牦牛是青藏高原重要的动物资源,是牛属动物中唯一对低氧环境具有强适应能力的动物[1],其繁殖力低下,体外卵母细胞成熟率和胚胎发育能力均较低,严重影响其体外受精、体细胞克隆等繁殖技术的发展[2]。成熟卵母细胞的产生对体外受精具有非常重要的作用,其位于原始卵泡中,由少量鳞状颗粒细胞(granulosa cells, GCs)包围的小卵母细胞形成[3-5]。生殖激素与颗粒细胞相结合可促进卵母细胞的发育,如促卵泡素(follicle-stimulating hormone, FSH)和促黄体素(luteinizing hormone, LH)等[6]。FSH是由垂体前叶的促性腺激素产生的糖蛋白,对体内卵泡的生长具有重要作用[7]。卵母细胞在体外成熟期间FSH可促进卵丘-卵母细胞复合体(cumulus-oocyte complexes, COCs)扩增和核成熟[8],FSH还可使胚胎囊胚率和孵化囊胚率均有所增加[9]。Hiradate等[10]发现,SH和表皮生长因子(epidermal growth factor, EGF)可通过MAPK依赖性途径而调节神经降亚素(neurotensin, NT)的表达,而NT可以充当精子获能的启动子使其在雌性生殖道中发生顶体反应。Prochazka等[11]研究发现,PKA和MAPK14途径对于FSH诱导表皮生长因子受体(epidermal growth factor receptor, EGFR)的反式激活起着至关重要的作用。对FSH在卵母细胞发育中发挥作用的研究越来越多,有研究表明,卵母细胞中FSH可改变基因的表达模式[12],体外受精-胚胎移植(IVF-ET)周期血清中, FSH水平较高可能会导致卵母细胞的棕色透明带厚度增加,胚胎质量降低,妊娠率降低[13]。FSH与卵泡中EGF相互作用以激活卵母细胞中磷脂酰肌醇3-磷酸/Akt级联,以控制其翻译和发育能力[14]。
有研究表明,牛卵母细胞早期凋亡与其发育能力有关[15],FSH和EGF的协同作用可以更好地阻止猪颗粒细胞多层膜共培养中颗粒细胞的凋亡[16]。徐庚全等[17]研究表明,FSH可通过调节牦牛颗粒细胞的凋亡及分泌功能,在调控卵泡发育及排卵过程中发挥重要作用。B细胞淋巴瘤/白血病基因伴随X蛋白(B cell lymphoma/leukemia-2 associated X, Bax)和B-细胞淋巴瘤/白血病-2原癌基因(B cell lymphoma/leukemia-2, Bcl-2)在调节细胞凋亡中起关键作用[18],潘阳阳等[2]研究表明, EGF和EGFR作为牦牛COCs体外成熟过程中重要的自分泌因子,且外源的EGF可以显著提高卵母细胞成熟率、卵裂率及囊胚率,其作用机制可能与调控凋亡相关基因Bax和Baxi表达有关。综上表明,了解激素对生长因子的作用以及卵母细胞凋亡的影响,对提高卵母细胞质量和附植前胚胎的发育具有重要意义,但目前在国内外尚未见关于FSH对牦牛卵母细胞EGF、EGFR表达及其细胞凋亡的影响方面的报道,本试验通过在牦牛卵母细胞体外培养,加入不同浓度的FSH,检测卵母细胞成熟率,采用Real-time PCR、蛋白免疫印迹法和免疫荧光染色法检测卵母细胞成熟过程中Bax和Bcl-2、EGF及EGFR在基因和蛋白水平的表达动态,以期阐明FSH与EGF与EGFR的关系,为进一步探讨FSH在牦牛生殖过程中发挥的作用以及EGF和EGFR对牦牛卵母细胞成熟和后期胚胎发育的影响提供理论依据。
