2. 农业部养猪科学重点实验室, 重庆 402460
2. Key Laboratory of Pig Industry Sciences of Ministry of Agriculture, Chongqing 402460, China
共轭亚油酸(conjugated linoleic acids,CLA)是一类含有共轭双键的十八碳脂肪酸的统称,常见于反刍动物的脂肪中,具有抗肿瘤、改善动物免疫、抑制氧化应激、减少体内脂肪沉积和调控肌肉生长等多种生理功能[1-3]。肌肉组织是动物体内最重要的组织之一,研究表明,CLA可通过改变骨骼肌的肌纤维类型来改善猪肉品质;CLA还能通过影响肌肉的生理变化如调控肌肉代谢信号传导通路、影响肌肉能量代谢,从而增加动物机体瘦肉重和改变肌肉蛋白质含量[4-6]。
microRNAs(miRNAs)是一类长度为20~24 nt的非编码小RNA。miRNA通过碱基互补配对与靶基因3′非编码区结合,降解靶基因mRNA或抑制其翻译,在转录后水平调节基因表达,从而参与多种生物学功能[7]。研究证实,miRNA在肌肉细胞分化和组织生长发育过程中发挥重要作用,而动物膳食结构的改变能影响其肌肉组织miRNA的表达;如肥胖小鼠饲粮中添加烟酸可显著改变骨骼肌中42个miRNAs表达[8-9],生长猪饲喂不同能量来源的饲粮能显著影响肌肉组织中miR-23a、miR-409和miR-208b的表达[10]。饲粮中添加CLA也能影响机体组织miRNA表达,但目前CLA对miRNA表达影响的研究仅集中在脂肪上,Parra等[11-12]发现,小鼠饲粮中添加CLA能显著改变脂肪组织中miR-143、miR-103和miR-107等的表达,猪饲粮中添加CLA则能显著下调脂肪组织miR-143和miR-27的表达。
本实验室前期研究发现,生长育肥期饲粮中添加1%~1.5% CLA能显著促进生长猪背最长肌的重量,增加肌内脂肪的含量并改变肌肉中脂肪酸的组成[13-14]。Corino等[15]研究证实,母猪妊娠期和泌乳期饲粮添加CLA可显著提高仔猪生长性能。然而,饲粮中长期添加CLA对猪肌肉miRNA表达谱造成怎样的影响及其存在的分子机理目前还未见报道。因此,本试验在母猪及其子代饲粮中添加1.5% CLA,研究其对于子代肌肉组织miRNA表达谱的影响,从而探明CLA对猪肌肉组织中miRNA的调控作用。
1 材料与方法 1.1 试验材料和样品采集试验选取12头平均体重为60.83 kg和背膘厚为24.16 mm的初产荣昌母猪,随机分为对照组和CLA组,每组6个重复,每个重复1头母猪。CLA组母猪的饲粮从妊娠初期开始添加1.5% CLA混合物,持续到仔猪28日龄断奶;仔猪断奶后,按照公母各半的原则分别从对照组和CLA组挑选健康仔猪各30头,分成6个重复,每个重复5头仔猪;断奶后原CLA组仔猪饲粮中继续添加1.5% CLA混合物,对照组仔猪饲粮中不添加。对照组母猪和断奶后仔猪的饲养水平参照荣昌猪饲养标准(GB/T 7223-2008)和中国猪饲养标准(NY/T65-2004)进行配制,试验组饲粮中的CLA等比例替代基础饲粮中的大豆油,其他组分和营养成分与对照组一致。仔猪30 kg左右时,每组选择6头仔猪(3头母猪和3头公猪)进行屠宰,采集背最长肌(背肌)及腿肌组织样品,于液氮速冻后转移至-80 ℃冰箱中保存备用。
