猪繁殖与呼吸综合征(porcine reproductive and respiratory syndrome, PRRS)是一种以猪繁殖与呼吸综合征病毒(porcine reproductive and respiratory syndrome virus, PRRSV)为病原体的疾病,可造成仔猪和育成猪呼吸障碍、母猪流产,具有较高的致死率,给全世界养猪产业造成巨大经济损失[1-3]。目前关于PRRSV的研究多集中于病毒学特性、病毒的起源、进化以及猪体对其产生的免疫应答等方面[4-5],而关于PRRSV如何依赖宿主因子完成吸附、入侵、RNA脱壳、核酸蛋白质等生物大分子合成、子代病毒组装和释放生活周期的分子机制的研究尚不充分。
组织蛋白酶(cathepsin)是在各种动物组织的细胞内(特别是溶酶体部分)发现的一类蛋白酶,是半胱氨酸蛋白酶家族的主要成员,在生物界已发现20余种,人体中主要存在11种,是近年来备受关注的一类靶标蛋白酶[6-7]。研究表明cathepsin与多种病理过程相关,例如cathepsin G通过切割白细胞介素6受体、水解活化细胞因子等途径参与血管性炎症、急性肺损伤等炎症性疾病的发生[8];由于cathepsin D的表达水平与胃癌的淋巴结转移之间的相关性,其表达水平的变化可以作为胃癌预后的判断指标之一[9]。而cathepsin在病毒复制周期中的作用也陆续被发现,例如埃博拉病毒(Ebola virus, EBOV)入侵宿主细胞时,需要借助cathepsin B和cathepsin L使病毒表面糖蛋白构象改变从而使病毒可以将遗传物质“注入”宿主细胞[10-11];在肝癌细胞中,乙肝病毒的x基因(HBx)表达量与cathepsin S的表达量正相关,在瞬时过表达HBx的HepG2细胞中,HBx可能是通过上调cathepsin S的表达量促进细胞增殖[12];PRRSV通过激活NF-κB通路和cathepsin L来上调乙酰肝素酶的表达,降解硫酸乙酰肝素从而促进病毒的释放[13]。由此,笔者推测其它组织蛋白酶也可能参与PRRSV的生活周期。本研究构建了针对猴源cathepsin基因的shRNA真核慢病毒表达载体,与包装质粒(pMD2.G, psPAX)共同转染HEK293T/17细胞后收取慢病毒上清液,感染源于MA-104的非洲绿猴胚胎肾上皮细胞Marc145细胞系(对PRRSV易感),获得高效且稳定敲减cathepsin基因的细胞株。该稳定细胞系的建立为后续探究cathepsin在PRRSV生活周期中的作用奠定了基础。
1 材料与方法 1.1 实验材料pLKO.1载体、慢病毒包装载体psPAX2、pMD2.G、Ampicillin和Puromycin购自Sigma公司;人胚胎肾细胞HEK293T/17购自ATCC公司;猴胚胎肾上皮细胞Marc145来自河南省动物免疫学重点实验室;PRRSV-GFP毒株由西北农林科技大学周恩民教授馈赠;DMEM、FBS以及胰酶购自Gibco;Prime Script RT Reagent Kit、SYBR Premix Ex Taq Ⅱ、rTaq酶、DL10000 DNA Marker、DL2000 DNA Marker购自TaKaRa公司;限制性内切酶EcoRⅠ-HF、AgeⅠ-HF及T4连接酶购自NEB公司;Lipofectamine®3000 Transfection Kit购自Promega公司;质粒中提试剂盒为QIAGEN产品;TOP10大肠杆菌感受态为实验室自制。引物由上海生工生物工程有限公司合成;DNA测序由上海英潍捷基生物公司完成。