1 材料与方法 1.1 材料和试剂促卵泡素(FSH)、EGF、雌二醇(E2)、促黄体素(LH)、透明质酸酶以及M199培养液为Sigma公司产品,胎牛血清FBS购自Hyclone公司。微量样品总RNA提取试剂盒(Omega)、两步法反转录试剂盒(Promega)、SYBR Green Ⅱ荧光定量PCR试剂盒(宝生物),免疫荧光染色所用试剂购自南京碧云天生物公司,其他试剂均为国产分析纯。EGF抗体(ab9862, Munich, Germany)、EGFR抗体(ab2430, Munich, Germany)、Bax抗体(ab77566,Munich, Germany)和Bcl-2抗体(ab117115, Munich, Germany)为Abcam公司产品,所有二抗为北京博奥森公司产品。荧光定量PCR仪(ABI ViiA7,Life technologies公司,美国),倒置荧光显微镜(Olympus,日本)。
1.2 牦牛卵巢及卵母细胞的采集牦牛的卵巢采自青海省西宁市乐家湾屠宰场。将从屠宰场采集的牦牛卵巢置于37 ℃生理盐水中,4 h内带回实验室,用生理盐水清洗3遍,用带18G针头注射器采集卵巢表面直径约3~10 mm卵泡,体视显微镜下挑选形态完好、细胞质均匀,周围带有3层以上致密卵丘细胞的卵丘-卵母细胞复合体(cumulus-oocyte complexes,COCs),用洗卵液(DPBS+ 5% FBS)清洗3遍后,备用。
1.3 卵母细胞体外成熟配制卵母细胞成熟液(M199+10 mmol·L-1 Hepes+10% FBS+1 μg·mL-1E2+1 mg·mL-1 BSA),分别加入不同浓度的FSH,使其最终浓度为0、2、5、10 μg·mL-1。将COCs随机分为4组,分别放入含不同浓度FSH的卵母细胞成熟液中,于38 ℃、5% CO2及饱和湿度的培养箱中进行成熟培养,培养24 h后,将部分成熟COCs进行冻存和固定,每个试验组重复5次。
1.4 卵母细胞成熟率统计在光学显微镜下,观察COCs的形态,根据卵丘细胞的扩张情况判断卵母细胞的成熟。将成熟的COCs放入0.1%透明质酸酶中,用移液枪轻微吹打5 min,以去除卵丘细胞而得到裸卵,用玻璃针拨动其卵母细胞,根据第一极体排出来统计成熟卵母细胞(M Ⅱ期),每个试验组重复5次。
1.5 RNA提取及反转录将每个试验组中的5个COCs用PBS清洗3次后,用微量样品总RNA提取试剂盒提取总RNA,再用两步法反转录试剂盒将总RNA反转录为cDNA[19],放入-80 ℃保存备用。
1.6 qRT-PCR检测Bax、Bcl-2、EGF和EGFR基因的表达根据GenBank数据库中检索的牛EGF(AY192564)、EGFR(HM749883)、Bax (NM173894)、Bcl-2(NM001166486)和β-actin(JF830811)的序列,用Primer Premier 6.0软件设计了5对引物(表 1),引物均由上海华大基因科技有限公司合成。采用实时荧光定量PCR仪检测EGF、EGFR、Bax和Bcl-2基因mRNA水平的表达。反应体系为20 μL:10 μL FastStart Master SYBR Green Mix,1 μL cDNA,上下游引物各0.5 μL,ROX Reference Dye Ⅱ(50×)0.5 μL,无菌去离子水7.5 μL。反应条件:95 ℃预变性10 s,95 ℃变性4 s,60 ℃退火(EGF、EGFR、β-actin),57 ℃(Bax)和64 ℃(Bcl-2)退火20 s,72 ℃延伸10 s,40个循环;每个样品重复3次。
|
|
表 1 目的基因和内参基因引物序列 Table 1 Primer sequences of target and house-keeping genes |
将不同处理组的COCs用免疫染色固定液在室温下固定1 h,然后在室温条件下,用免疫染色洗涤液洗3次,用含0.2% TritonX-100的免疫染色封闭液封闭1 h,将COCs置于用含2%BSA的PBS稀释成5 μg·mL-1的一抗EGF和EGFR中(EGF和EGFR抗体分别是山羊和兔多克隆抗体),在4 ℃孵育过夜,再用含2%BSA的PBS洗3次,先后移入含有APC标记的驴抗山羊抗体和FITC标记的山羊抗兔抗体中孵育2 h,清洗3次后,封片,再用荧光显微镜观察并照相。同时,使用含有2%BSA的PBS替代阴性一抗作为对照组,其他步骤与试验组保持一致。