1.2 RNA文库的构建Trizol试剂法提取肌肉组织总RNA,每组3份样品进行等量混合,检测混合后总RNA质量,保证RIN(RNA integrity number)值为7.5~9.0。使用PAGE胶分离不同片段大小的RNA,切取18~30 nt之间的条带进行回收;分别配制3′和5′连接体系,混匀离心后适温连接;配制反转录体系,在PCR仪上适温反应一定时间,使连接产物反转录成双链,再配制PCR反应体系,在PCR仪上按照一定程序进行扩增;使用PAGE胶对PCR产物进行切胶回收纯化,完成文库构建。分别对对照组和CLA组的背肌和腿肌(混样)进行建库,每个组设定一个重复,因此共有8个库,包括背肌对照组Ⅰ、背肌对照组Ⅱ、背肌CLA组Ⅰ、背肌CLA组Ⅱ、腿肌对照组Ⅰ、腿肌对照组Ⅱ、腿肌CLA组Ⅰ和腿肌CLA组Ⅱ。构建好的文库进行质检后在Illumina HiSeq 2000平台进行高通量测序,测序工作由深圳华大基因科技有限公司完成。
1.3 已知miRNA的鉴定测序原始序列(raw reads)去除低质量、接头污染和长度小于18 nt的序列,获得clean reads。通过SOAP和bowtie将筛选后的sRNA定位到猪参考基因组上(ftp://ftp.ensembl.org/pub/release-87/gtf/sus_scrofa/),去除rRNA、tRNA、scRNA、snRNA和snoRNA等小RNA。然后将剩余序列与miRBase 21.0 (http://www.mirbase.org/)中猪的已知miRNA进行比对,确定已知的miRNA,用mirdeep软件预测新miRNA。
1.4 差异表达miRNA筛选和候选靶基因功能分析对样本中已知miRNA进行表达量的统计,用TPM[16]进行表达量归一化处理,采用SPSS 18.0中的描述统计(descriptive statistics)分析数据,以“平均值±标准差”表示miRNA表达量。使用ExpDiff方法比较对照组和CLA组之间miRNA表达量的差异,分析对照组和CLA组中已知miRNA的差异倍数(fold change)及P值,筛选差异表达的miRNA。用miRanda和RNAhybrid软件对差异表达的已知miRNA进行靶基因预测,取交集作为预测结果。使用KEGG代谢通路分析确定候选靶基因参与的主要生化代谢途径和信号转导途径。
1.5 荧光定量PCR验证测序为验证高通量测序结果的准确性,在背肌和腿肌各选择3个差异表达的miRNAs进行荧光定量PCR试验。按照反转录SYBR® Prime Script RM miRNA RT-PCR试剂盒(TaKaRa,Japan)的说明书步骤获得cDNA,特异性上游引物根据所选miRNA的自身序列进行设计,由上海生工生物工程股份有限公司合成(表 1),下游引物为通用引物,选择U6为内参基因。用SYBR® Premix Ex TaqTM Ⅱ试剂盒(TaKaRa,Japan)进行荧光定量PCR反应,体系:模板2.0 μL cDNA,10 μL 2×SYBR Premix Ex Taq Ⅱ,0.4 μL ROX Reference Dye, 0.8 μL miRNA特异引物,0.8 μL下游引物(试剂盒自带),加ddH2O使总体积为20 μL。所有的反应设置3个重复,数据结果通过2-△△CT法进行相对定量的统计分析。
|
|
表 1 荧光定量PCR引物序列 Table 1 The primers sequence for qRT-PCR |
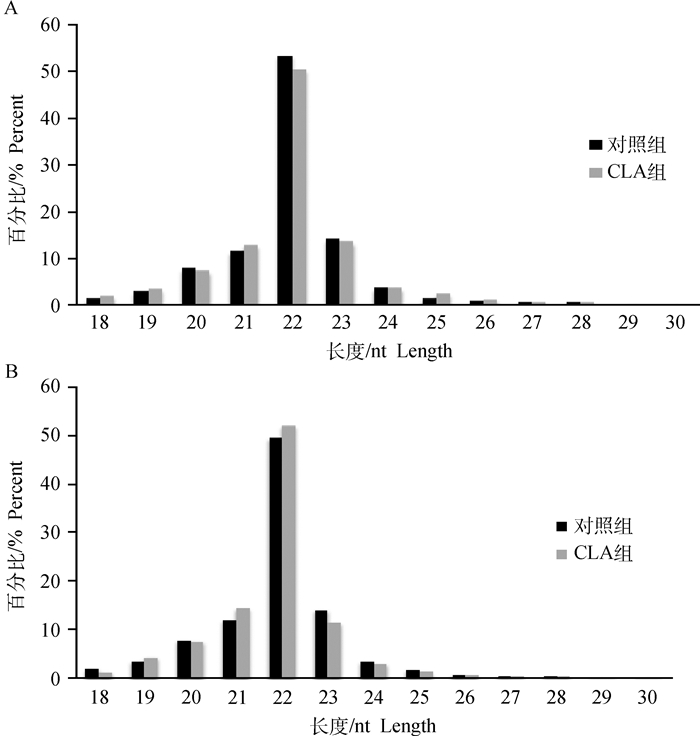
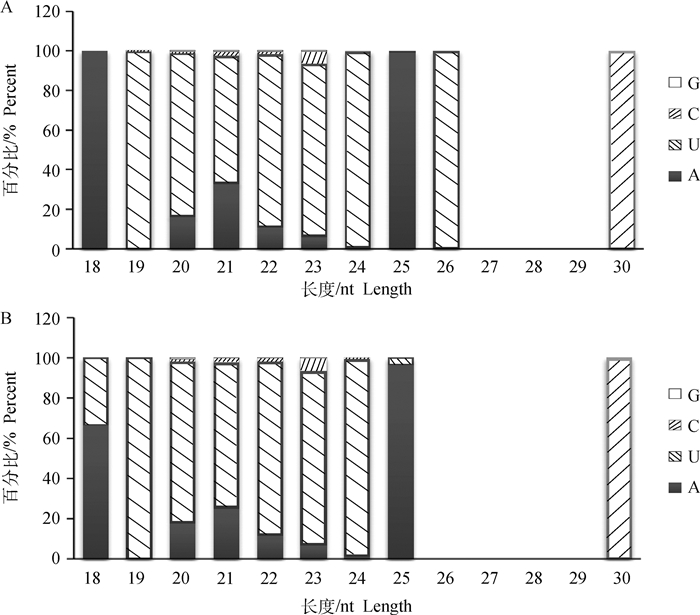