1.2 方法 1.2.1 猴源cathepsin基因shRNA重组质粒的构建和鉴定RNA干扰(RNA interference, RNAi)是一种进化上保守的抵御外来病毒或转基因的防御机制[14-15]。将与靶基因mRNA同源的双链RNA (double strand RNA, dsRNA)导入细胞,shRNA的发卡结构可被细胞切割成siRNA,然后siRNA结合到RNA诱导沉默复合物上(RNA-induced silencing complex,RISC),该复合物能够结合到目的mRNA上并将其特异性地降解,从而产生相应的功能表型缺失[16-19]。本研究利用慢病毒载体把shRNA导入细胞,其在感染后可以整合到细胞的基因组上;并且载体中的U6启动子确保了shRNA长时间表达;这种整合的shRNA序列可随细胞DNA一起复制并被传递到子代细胞中去,从而使靶基因长期稳定沉默。
首先,查找NCBI数据库得到非洲绿猴(Chlorocebus sabaeus)的全部cathepsin基因;然后,使用Invitrogen公司在线软件BLOCK-iT RNAi Designer,针对每种猴源cathepsin基因设计3条长度21 bp的shRNA序列,每条序列作用于不同靶点。shRNA退火形成双链,与pLKO.1载体(经EcoRⅠ-HF、AgeⅠ-HF处理)连接成重组质粒(如图 1);转化大肠杆菌,37 ℃振荡培养1 h,涂布于含100 μg·mL-1 Ampicillin的固体LB培养基倒置培养过夜。次日挑选阳性单克隆,以菌液为模板,PCR扩增鉴定插入片段。菌液PCR上游引物:5′-TCGACGGTATCGATCACGAGACTAG-3′;下游引物:5′-AATTGTGGATGAATACTGCCATTTG-3′。阳性结果经上海英潍捷基生物公司测序正确后,按照中提试剂盒说明书抽提质粒,置于-20 ℃保存备用。
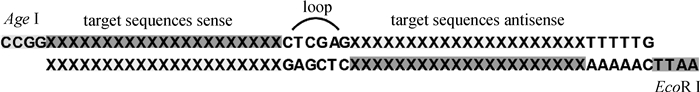
|
图 1 shRNA表达原理 Figure 1 Illustration of shRNA |
将HEK293T/17细胞以1.6×106·瓶-1的密度接种于T25细胞培养瓶,培养至细胞融合度40%左右,按照Lipofectamine® 3000 Transfection Kit说明书将重组质粒与包装质粒psPAX2、pMD2.G共转染。分别于48、72 h收取上清中的慢病毒,分装冻存于-80 ℃。
1.2.3 稳定转染Marc145细胞系建立以2×105·孔-1的密度接种Marc145细胞于6孔板,培养至细胞融合度30%~40%,弃去1 mL培养基,加入1 mL慢病毒感染细胞。48 h后用含10 μg·mL-1 puromycin的DMEM完全培养基持续筛选至对照组细胞全部死亡。继续使用含2 μg·mL-1 puromycin的DMEM完全培养基筛选维持,待感染效果鉴定。
1.2.4 实时荧光定量PCR检测Trizol法抽提细胞总RNA,反转录得到cDNA;以实时荧光定量PCR方法测定cathepsin mRNA表达量的变化,得到敲减效率最高的细胞株。实时荧光定量PCR引物见表 1。
|
|
表 1 非洲绿猴cathepsin基因实时荧光定量PCR引物序列 Table 1 Sequence of quantitative real-time PCR primer for Chlorocebus sabaeus cathepsin genes |
将筛选得到cathepsin shRNA-Marc145细胞株以1×105·孔-1的密度接种于12孔板,培养至细胞融合度80%。感染PRRSV-GFP病毒(MOI=1),37 ℃吸附1 h后,用1%FBS的DMEM培养基培养48 h,置于倒置荧光显微镜观察,并用酶标仪检测荧光结果。