1.8 Western blotting检测Bax和Bcl-2蛋白的表达提取不同处理组的总蛋白,将其与上样缓冲液(4×)按3:1混合,100 ℃煮10 min,冰上5 min,进行SDS-PAGE电泳;再用半干法将其转移至聚偏二氟乙烯(PVDF)膜上,然后用5%的脱脂奶粉溶液室温封闭1.5 h(摇床振动,70 r·min-1),用Bax和Bcl-2的抗体4 ℃孵育过夜,用二抗室温孵育1.5 h,每步完成后,用TBST洗膜,每次洗3遍,每遍5 min,最后用ECL避光显色,X光片显影、定影,再扫描蛋白印迹条带。试验重复3次,对扫描的蛋白印迹条带用Image J软件进行图像分析。
1.9 数据分析采用SPSS21.0统计软件对试验数据进行单因素方差分析,分析不同COCs组卵母细胞中EGF、EGFR、Bax和Bcl-2 mRNA以及蛋白质的表达差异,成熟率以卵母细胞数为基数进行比较,每组至少3次重复。结果以“平均值±标准差(Mean±SE)”表示,P<0.05表示差异显著。
2 结果 2.1 FSH对牦牛卵母细胞成熟率的影响根据卵丘细胞的扩散(图 1)和第一极体(图 2)来判断卵母细胞的成熟情况,随着卵母细胞的成熟,卵丘细胞扩散越明显(图 1),成熟卵母细胞的第一极体为圆形,胞质均质,膜表面平滑完整(图 2)。消化卵丘细胞后,根据成熟卵母细胞排出第一极体来统计卵母细胞的成熟率(表 2),发现成熟培养液中加入FSH均可提高COCs的成熟率,当成熟液中加FSH为5 μg·mL-1时成熟率最高,达到76.84%,其显著高于其他组(P<0.05);2和10 μg·mL-1 FSH组成熟率分别为67.41%和57.23%;无FSH (0 μg·mL-1)阴性对照组的成熟率最低,为51.97%。10 μg·mL-1 FSH组与无FSH(0 μg·mL-1)阴性对照组之间差异不显著(P>0.05),而其他组别之间均差异显著(P<0.05)。
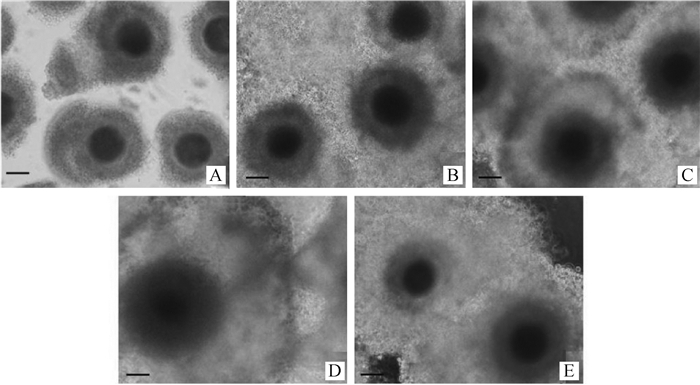
|
A.未成熟的卵母细胞;B.阴性对照卵母细胞(FSH 0 μg·mL-1);C.2 μg·mL-1 FSH;D.5 μg·mL-1 FSH;E.10 μg·mL-1 FSH A.Immature oocytes; B.The negative control oocytes (0 μg·mL-1 FSH); C. 2 μg·mL-1 FSH; D. 5 μg·mL-1 FSH; E. 10 μg·mL-1 FSH 图 1 牦牛未成熟的卵母细胞和4种不同浓度FSH处理24 h后的成熟卵母细胞(200×, 标尺50 μm) Figure 1 Development of yak oocytes in vitro with 4 different FSH concentrations for 24 h and immaturate oocytes (200×, Bar 50 μm) |
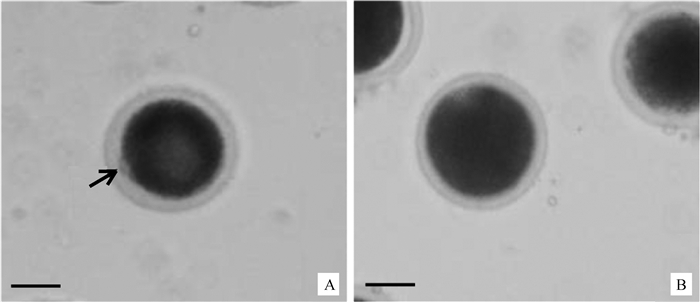
|
A.成熟卵母细胞;B.未成熟卵母细胞。↗.第一极体 A. Mature oocytes; B.Immature oocytes. ↗.The first polarbody 图 2 根据第一极体判断卵母细胞的成熟(200×,标尺50 μm) Figure 2 The maturation of oocytes were assessed based on first polar body (200×, Bar 50 μm) |