经过Solexa测序得到的raw data数据进行过滤处理,主要包括去除接头、污染及低质量序列等。本试验一共构建8个文库,背肌组4个文库总共获得了44 869 982条clean reads,占总数的95.10%;而腿肌组4个文库则获得了45 105 806条clean reads,占总数的95.09%(表 2)。背肌和腿肌中小RNA序列长度分布见图 1,绝大多数序列长度为20~23 nt,其中22 nt长度序列占的比例最大。miRNA在由前体发育为成熟体时其过程是由Dicer酶切完成的,酶切位点的特异性使得miRNA成熟体的首位碱基具有很强的偏好性,图 2统计了背肌和腿肌中长度为18~30 nt miRNA首位碱基分布情况,发现第一个碱基对U具有强烈的偏好性。
|
|
表 2 测序文库的小RNA片段质量分类 Table 2 The classification of small RNA tags in sequencing libraries |
|
图 1 背肌(A)和腿肌(B)中小RNA序列长度分布 Figure 1 Length distribution of small RNA sequence in back muscle(A) and leg muscle(B) |
|
图 2 背肌(A)和腿肌(B)中18~30 nt miRNA的首位碱基偏向性 Figure 2 First nucleotide bias of miRNA with 18-30 nt in back muscle(A) and leg muscle(B) |
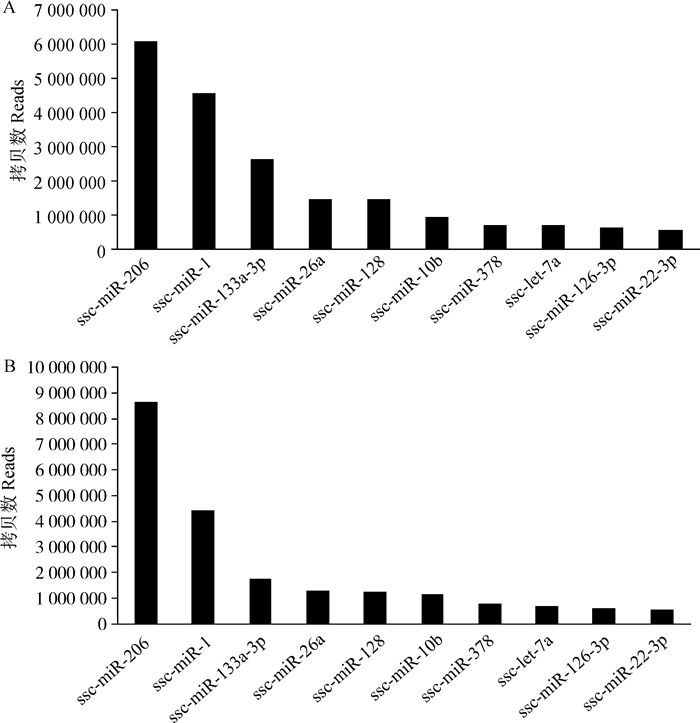
通过blast或bowtie将sRNA与miRBase 21.0中猪(Sus scrofa)miRNAs序列进行比对,背肌和腿肌组织中分别鉴定出306和304个已知miRNAs,有295个miRNAs共表达,其中ssc-miR-206、ssc-miR-1、ssc-miR-133a-3p、ssc-miR-26a、ssc-miR-10b和ssc-miR-128等多个miRNAs在背肌和腿肌组织中均高表达。作为肌肉特异性miRNA, ssc-miR-1、ssc-miR-206和ssc-miR-133a-3p的表达量均超过百万拷贝数(图 3)。
|
图 3 背肌(A)和腿肌(B)表达量前10的miRNAs Figure 3 The top 10 expressed miRNAs in back muscle(A) and leg muscle(B) |
在饲粮中持续添加CLA影响猪背肌和腿肌miRNA表达,对CLA组和对照组标准化之后的表达值进行差异比较,并把差异倍数取2为底的对数,以log2(Fold change)表示,正值代表上调,负值代表下调。结果表明,添加CLA后背肌中有151个miRNA表达上调,155个表达下调;腿肌中有141个miRNA表达上调,163个表达下调。腿肌中有17个miRNAs的log2(Fold change)大于1,而背肌中只有7个;腿肌中有13个miRNA的log2(Fold change)小于-1,而背肌中只有6个(表 3)。说明CLA对腿肌miRNA表达的影响要强于背肌。
|
|
表 3 添加CLA后对背肌和腿肌中miRNAs差异表达倍数的影响 Table 3 Effects of CLA supplementation on log2(Fold change) of miRNAs in back muscle and leg muscle |