1.2.6 统计学处理用t检验比较各组mRNA以及荧光结果差异,试验数据用Microsoft Excel 2010进行处理,计量数据以平均数±标准差(x±s)表示,P < 0.001表示差异有统计学意义,**P < 0.001,***P < 0.000 1。
2 结果 2.1 猴源cathepsin基因的shRNA靶序列所构建cathepsin基因shRNA文库包含15种猴源cathepsin 基因,针对每种cathepsin基因分别设计的3个shRNA靶点序列如表 2。
|
|
表 2 猴源cathepsin基因shRNA靶序列 Table 2 Targeting sequence of shRNA for Chlorocebus sabaeus cathepsin genes |
用限制性内切酶EcoRⅠ-HF和Age Ⅰ-HF对pLKO.1载体进行双酶切,经1%琼脂糖凝胶电泳分离出7 500 bp左右的目的条带(如图 2),回收该条带得到pLK0.1酶切产物。猴源cathepsin基因shRNA文库共包含45对shRNA寡核苷酸链退火杂交后形成双链DNA,亚克隆至pLKO.1得到45个pLKO.1-cathepsin shRNA重组质粒。该重组质粒转化至大肠杆菌TOP10感受态,挑取阳性单克隆,菌液PCR鉴定得到273 bp产物的阳性菌(图 3),测序。
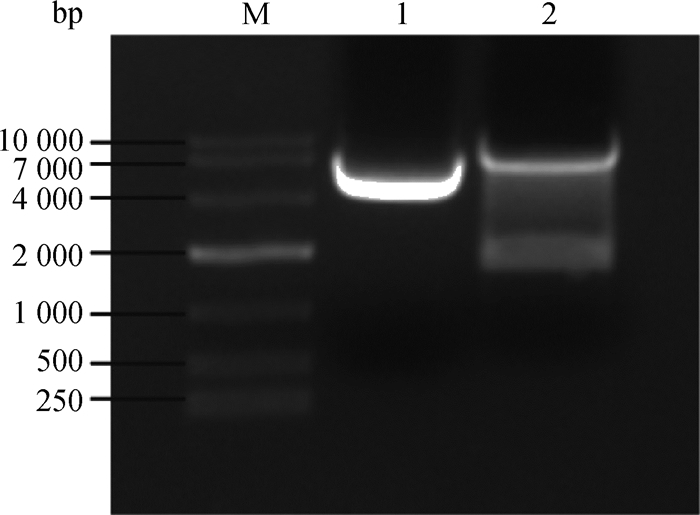
|
M. DL10000相对分子质量标准;1.阴性对照;2. pLKO.1质粒双酶切产物 M. DL10000 marker; 1. Negative control; 2. The double-restriction endonuclease digestion products of pLKO.1 plasmids 图 2 pLKO.1质粒的双酶切 Figure 2 Restriction enzymes digestion of pLKO.1 plasmids |
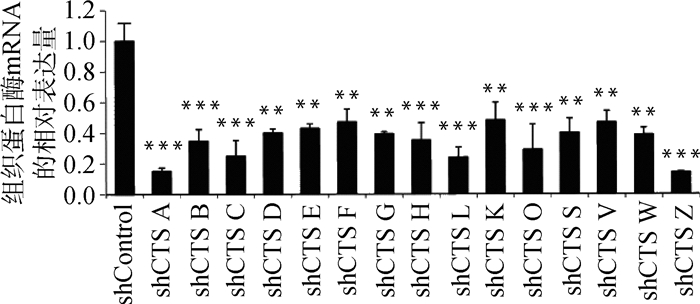
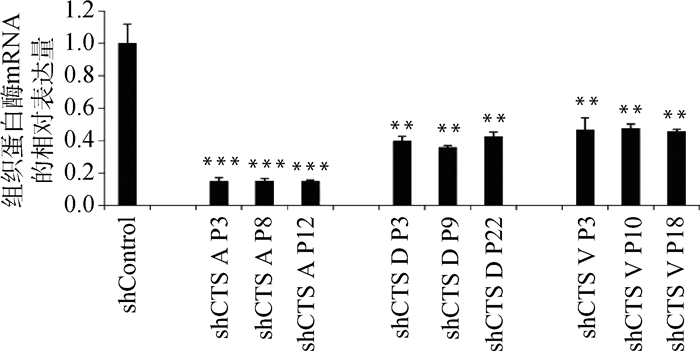
慢病毒感染Marc145细胞48 h,用含10 μg·mL-1 puromycin的DMEM完全培养液培养细胞至对照组细胞死亡,换含2 μg·mL-1 puromycin DMEM完全培养基培养维持。抽提对照细胞和敲减细胞全部RNA反转录得到cDNA,实时荧光定量PCR分析cathepsin mRNA表达量。以β-actin作内参,比较对照细胞与不同靶点敲减cathepsin基因的细胞mRNA表达量(如图 4),选出敲减效率最高的细胞株(图 5),各cathepsin shRNA-Marc145细胞株中cathepsin mRNA的表达量均明显下降,表明成功构建cathepsin基因敲减细胞系。为证明该细胞系的稳定性,选取同一细胞株不同代次细胞进行敲减效率检测(图 6),发现不同代次细胞敲减效率稳定。进一步证明所构建细胞系高效且稳定敲减cathepsin基因。
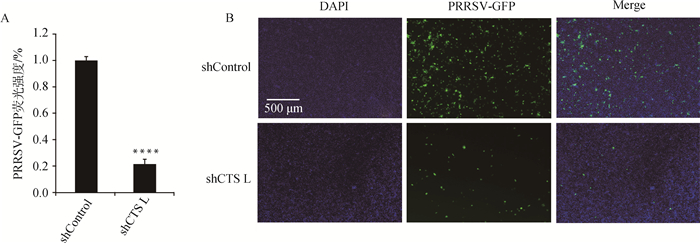
2.4 cathepsin L shRNA-Marc145细胞抑制PRRSV-GFP复制将筛选得到的cathepsin shRNA-Marc145细胞接种于12孔板,培养至细胞融合度80%,按上述方法用PRRSV-GFP分别感染shControl-Marc145细胞和cathepsin L shRNA-Marc145细胞,48 h后置于荧光显微镜观察,发现cathepsin L shRNA-Marc145细胞中PRRSV荧光强度明显下降(图 7B),酶标仪检测结果与荧光结果一致(图 7A),表明敲减cathepsin L会显著抑制PRRSV-GFP的增殖。