|
|
表 2 不同浓度FSH对卵母细胞成熟率的影响 Table 2 Rates of mature yak oocytes after addition of FSH with various concentrations |
通过实时荧光定量对EGF和EGFR的相对表达量进行分析表明(图 3),在0、2和5 μg·mL-1 FSH组中,随着FSH浓度增加,EGF和EGFR表达量逐渐升高;然而,在10 μg·mL-1 FSH组中,EGF和EGFR的表达水平降低,其中在5 μg·mL-1 FSH组中,EGF和EGFR的表达均显著高于0、2和10 μg·mL-1 FSH组(P<0.05)。
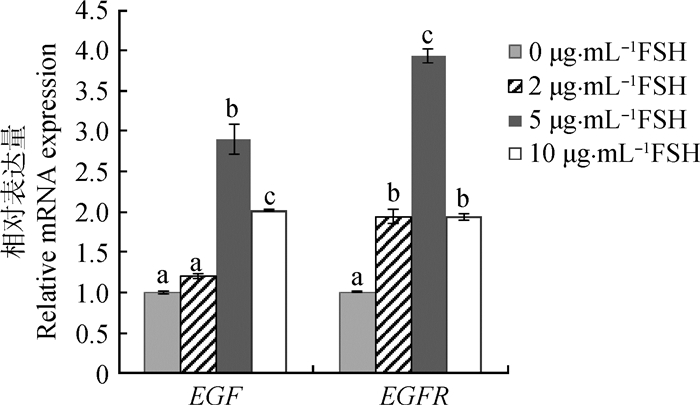
|
误差线上不同的字母表示不同浓度组间差异显著(P<0.05);相同的字母表示不同浓度组间差异不显著(P>0.05)。下同 Bars with different superscripts mean significant difference among different concentration groups (P < 0.05); Bars with same superscripts mean no significant difference among different concentration groups (P > 0.05). The same as below 图 3 不同浓度FSH的成熟液处理后牦牛卵母细胞EGF和EGFR的相对表达量 Figure 3 Relative abundance of EGF and EGFR mRNA in yak oocytes with different concentrations of FSH |
通过实时荧光定量对Bax和Bcl-2的相对表达量进行分析表明(图 4),在2和5 μg·mL-1 FSH组中,Bax和Bcl-2的表达显示明显的逆向模式,Bcl-2的表达水平逐渐升高,Bax的表达水平逐渐降低。5 μg·mL-1 FSH组Bax的表达量最低,其显著低于0、2和10 μg·mL-1 FSH组(P<0.05),而Bcl-2表达量最高,其显著高于0、2和10 μg·mL-1 FSH组(P<0.05)。2 μg·mL-1 FSH组中Bax的表达水平低于0和10 μg·mL-1 FSH组,Bcl-2在2 μg·mL-1 FSH组中的表达均比0和10 μg·mL-1 FSH组高。
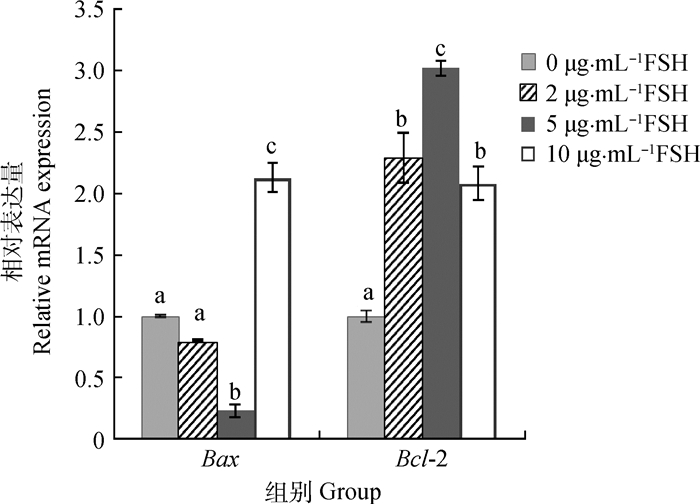
|
图 4 不同浓度FSH的成熟液处理后牦牛卵母细胞Bax和Bcl-2的相对表达量 Figure 4 Relative abundance of Bax and Bcl-2 mRNA in yak oocytes with different concentrations of FSH |
对EGF和EGFR蛋白分别用蓝色和绿色进行荧光染色(图 5)。FSH浓度较低时,EGF和EGFR的荧光强度较低,卵丘细胞扩增不完整(图 5 A1,图 5 A2, 图 5 B1和图 5B2)随着FSH浓度增加时,EGF和EGFR的荧光强度逐渐增强,其中5 μg·mL-1 FSH组中EGF和EGFR表达水平最高,并且卵丘细胞的扩增比其他组的细胞扩增完整(图 5 C1和图 5 C2),但当FSH浓度增加到10 μg·mL-1时,而荧光强度降低(图 5 D1,图 5 D2)。通过免疫荧光检测发现,EGF主要位于卵丘细胞中,而EGFR在卵丘细胞和卵母细胞上均可检测到(图 5)。
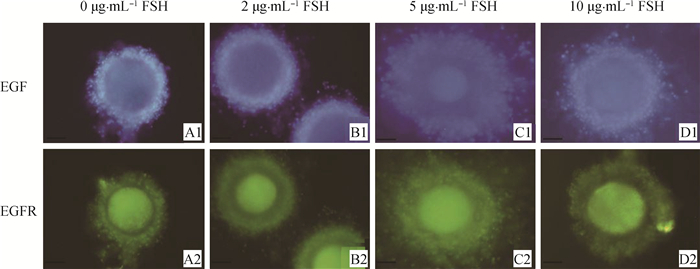