以log2(Fold change)>1或 < -1,P < 0.05为标准筛选对照组和CLA组之间显著差异表达的miRNA,发现饲粮中添加CLA显著改变了背肌中ssc-miR-224、ssc-miR-9841-3p、ssc-miR-4332、ssc-miR-628和ssc-miR-339-3p的表达;显著改变了腿肌中12个miRNAs的表达,其中5个表达上调,7个表达下调,其中只有ssc-miR-224在猪背肌和腿肌都差异表达(表 4)。此外,背肌和腿肌组织中有8个差异表达miRNAs的差异倍数发生了4倍以上的改变,包括背肌中的ssc-miR-9841-3p和ssc-miR-339-3p,以及腿肌中的ssc-miR-151-5p、ssc-miR-215、ssc-miR-194b-5p、ssc-miR-452、ssc-miR-205和ssc-miR-545-3p。
|
|
表 4 添加CLA后肌肉中显著差异表达的miRNAs Table 4 Significant differentially expressed miRNAs in muscle responsing to CLA treatment |
CLA组猪的背肌和腿肌中分别有5个和12个miRNAs显著差异表达,其中ssc-miR-224在两种组织中都差异表达,因此共有16个差异表达miRNAs。用RNAhybrid和miRanda分析软件对差异表达的16个miRNAs进行靶基因预测,共预测到5 155个靶基因。对靶基因进行KEGG功能分析,发现其共富集到292条生物学通路中,其中有24个显著富集通路(P < 0.05,表 5)。显著富集通路中,Notch信号通路和MAPK信号通路都曾报道与肌肉发育和代谢密切相关。靶基因富集最多的前5个通路包括:代谢通路(376个)、肌动蛋白细胞骨架调控(182个)、癌症通路(160个)、黏着斑(148个)、MAPK信号通路(138个),其中,肌动蛋白细胞骨架调控、黏着斑和MAPK信号通路都为显著富集通路(P < 0.05)。
|
|
表 5 16个差异表达miRNAs靶基因的KEGG通路富集分析 Table 5 KEGG pathway analysis for target genes of 16 differentially expressed miRNAs |
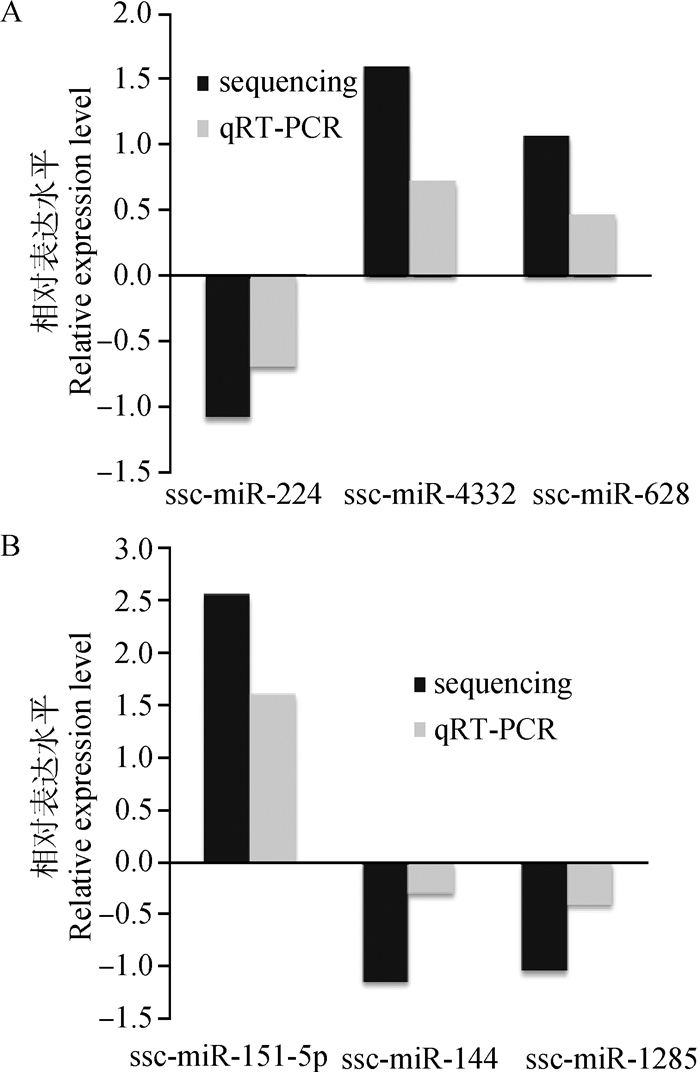
在表 4中随机选择6个差异表达的miRNAs,进行荧光定量PCR(qRT-PCR)验证。在背肌中检测ssc-miR-224、ssc-miR-4332和ssc-miR-628的表达,在腿肌中则检测ssc-miR-151-5p、ssc-miR-144和ssc-miR-1285的表达。qRT-PCR结果采用2-△△CT进行计算,并以log2(CLA组/对照组)得出相对表达情况。由图 4可知,miRNA的表达变化趋势与测序结果基本一致,但qRT-PCR检测到的表达水平变化幅度要小于测序。
|
A.背肌;B.腿肌 A.Back muscle; B. Leg muscle 图 4 差异表达miRNA的qRT-PCR验证 Figure 4 qRT-PCR validation for the differentially expressed miRNA |
骨骼肌是机体进行能量代谢的主要场所,主要作用是控制运动及维持姿势。所有的身体活动和体育活动,都是由骨骼肌收缩完成[17]。miRNA作为重要的调控因子,与骨骼肌的发育代谢密切相关[18]。本研究中,背肌和腿肌表达量前10的miRNAs包括ssc-miR-206、ssc-miR-1、ssc-miR-133a-3p、ssc-miR-26a、ssc-miR-10b、ssc-miR-128、ssc-miR-378、ssc-let-7a、ssc-miR-126-3p和ssc-miR-22-3p。其中,miR-206、miR-1和miR-133a-3p属于肌源miRNA,主要参与肌细胞增殖和分化等过程[19]。Qin等[20]研究发现,miR-26a、miR-10b、miR-128、miR-378和let-7a在猪骨骼肌中高表达,与本研究的结果一致。另外两个高表达miRNAs,miR-126-3p和miR-22-3p则报道与平滑肌细胞的增殖有关[21-22]。结果说明,这些高表达的miRNA在肌肉组织中发挥着重要的生物学功能。