3 讨论非洲绿猴体内可以表达cathepsin A、B、C、D、E、F、G、H、K、L、O、S、V、W、Z,本研究设计了针对这些cathepsin基因的shRNA引物(表 2),构建得到了45个重组质粒,命名为pLKO.1-cathepsin shRNA(图 2、图 3)。利用该重组质粒包装慢病毒颗粒并感染Marc145细胞,筛选鉴定出45株稳定且高效敲减cathepsin的细胞株,命名为cathepsin shRNA-Marc145细胞株(图 4~6)。这些细胞系经PRRSV-GFP病毒感染,初步筛选出对PRRSV复制有影响的cathepsin(图 7)。Guo等[13]的研究表明PRRSV感染诱导乙酰肝素和cathepsin L表达,调节乙酰肝素转运到细胞表面降解HS,从而促进PRRSV子代病毒的释放,这说明cathepsin L在PRRSV的释放中发挥着重要作用。笔者的试验也证实了这一点,如图 7所示,敲减cathepsin L会显著抑制PRRSV的增殖。因其他有效果cathepsin的分子机制正在进一步解析中,所以在本文中没有显示。
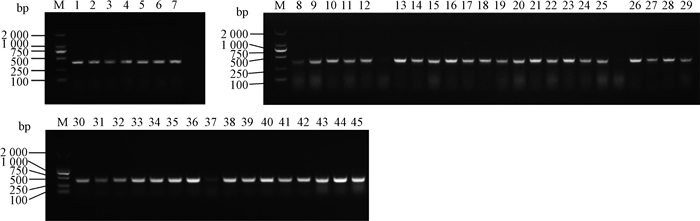
|
M. DL2000相对分子质量标准(DL2000 marker);1. cathepsin A-1 shRNA-pLKO.1; 2. cathepsin A-2 shRNA-pLKO.1; 3. cathepsin O-1 shRNA-pLKO.1; 4. cathepsin O-2 shRNA-pLKO.1; 5. cathepsin D-1 shRNA-pLKO.1; 6.cathepsin D-2 shRNA-pLKO.1; 7.cathepsin Z-1 shRNA-pLKO.1; 8. cathepsin Z-2 shRNA-pLKO.1; 9.cathepsin B-1 shRNA-pLKO.1; 10. cathepsin B-2 shRNA-pLKO.1; 11. cathepsin C-1 shRNA-pLKO.1; 12. cathepsin C-2 shRNA-pLKO.1; 13. cathepsin E-1 shRNA-pLKO.1; 14. cathepsin E-2 shRNA-pLKO.1; 15. cathepsin F-1 shRNA-pLKO.1; 16. cathepsin F-2 shRNA-pLKO.1; 17. cathepsin G-1 shRNA-pLKO.1; 18. cathepsin G-2 shRNA-pLKO.1; 19. cathepsin H-1 shRNA-pLKO.1; 20. cathepsin H-2 shRNA-pLKO.1; 21. cathepsin L-1 shRNA-pLKO.1; 22. cathepsin L-2 shRNA-pLKO.1; 23. cathepsin K-1 shRNA-pLKO.1; 24. cathepsin K-2 shRNA-pLKO.1; 25. cathepsin S-1 shRNA-pLKO.1; 26. cathepsin S-2 shRNA-pLKO.1; 27. cathepsin V-1 shRNA-pLKO.1; 28. cathepsin V-2 shRNA-pLKO.1; 29. cathepsin W-1 