|
图 5 不同浓度FSH的成熟液处理后牦牛COCs中EGF、EGFR蛋白分布(200×) Figure 5 The distribution of EGF and EGFR proteins in yak COCs with different concentrations of FSH(200×) |
Western blotting方法检测, 添加FSH显著增加了牦牛COCs中抗凋亡分子Bcl-2蛋白水平的表达,同时也明显降低了牦牛COCs中促凋亡分子Bax蛋白水平的表达(10 μg·mol-1 FSH组除外)(图 6A)。在5 μg·mL-1 FSH组中Bax表达量最低,其显著低于0 (阴性对照)、2和10 μg·mL-1 FSH组(P<0.05),而在10 μg·mL-1 FSH组中Bax表达量最高(图 6B);Bcl-2蛋白在5与10 μg·mL-1 FSH组中相比其表达量接近,其差异性不显著(P>0.05)(图 6B)。
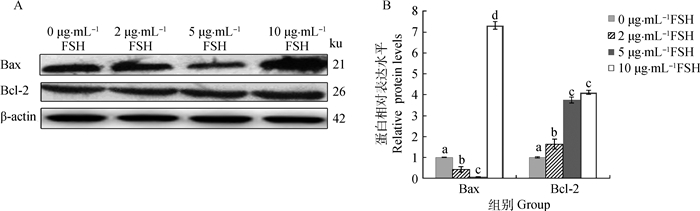
|
图 6 Western blotting检测(A)及不同组Bax和Bcl-2蛋白水平的表达(B) Figure 6 Detected the expression levels (B) of Bax and Bcl-2 proteins by Western blotting(A) in different groups |
了解生殖激素对成熟卵母细胞的调节作用对于开发有效的体外成熟(IVM)体系是非常重要的。本研究探讨了FSH对牦牛卵母细胞IVM的特异性作用及其对EGF、EGFR、Bax和Bcl-2等生长和凋亡相关因子的作用。EGFR的特异性抑制剂tyrphostin AG 1478有效阻断FSH诱导的猪卵母细胞减数分裂的恢复,表明EGFR或EGF受体信号通路与FSH的作用有关[20]。此外,EGF和FSH对猪卵母细胞细胞质的成熟具有协同作用[21]。本研究发现,FSH提高了牦牛COCs中细胞核的成熟率(表 2),并且在体外条件下,诱导了牦牛卵母细胞成熟过程中EGF和EGFR的表达(图 3和图 5),而且其与FSH的浓度有关;当FSH为5 μg·mL-1时,卵母细胞中EGF和EGFR水平最高,卵母细胞成熟率最高,卵丘细胞的扩增比其他各组均完整(图 1和图 5),但当FSH为10 μg·mL-1时,卵母细胞成熟率及EGF、EGFR、Bcl-2表达均下降(表 2,图 6),而Bax增强(图 4和6)。因此,本研究发现牦牛卵母细胞体外成熟过程中FSH的最适浓度为5 μg·mL-1。
本研究结果表明,FSH诱导牦牛卵丘细胞的扩张(图 1和图 5)。前人研究表明,FSH在卵母细胞发育中起关键作用[22],其可以增强卵丘细胞的扩张[23],这与本研究相一致;EGF和EGFR在牦牛卵母细胞成熟过程中受FSH的诱导表达,并且牦牛卵丘细胞的增殖与其表达水平一致。FSH主要通过激活cAMP依赖性信号通路后激活其周围卵丘细胞中的丝裂原活化蛋白激酶(mitogen-activated protein kinase, MAPK)[24],卵泡发生~早期窦性阶段被认为FSH是独立的,而窦性(晚期)卵泡发生完全依赖FSH[25],但是,cAMP不能直接激活MAPK[26],cAMP激活MAPK的具体过程尚未完全了解。FSH通过p38 MAPK诱导信号转导和转录激活因子1(STAT1)丝氨酸727去磷酸化作用可以下调细胞色素P450 1B1(cytochrome P450 B1, Cyp1b1)表达并维持小鼠优势卵泡中的雌二醇水平[27],FSH和17β-雌二醇联合作用时,通过cAMP、Ca2+内流和ERK1/2途径参与了FSH和17β-雌二醇对细胞周期蛋白D1(Cyclin D1)mRNA和细胞周期蛋白E1(Cyclin E1)mRNA在细胞中的表达[28]。