3.2 添加CLA后肌肉中差异表达miRNA功能分析CLA对miRNA表达影响的研究主要集中在脂肪上,目前尚未有CLA对肌肉组织miRNA表达谱影响的报道。本研究通过在猪饲粮中添加1.5% CLA,对猪背肌和腿肌组织进行miRNA测序和生物信息学分析,旨在探明miRNA在CLA调控肌肉发育和代谢中发挥何种作用。结果显示,添加CLA对腿肌miRNA表达的影响要强于背肌,说明CLA对不同的部位肌肉影响效果不同。背肌和腿肌组织中共发现16个差异显著表达的miRNAs,其中只有ssc-miR-224在两种组织中都差异表达。有报道称,miR-224与动物脂肪细胞的成脂分化相关,可通过长链酰基辅酶A合成酶4(ACSL4)调节脂肪酸代谢;也有报道认为,miR-224通过调控过氧化物酶体增殖物激活受体-α(PPARα)的转录水平,从而影响脂肪酸分解代谢并控制脂肪堆积[23-24]。另外15个差异表达的miRNAs中,Wang等[25-29]研究认为,miR-4332能调控猪肌肉中脂肪沉积,miR-122、miR-215、miR-194和miR-205等则被证实与脂代谢相关;其他一些miRNAs,如miR-628、miR-151-5p和miR-339-3p等报道与肌肉的发育或疾病的发生有关[30-32]。说明CLA可能通过影响肌肉中重要miRNA表达调控肌肉脂肪沉积和发育代谢。
3.3 差异表达miRNAs靶基因功能和通路分析通过对16个差异表达miRNAs靶基因进行功能分析,发现5 155个靶基因显著富集到24条代谢通路中,其中MAPK信号通路和Notch信号通路与肌肉代谢密切相关。MAPK家族成员主要包括P38 MAPK、ERK 1/2和JNK,这些因子参与到细胞的生长发育、增殖分化、凋亡等多种生理过程[33-34]。Segalés等[35-37]发现,这3条MAPK通路在调控肌细胞的成脂转分化中起到不同的作用,抑制P38 MAPK能促进细胞成脂分化,增强脂肪代谢;阻断ERK 1/2抑制脂肪形成;阻断JNK则刺激脂肪分解,促进细胞凋亡。另外,P38 MAPK还具有促进肌细胞成肌分化,抑制其增殖的作用。Lee等[38-39]研究证实,肌细胞增殖过程中,添加c9, t11-CLA能增加细胞ERK 1/2和JNK的磷酸化水平,增加细胞分化过程中ERK 1/2的磷酸化水平,说明CLA可能通过影响MAPK通路中关键蛋白来调节肌细胞的增殖和分化。
Notch通路在调节骨骼肌发育和再生中起关键作用,Bi等[40-41]发现,Notch1通过激活效应基因Hes5,来调节猪骨骼肌卫星细胞增殖并维持细胞状态;激活Notch信号通路能促进肌细胞增殖,但抑制肌细胞分化。有报道显示,Notch通路还能调控肌肉糖代谢[42]。目前未见关于CLA对肌细胞中Notch通路影响的相关报道,但添加CLA能显著提高肌肉中棕榈酸的含量[14],而体外试验证实棕榈酸能提高肌细胞趋化因子配体1(CXCL1)表达水平,敲除CXCL1可减少肌细胞增殖,显著降低Notch蛋白质水平,因此推测,CLA可能是通过影响肌肉中其他脂肪酸含量变化来调控Notch信号通路的[43]。