shRNA-pLKO.1; 30. cathepsin W-2 shRNA-pLKO.1; 31. cathepsin A-3 shRNA-pLKO.1; 32. cathepsin B-3 shRNA-pLKO.1; 33. cathepsin C-3 shRNA-pLKO.1; 34. cathepsin D-3 shRNA-pLKO.1; 35. cathepsin E-3 shRNA-pLKO.1; 36. cathepsin F-3 shRNA-pLKO.1; 37. cathepsin G-3 shRNA-pLKO.1;38. cathepsin H-3 shRNA-pLKO.1; 39. cathepsin L-3 shRNA-pLKO.1; 40. cathepsin K-3 shRNA-pLKO.1; 41. cathepsin O-3 shRNA-pLKO.1; 42. cathepsin S-3 shRNA-pLKO.1; 43. cathepsin V-3 shRNA-pLKO.1; 44. cathepsin W-3 shRNA-pLKO.1; 45. cathepsin Z-3 shRNA-pLKO.1 图 3 重组质粒的PCR鉴定 Figure 3 PCR results of recombinant plasmid (portion) |
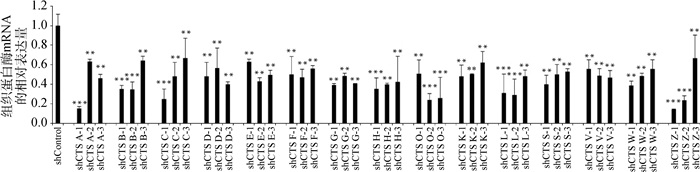
|
与shControl相比,**.P < 0.001,***.P < 0.000 1 Compared with shControl, **.P < 0.001,***.P < 0.000 1 图 4 cathepsin基因不同靶点的敲减效率比较 Figure 4 Comparison of knockdown efficiency of cathepsin genes at different targets |
|
与shControl相比,**.P < 0.001,***.P < 0.000 1 Compared with shControl, **.P < 0.001,***.P < 0.000 1 图 5 cathepsin shRNA-Marc145细胞株相应cathepsin mRNA相对表达量 Figure 5 The relative mRNA expression of cathepsin in Marc145 cell lines |
|
shControl.对照; shCTS A P3. cathepsin A shRNA-Marc145细胞株第3代细胞; shCTS A P8. cathepsin A shRNA-Marc145细胞株第8代细胞; shCTS A P12. cathepsin A shRNA-Marc145细胞株第12代细胞; shCTS D P3. cathepsin D shRNA-Marc145细胞株第3代细胞; shCTS D P9. cathepsin D shRNA-Marc145细胞株第9代细胞: shCTS D P22. cathepsin D shRNA-Marc145细胞株第22代细胞; shCTS V P3. cathepsin V shRNA-Marc145细胞株第3代细胞; shCTS V P10. cathepsin V shRNA-Marc145细胞株第10代细胞; shCTS V P18. cathepsin V shRNA-Marc145细胞株第18代细胞,与shControl相比,**.P < 0.001, ***.P < 0.000 1 