蛋白激酶A Ⅱ(protein kinase A Ⅱ, PKAII)和蛋白激酶C(protein kinase C, PKC)是FSH通过MAPK激活而起作用的可能途径[29]。综上,EGF和EGFR在FSH诱导的牦牛卵母细胞发育过程中发挥重要作用。然而,在大多数组中EGFR的表达水平高于EGF(图 3),这可能是由许多激活EGFR配体引起的,包括EGF、TGFα、双调蛋白(Ar)、肝素结合的EGF (HB-EGF)和钙结合蛋白-D9k(CaBP-9k)等[30-31]。因此,FSH对COCs分泌EGFR配体的影响不容忽视,本研究用荧光标记物标记的EGF和EGFR在牦牛COCs中具有特异性的表达,其在卵丘细胞和卵母细胞上均可检测到EGFR,而EGF仅在卵丘细胞上检测到(图 5),这与卵丘细胞的存在对于EGF作用是必需的[21]研究相一致。
卵母细胞质量是决定胚胎发育的最重要参数,结合COCs的形态学研究凋亡基因的表达是判断COCs质量的重要标准。本研究在卵丘细胞完全扩张组中Bcl-2基因表达量最高,而Bax表达最低,同时卵母细胞的成熟率也最高,这表明FSH通过调控Bax、Bcl-2等凋亡相关基因的表达,抑制了卵母细胞凋亡。有研究表明,Bax和Bcl-2表达与体外培养的牛卵母细胞和胚胎质量有关[18],Bax与Bcl-2的比率可以用来测量卵母细胞和胚胎存活或凋亡的趋势。然而,调控卵母细胞质量并影响卵母细胞和胚胎发育的细胞凋亡基因很多[32],FSH在哺乳动物卵母细胞成熟和胚胎发育过程中是否诱导其他凋亡基因尚不清楚。
本研究FSH诱导EGF、EGFR和Bcl-2表达趋势在牦牛卵母细胞发育过程是一致的,而Bax的表达恰恰与其相反。Kawamura等[33]研究表明,EGF作为自分泌/旁分泌的生长因子可促进人早期胚胎发育,EGF对凋亡相关基因是可以进行调控的[34]。本研究结果表明,在牦牛卵母细胞成熟过程中,FSH可调节EGF、EGFR、Bcl-2和Bax的表达。因此,推测EGF、EGFR、Bcl-2和Bax在FSH诱导的卵母细胞成熟和抑制细胞凋亡中起重要作用,但Bax和Bcl-2变化是否受FSH诱导的EGF和EGFR调节,以及FSH信号传导通路与EGF、EGFR、Bax和Bcl-2的关系有待进一步研究。
4 结论在牦牛卵母细胞体外成熟过程中,FSH提高卵母细胞发育能力并诱导EGF和EGFR表达,而且通过调控Bax、Bcl-2等凋亡相关因子的表达,抑制卵母细胞凋亡,推测EGF、EGFR、Bcl-2和Bax在FSH诱导卵母细胞成熟和抑制细胞凋亡中起重要作用,为进一步探讨FSH在牦牛生殖过程中发挥的作用以及EGF和EGFR对牦牛卵母细胞成熟和后期胚胎发育的影响提供理论依据。
| [1] |
何翃闳, 崔燕, 潘阳阳, 等. 牦牛HSP27基因的克隆及其在雌性生殖器官中的表达[J]. 中国农业科学, 2015, 48(20): 4178–4187.
HE H H, CUI Y, PAN Y Y, et al. Cloning of bos grunniens HSP27 gene and its expression in the female yak reproductive organs[J]. Scientia Agricultura Sinica, 2015, 48(20): 4178–4187. DOI: 10.3864/j.issn.0578-1752.2015.20.017 (in Chinese) |
| [2] |
潘阳阳, 李秦, 崔燕, 等. EGF、EGFR在牦牛卵母细胞中的表达及对胚胎发育能力的作用[J]. 中国农业科学, 2015, 48(12): 2439–2448.