4 结论猪饲粮中持续添加1.5% CLA可以显著改变背肌和腿肌组织miRNA的表达,背肌和腿肌组织中共得到16个差异表达的miRNAs,且其靶基因显著富集到24个信号通路,包括MAPK信号通路和Notch信号通路,提示CLA可通过改变肌肉miRNA表达调控肌肉重要代谢通路,进而影响猪肌肉生长发育。
| [1] | LEHNEN T E, DA SILVA M R, CAMACHO A, et al. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism[J]. J Int Soc Sports Nutr, 2015, 12: 36. DOI: 10.1186/s12970-015-0097-4 |
| [2] | WANG Y W, JONES P J H. Dietary conjugated linoleic acid and body composition[J]. Am J Clin Nutr, 2004, 79(6): 1153S–1158S. DOI: 10.1093/ajcn/79.6.1153S |
| [3] | FERLAY A, BERNARD L, MEYNADIER A, et al. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health:a review[J]. Biochimie, 2017, 141: 107–120. DOI: 10.1016/j.biochi.2017.08.006 |
| [4] | QI R L, YANG F Y, HUANG J X, et al. Supplementation with conjugated linoeic acid decreases pig back fat deposition by inducing adipocyte apoptosis[J]. BMC Vet Res, 2014, 10: 141. DOI: 10.1186/1746-6148-10-141 |
| [5] | KIM Y, KIM J, WHANG K Y, et al. Impact of conjugated linoleic acid (CLA) on skeletal muscle metabolism[J]. Lipids, 2016, 51(2): 159–178. DOI: 10.1007/s11745-015-4115-8 |
| [6] |
黄金秀, 杨飞云, 刘作华, 等. 共轭亚油酸对体外培养的猪骨骼肌肌纤维类型组成的影响[J]. 畜牧兽医学报, 2010, 41(3): 295–300.
HUANG J X, YANG F Y, LIU Z H, et al. Effect of conjugated linoleic acid on the composition of myofiber types in skeletal muscle cells of pigs in vitro[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(3): 295–300. (in Chinese) |
| [7] | VIENBERG S, GEIGER J, MADSEN S, et al. microRNAs in metabolism[J]. Acta Physiol, 2017, 219(2): 346–361. DOI: 10.1111/apha.2017.219.issue-2 |
| [8] |
盛熙晖, 邓桂馨, 倪和民, 等. microRNAs调控动物骨骼肌发育的研究进展[J]. 畜牧兽医学报, 2015, 46(2): 179–185.