shControl. Control; shCTS A P3. The 3rd generation of cathepsin A shRNA-Marc145 cell line; shCTS A P8. The 8th generation of cathepsin A shRNA-Marc145 cell line; shCTS A P12. The 12th generation of cathepsin A shRNA-Marc145 cell line; shCTS D P3. The 3rd generation of cathepsin D shRNA-Marc145 cell line; shCTS D P9. The 9th generation of cathepsin D shRNA-Marc145 cell line: shCTS D P22. The 22nd generation of cathepsin D shRNA-Marc145 cell line; shCTS V P3. The 3rd generation of cathepsin V shRNA-Marc145 cell line; shCTS V P10. The 10th generation of cathepsin V shRNA-Marc145 cell line; shCTS V P18. The 18th generation of cathepsin V shRNA-Marc145 cell line.Compared with shControl, **.P < 0.001, ***.P < 0.000 1 图 6 不同代次cathepsin shRNA-Marc145细胞株相应cathepsin mRNA相对表达量(部分) Figure 6 The relative mRNA expression of cathepsin in different generations of Marc145 cell lines (partial) |
|
****.P < 0.000 1 图 7 cathepsin L基因敲减抑制PRRSV-GFP复制 Figure 7 cathepsin L knockdown inhibits PRRSV-GFP replication |
PRRSV是高度变异的单股正链RNA囊膜病毒,其毒株的多样加剧了蓝耳病的临床复杂性[20-21]。因此,单纯针对其囊膜抗原研制的疫苗很难满足生产上的防控需求[22-23]。研究显示,PRRSV复制周期的每一步都依赖于宿主细胞蛋白:病毒首先结合细胞表面的硫酸类肝素和葡萄糖胺聚糖;然后囊膜蛋白与CD163结合使病毒发生细胞内化;最后在网格蛋白介导下形成内吞体进入细胞质[24-26]。进入细胞质的病毒粒子需进一步脱壳释放出RNA,进行病毒的复制、组装以及释放。所以,通过调控宿主靶向蛋白来抑制病毒复制的思路成为一个新的研究方向[27]。
本研究所探究的组织蛋白酶作为细胞中重要的蛋白酶类,同胞外蛋白酶一样,可以重塑胞外基质、降解胞内不必要的细胞器及蛋白质、降解胞内基质、传递细胞信号、调节细胞凋亡[28-32],极有可能在病毒与宿主的相互作用起关键作用。因此,未来工作的重点将集中在回答以下问题:1)PRRSV的表面蛋白是否需要经细胞cathepsin切割,暴露出真正的抗原表位与细胞受体结合?2)PRRSV的核衣壳需要哪种cathepsin发挥功能,才能使位于其中的病毒RNA释放到细胞核周区域?3)病毒RNA指导合成的病毒蛋白质,是否需要细胞cathepsin的功能形成正确的空间结构以有利于子代病毒的组装?4)具体的分子机制又是如何?