PAN Y Y, LI Q, CUI Y, et al. The expression of EGF and EGFR in yak oocyte and its function on development competence of embryo[J]. Scientia Agricultura Sinica, 2015, 48(12): 2439–2448. DOI: 10.3864/j.issn.0578-1752.2015.12.017 (in Chinese) |
| [3] | BINELLI M, MURPHY B D. Coordinated regulation of follicle development by germ and somatic cells[J]. Reprod Fertil Dev, 2009, 22(1): 1–12. |
| [4] | OKTEM O, URMAN B. Understanding follicle growth in vivo[J]. Hum Reprod, 2010, 25(12): 2944–2954. DOI: 10.1093/humrep/deq275 |
| [5] | DEMEESTERE I, STREIFF A K, SUZUKI J, et al. Follicle-stimulating hormone accelerates mouse oocyte development in vivo[J]. Biol Reprod, 2012, 87(1): 1–11. |
| [6] | SU J M, WANG Y S, ZHANG L, et al. Oocyte-secreted factors in oocyte maturation media enhance subsequent development of bovine cloned embryos[J]. Mol Reprod Dev, 2014, 81(4): 341–349. DOI: 10.1002/mrd.22302 |
| [7] | HUNZICKER-DUNN M, MAIZELS E T. FSH signaling pathways in immature granulosa cells that regulate target gene expression:branching out from protein kinase A[J]. Cell Signal, 2006, 18(9): 1351–1359. DOI: 10.1016/j.cellsig.2006.02.011 |
| [8] | SINGH B, MENG L, RUTLEDGE J M, et al. Effects of epidermal growth factor and follicle-stimulating hormone during in vitro maturation on cytoplasmic maturation of porcine oocytes[J]. Mol Reprod Dev, 1997, 46(3): 401–407. DOI: 10.1002/(ISSN)1098-2795 |
| [9] | VERAGUAS D, GALLEGOS P F, VELASQUEZ A E, et al. FSH stimulation of anestrous cats improves oocyte quality and development of parthenogenetic embryos[J]. Theriogenology, 2016, 87: 25–35. |
| [10] | HIRADATE Y, INOUE H, KOBAYASHI N, et al. Neurotensin enhances sperm capacitation and acrosome reaction in mice[J]. Biol Reprod, 2014, 91(2): 53. |
| [11] | PROCHAZKA R, BLAHA M, NEMCOVA L. Signaling pathways regulating FSH-and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig[J]. Reproduction, 2012, 144(5): 535–546. DOI: 10.1530/REP-12-0191 |
| [12] | SÁNCHEZ F, ADRIAENSSENS T, ROMERO S, et al. Different follicle-Stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice[J]. Biol Reprod, 2010, 83(4): 514–524. DOI: 10.1095/biolreprod.109.083311 |
| [13] | XU H, DENG K, LUO Q, et al. High serum FSH is associated with brown oocyte formation and a lower pregnacy rate in human IVF parctice[J]. Cell Physiol Biochem, 2016, 39(2): 677–684. DOI: 10.1159/000445658 |
| [14] | FRANCIOSI F, MANANDHAR S, CONTI M. FSH regulates mRNA translation in mouse oocytes and promotes developmental competence[J]. Endocrinology, 2016, 157(2): 872–882. DOI: 10.1210/en.2015-1727 |
| [15] | LI H J, LIU D J, CANG M, et al. Early apoptosis is associated with improved developmental potential in bovine oocytes[J]. Anim Prod Sci, 2009, 114(1-3): 89–98. |
| [16] | PALMERINI M G, NOTTOLA S A, TUNJUNG W A S, et al. EGF-FSH supplementation reduces apoptosis of pig granulosa cells in co-culture with cumulus-oocyte complexes[J]. Biochem Biophys Res Commun, 2016, 481(1-2): 159–164. DOI: 10.1016/j.bbrc.2016.10.151 |
| [17] |
徐庚全, 樊江峰, 杨世华, 等. FSH、LH对体外培养的牦牛卵泡颗粒细胞凋亡及E2、P分泌功能的影响[J]. 畜牧兽医学报, 2015, 46(6): 932–939.
XU G Q, FAN J F, YANG S H, et al. The effects of FSH, LH on apoptosis and E2, P secreting of yak's granulosa cells cultured in vitro[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(6): 932–939. (in Chinese) |
| [18] | YANG M Y, RAJAMAHENDRAN R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro[J]. Anim Prod Sci, 2002, 70(3-4): 159–169. |
| [19] |
张译夫, 潘阳阳, 温泽星, 等. 表皮生长因子对牦牛卵丘细胞低氧诱导因子-1α表达的影响及与凋亡的关联性分析[J]. 畜牧兽医学报, 2016, 47(6): 1154–1161.
