, WANG Z X, CAO J, et al. Monochromatic light affects the development of chick embryo liver via an anti-oxidation pathway involving melatonin and the melatonin receptor Mel1c[J]. Can J Anim Sci, 2017, 94(3): 391–400. |
| [22] | YU Y, WANG Z X, CAO J, et al. Effects of monochromatic light stimuli on the development and Muc2 expression of goblet cells in broiler small intestines during embryogenesis[J]. Poult Sci, 2014, 93(7): 1801–1808. DOI: 10.3382/ps.2013-03805 |
| [23] | KE Y Y, LIU W J, WANG Z X, et al. Effects of monochromatic light on quality properties and antioxidation of meat in broilers[J]. Poult Sci, 2011, 90(11): 2632–2637. DOI: 10.3382/ps.2011-01523 |
| [24] | WANG Y, BAI X J, WANG Z X, et al. Various LED wavelengths affected myofiber development and satellite cell proliferation of chick embryos via the IGF-1 signaling pathway[J]. Photochem Photobiol, 2017, 93(6): 1492–1501. DOI: 10.1111/php.2017.93.issue-6 |
| [25] | MOLETTE C, RÉMIGNON H, BABILÉ R. Maintaining muscles at a high post-mortem temperature induces PSE-like meat in turkey[J]. Meat Sci, 2003, 63(4): 525–532. DOI: 10.1016/S0309-1740(02)00114-6 |
| [26] | BAI X J, WANG Y, WANG Z X, et al. In ovo exposure to monochromatic lights affect posthatch muscle growth and satellite cell proliferation of chicks:role of IGF-1[J]. Growth Factors, 2016, 34(3-4): 107–118. DOI: 10.1080/08977194.2016.1199553 |