SHENG X H, DENG G X, NI H M, et al. Research progress on microRNAs regulating animal skeletal muscle development[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(2): 179–185. (in Chinese) |
| [9] | COUTURIER A, KELLER J, MOST E, et al. Niacin in pharmacological doses alters microRNA expression in skeletal muscle of obese Zucker rats[J]. PLoS One, 2014, 9(5): e98313. DOI: 10.1371/journal.pone.0098313 |
| [10] | LI Y J, LI J L, ZHANG L, et al. Effects of dietary energy sources onPost mortem glycolysis, meat quality and muscle fibre type transformation of finishing pigs[J]. PLoS One, 2015, 10(6): e0131958. DOI: 10.1371/journal.pone.0131958 |
| [11] | PARRA P, SERRA F, PALOU A. Expression of adipose microRNAs is sensitive to dietary conjugated linoleic acid treatment in mice[J]. PLoS One, 2010, 5(9): e13005. DOI: 10.1371/journal.pone.0013005 |
| [12] | QI R L, CHEN Y, HUANG J X, et al. Effects of conjugated linoleic acid on the expression levels of miR-27 and miR-143 in pig adipose tissue[J]. Genet Mol Res, 2015, 14(2): 6985–6992. DOI: 10.4238/2015.June.26.7 |
| [13] | HUANG J X, QI R L, CHEN X L, et al. Improvement in the carcass traits and meat quality of growing-finishing Rongchang pigs by conjugated linoleic acid through altered gene expression of muscle fiber types[J]. Genet Mol Res, 2014, 13(3): 7061–7069. DOI: 10.4238/2014.March.24.25 |
| [14] |
王琪, 齐仁立, 王敬, 等. 从胚胎期到育肥期饲粮添加共轭亚油酸对猪肉品质、脏器指数及脂肪酸组成的影响[J]. 中国畜牧杂志, 2017, 53(2): 79–84.
WANG Q, QI R L, WANG J, et al. Effects of CLA supplementation on meat quality, organ index and fatty acid composition of pigs from embryo to finishing period[J]. Chinese Journal of Animal Science, 2017, 53(2): 79–84. (in Chinese) |
| [15] | CORINO C, PASTORELLI G, ROSI F, et al. Effect of dietary conjugated linoleic acid supplementation in sows on performance and immunoglobulin concentration in piglets[J]. J Anim Sci, 2009, 87(7): 2299–2305. DOI: 10.2527/jas.2008-1232 |
| [16] | ZHOU L, CHEN J, LI Z, et al. Integrated profiling of microRNAs and mRNAs:microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma[J]. PLoS One, 2010, 5(12): e15224. DOI: 10.1371/journal.pone.0015224 |
| [17] | FRONTERA W R, OCHALA J. Skeletal muscle:a brief review of structure and function[J]. Calcif Tissue Int, 2015, 96(3): 183–195. DOI: 10.1007/s00223-014-9915-y |
| [18] |
吴望军, 陈杰, 黄瑞华, 等. 高通量技术挖掘猪microRNA的研究进展[J]. 畜牧兽医学报, 2013, 44(12): 1857–1866.
WU W J, CHEN J, HUANG R H, et al. Advances in porcine microRNA research using high-throughput technologies[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(12): 1857–1866. (in Chinese) |
| [19] | GE Y J, CHEN J. microRNAs in skeletal myogenesis[J]. Cell Cycle, 2011, 10(3): 441–448. DOI: 10.4161/cc.10.3.14710 |
| [20] | QIN L J, CHEN Y S, LIU X H, et al. Integrative analysis of porcine microRNAome during skeletal muscle development[J]. PLoS One, 2013, 8(9): e72418. DOI: 10.1371/journal.pone.0072418 |
| [21] | JANSEN F, STUMPF T, PROEBSTING S, et al. Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6[J]. J Mol Cell Cardiol, 2017, 104: 43–52. DOI: 10.1016/j.yjmcc.2016.12.005 |
| [22] | HUANG S C, WANG M, WU W B, et al. Mir-22-3p Inhibits arterial smooth muscle cell proliferation and migration and neointimal hyperplasia by targeting HMGB1 in arteriosclerosis obliterans[J]. Cell Physiol Biochem, 2017, 42(6): 2492–2506. DOI: 10.1159/000480212 |
| [23] | PENG Y D, XIANG H, CHEN C, et al. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism[J]. Int J Biochem Cell Biol, 2013, 45(8): 1585–1593. DOI: 10.1016/j.biocel.2013.04.029 |
| [24] | STACHOWIAK M, SZYDLOWSKI M, FLISIKOWSKI K, et al. Polymorphism in 3' untranslated region of the pigPPARA gene influences its transcript level and is associated with adipose tissue accumulation[J]. J Anim Sci, 2014, 92(6): 2363–2371. DOI: 10.2527/jas.2013-7509 |
| [25] | WANG Z X, LI Q G, CHAMBA Y, et al. Identification of genes related to growth and lipid deposition from transcriptome profiles of pig muscle tissue[J]. PLoS One, 2017, 12(2): e0172930. DOI: 10.1371/journal.pone.0172930 |
| [26] |
陈晨, 胡雄贵, 朱吉, 等. 猪脂肪发育相关miRNAs的功能研究进展[J]. 畜牧兽医学报, 2015, 46(12): 2117–2126.