ZHANG Y F, PAN Y Y, WEN Z X, et al. The effect of epidermal growth factor on the expression of hypoxia inducible factor-1α in cumulus cells of yak (Bos grunniens) and its correlation analysis with apoptosis[J]. Acta Veterinaria et Zootechnica Sinica, 2016, 47(6): 1154–1161. (in Chinese) |
| [20] | CHEN X F, ZHOU B, YAN J, et al. Epidermal growth factor receptor activation by protein kinase C is necessary for FSH-induced meiotic resumption in porcine cumulus-oocyte complexes[J]. J Endocrinol, 2008, 197(2): 409–419. DOI: 10.1677/JOE-07-0592 |
| [21] | UHM S J, GUPTA M K, YANG J H, et al. Epidermal growth factor can be used in lieu of follicle-stimulating hormone for nuclear maturation of porcine oocytes in vitro[J]. Theriogenology, 2010, 73(8): 1024–1036. DOI: 10.1016/j.theriogenology.2009.11.029 |
| [22] | HUSSEIN T S, THOMPSON J G, GILCHRIST R B. Oocyte-secreted factors enhance oocyte developmental competence[J]. Dev Biol, 2006, 296(2): 514–521. DOI: 10.1016/j.ydbio.2006.06.026 |
| [23] | CALDER M D, CAVENEY A N, SMITH L C, et al. Responsiveness of bovine cumulus-oocyte-complexes (COC) to porcine and recombinant human FSH, and the effect of COC quality on gonadotropin receptor and Cx43 marker gene mRNAs during maturation in vitro[J]. Reprod Biol Endocrinol, 2003, 1: 14. DOI: 10.1186/1477-7827-1-14 |
| [24] | LIANG C G, HUO L J, ZHONG Z S, et al. Cyclic adenosine 3', 5'-monophosphate-dependent activation of mitogen-activated protein kinase in cumulus cells is essential for germinal vesicle breakdown of porcine cumulus-enclosed oocytes[J]. Endocrinology, 2005, 146(10): 4437–4444. DOI: 10.1210/en.2005-0309 |
| [25] | PALERMO R. Differential actions of FSH and LH during folliculogenesis[J]. Reprod Biomed Online, 2007, 15(3): 326–337. DOI: 10.1016/S1472-6483(10)60347-1 |
| [26] | WU J, DENT P, JELINEK T, et al. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3', 5'-monophosphate[J]. Science, 1993, 262(5136): 1065–1069. DOI: 10.1126/science.7694366 |
| [27] | DU X H, ZHOU X L, CAO R, et al. FSH-induced p38-MAPK-mediated dephosphorylation at serine 727 of the signal transducer and activator of transcription 1 decreases Cyp1b1, expression in mouse granulosa cells[J]. Cell Signal, 2015, 27(1): 6–14. DOI: 10.1016/j.cellsig.2014.10.002 |
| [28] |
王怡, 张姣姣, 汪勇, 等. FSH和17β-雌二醇联合作用对Cyclin D1 mRNA和Cyclin E1 mRNA表达的影响[J]. 畜牧兽医学报, 2013, 44(6): 858–865.
WANG Y, ZHANG J J, WANG Y, et al. Effect of combination of FSH and 17β-estradiol on the mRNA expression of Cyclin D1 and Cyclin E1 in the cultured immature boar sertoli cell[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(6): 858–865. (in Chinese) |
| [29] | YAMASHITA Y, KAWASHIMA I, YANAI Y, et al. Hormone-induced expression of tumor necrosis factor α-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor[J]. Endocrinology, 2007, 148(12): 6164–6175. DOI: 10.1210/en.2007-0195 |
| [30] | TOYODA H, KOMURASAKI T, UCHIDA D, et al. Epiregulin:a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes[J]. J Biol Chem, 1995, 270(13): 7495–7500. DOI: 10.1074/jbc.270.13.7495 |
| [31] | LINSE S, THULIN E, GIFFORD L K, et al. Domain organization of calbindin D28k as determined from the association of six synthetic EF-hand fragments[J]. Protein Sci, 1997, 6(11): 2385–2396. |
| [32] | ZHANG C H, QIAN W P, QI S T, et al. Maternal diabetes causes abnormal dynamic changes of endoplasmic reticulum during mouse oocyte maturation and early embryo development[J]. Reprod Biol Endocrinol, 2013, 11: 31. DOI: 10.1186/1477-7827-11-31 |
| [33] | KAWAMURA K, CHEN Y, SHU Y M, et al. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors[J]. PLoS One, 2012, 7(11): e49328. DOI: 10.1371/journal.pone.0049328 |
| [34] | FENG Y J, TEITELBAUM D H. Epidermal growth factor/TNF-α transactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(2): G236–G249. DOI: 10.1152/ajpgi.00142.2011 |