CHEN C, HU X G, ZHU J, et al. Progress on the research of miRNAs associated with fat development in pigs[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(12): 2117–2126. (in Chinese) |
| [27] | CASAS-AGUSTENCH P, FERNANDES F S, DO CARMO M G T, et al. Consumption of distinct dietary lipids during early pregnancy differentially modulates the expression of microRNAs in mothers and offspring[J]. PLoS One, 2015, 10(2): e0117858. DOI: 10.1371/journal.pone.0117858 |
| [28] | JEONG B C, KANG I H, HWANG Y C, et al. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFⅡ expression[J]. Cell Death Dis, 2014, 5: e1532. DOI: 10.1038/cddis.2014.485 |
| [29] | YU J W, CHEN Y S, QIN L M, et al. Effect of miR-205 on 3T3-L1 preadipocyte differentiation through targeting to glycogen synthase kinase 3 beta[J]. Biotechnol Lett, 2014, 36(6): 1233–1243. DOI: 10.1007/s10529-014-1491-8 |
| [30] | YU Y H, LI X, LIU L Y, et al. miR-628 promotes burn-induced skeletal muscle atrophy via targeting IRS1[J]. Int J Biol Sci, 2016, 12(10): 1213–1224. DOI: 10.7150/ijbs.15496 |
| [31] | ZHANG Y, WANG R J, DU W J, et al. Downregulation of miR-151-5p contributes to increased susceptibility to arrhythmogenesis during myocardial infarction with estrogen deprivation[J]. PLoS One, 2013, 8(9): e72985. DOI: 10.1371/journal.pone.0072985 |
| [32] | HENDGEN-COTTA U B, MESSIHA D, ESFELD S, et al. Inorganic nitrite modulates miRNA signatures in acute myocardialin vivo ischemia/reperfusion[J]. Free Radic Res, 2017, 51(1): 91–102. DOI: 10.1080/10715762.2017.1282158 |
| [33] | SELIM K A, ABDELRASOUL H, ABOELMAGD M, et al. The role of the MAPK signaling, topoisomerase and dietary bioactives in controlling cancer incidence[J]. Diseases, 2017, 5(2): 13. |
| [34] | KYOSSEVA S V. Targeting MAPK signaling in age-related macular degeneration[J]. Ophthalmol Eye Dis, 2016, 8: 23–30. |
| [35] | SEGALÉS J, PERDIGUERO E, MUÑOZ-CÁNOVES P. Regulation of muscle stem cell functions:a focus on the p38 MAPK signaling pathway[J]. Front Cell Dev Biol, 2016, 4: 91. |
| [36] | QI R, LIU H, WANG Q, et al. Expressions and regulatory effects of P38/ERK/JNK Mapks in the adipogenic trans-differentiation of C2C12 myoblasts[J]. Cell Physiol Biochem, 2017, 44(6): 2467–2475. DOI: 10.1159/000486169 |
| [37] | BRIEN P, PUGAZHENDHI D, WOODHOUSE S, et al. p38α MAPK regulates adult muscle stem cell fate by restricting progenitor proliferation during postnatal growth and repair[J]. Stem Cells, 2013, 31(8): 1597–1610. DOI: 10.1002/stem.1399 |
| [38] | LEE J H, TACHIBANA H, MORINAGA Y, et al. Modulation of proliferation and differentiation of C2C12 skeletal muscle cells by fatty acids[J]. Life Sci, 2009, 84(13-14): 415–420. DOI: 10.1016/j.lfs.2009.01.004 |
| [39] | HUANG W C, TU R S, CHEN Y L, et al. Conjugated linoleic acids suppress inflammatory response and ICAM-1 expression through inhibition of NF-κB and MAPK signaling in human bronchial epithelial cells[J]. Food Function, 2016, 7(4): 2025–2033. DOI: 10.1039/C5FO01037C |
| [40] | BI P P, YUE F, SATO Y, et al. Stage-specific effects of Notch activation during skeletal myogenesis[J]. eLife, 2016, 5: e17355. DOI: 10.7554/eLife.17355 |
| [41] | QIN L L, XU J, WU Z F, et al. Notch1-mediated signaling regulates proliferation of porcine satellite cells (PSCs)[J]. Cell Signal, 2013, 25(2): 561–569. DOI: 10.1016/j.cellsig.2012.11.003 |
| [42] | PAJVANI U B, QIANG L, KANGSAMAKSIN T, et al. Inhibition of Notch uncouples Akt activation from hepatic lipid accumulation by decreasing mTorc1 stability[J]. Nat Med, 2013, 19(8): 1054–1060. DOI: 10.1038/nm.3259 |
| [43] | MASUDA S, TANAKA M, INOUE T, et al. Chemokine (C-X-C motif) ligand 1 is a myokine induced by palmitate and is required for myogenesis in mouse satellite cells[J]. Acta Physiol, 2017, 222(3): e12975. |