综上所述,本研究所构建的猴源cathepsin基因稳定且高效敲减细胞系,为进一步筛选对PRRSV复制有影响的cathepsin奠定了基础,为研究宿主细胞与PRRSV相互作用的分子机制提供了新的见解,这可能有助于我们进一步了解PRRSV的发病机制。
4 结论本研究利用了RNAi原理,成功构建了猴源cathepsin基因的shRNA文库,并以对PRRSV易感的非洲绿猴胚胎肾上皮细胞Marc145为模型,筛选出了稳定敲减细胞株。所构建的shRNA文库中共有45个单克隆,涉及15种cathepsin基因;筛选出的细胞株经Q-PCR鉴定,cathepsin基因mRNA的表达量均显著下降,可以用于探究组织蛋白酶在PRRSV吸附、入侵、脱壳、生物合成、组装以及释放中的作用。
| [1] | ZHOU Z, WU J J, ZHANG S, et al. Analysis of genetic variation of two nadc30-like strains of porcine reproductive and respiratory syndrome virus in China[J]. Open Virol J, 2017, 11: 90–97. DOI: 10.2174/1874357901711010090 |
| [2] | WHITWORTH K M, ROWLAND R R R, EWEN C L, et al. Gene-edited pigs are protected from porcine reproductive and respiratory syndrome virus[J]. Nat Biotechnol, 2016, 34(1): 20–22. DOI: 10.1038/nbt.3434 |
| [3] |
郭振华, 陈鑫鑫, 李睿, 等. 中国猪繁殖与呼吸综合征病毒流行历史及现状[J]. 畜牧兽医学报, 2018, 49(1): 1–9.
GUO Z H, CHEN X X, LI R, et al. The prevalent history and current status of porcine reproductive and respiratory syndrome in China[J]. Acta Veterinaria et Zootechnica Sinica, 2018, 49(1): 1–9. (in Chinese) |
| [4] | LI X D, BAO H Y, WANG Y, et al. Widespread of NADC30-like PRRSV in China:another Pandora's box for Chinese pig industry as the outbreak of highly pathogenic PRRSV in 2006?[J]. Infect Genet Evol, 2017, 49: 12–13. DOI: 10.1016/j.meegid.2016.12.021 |
| [5] | CHEN N H, LIU Q R, QIAO M M, et al. Whole genome characterization of a novel porcine reproductive and respiratory syndrome virus 1 isolate:genetic evidence for recombination between Amervac vaccine and circulating strains in mainland China[J]. Infect Genet Evol, 2017, 54: 308–313. DOI: 10.1016/j.meegid.2017.07.024 |
| [6] |
曾广智, 谭宁华, 贾锐锐, 等. 组织蛋白酶及其抑制剂研究进展[J]. 云南植物研究, 2005, 27(4): 337–354.
ZENG G Z, TAN N H, JIA R R, et al. Cathepsins:structures, functions and inhibitors[J]. Acta Botanica Yunnanica, 2005, 27(4): 337–354. (in Chinese) |
| [7] | XU X, GREENLAND J R, GOTTS J E, et al. Cathepsin L helps to defend mice from infection with influenza A[J]. PLoS One, 2016, 11(10): e0164501. DOI: 10.1371/journal.pone.0164501 |
| [8] |
陈驹, 彭礼飞. 组织蛋白酶G在炎症性疾病发生发展中作用的研究进展[J]. 山东医药, 2017, 57(21): 98–101.
CHEN J, PENG L F. Research progress on the role of cathepsin g in the development of inflammatory diseases[J]. Shandong Medical Journal, 2017, 57(21): 98–101. DOI: 10.3969/j.issn.1002-266X.2017.21.034 (in Chinese) |
| [9] | TSUTSUMI S, OGINO I, MIYAJIMA M, et al. Role of cathepsin K in the development of chronic subdural hematoma[J]. J Clin Neurosci, 2017, 45: 343–347. DOI: 10.1016/j.jocn.2017.08.021 |
| [10] |
高秋月, 肖露平, 李海燕, 等. 埃博拉病毒及其免疫研究进展[J]. 生物学教学, 2009, 34(7): 7–9.
GAO Q Y, XIAO L P, LI H Y, et al. Advances in researches on ebola virus and its immunology[J]. Biology Teaching, 2009, 34(7): 7–9. (in Chinese) |
| [11] | ZHOU N, PAN T, ZHANG J S, et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of ebola virus, middle east respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV)[J]. J Biol Chem, 2016, 291(17): 9218–9232. DOI: 10.1074/jbc.M116.716100 |
| [12] |
张智, 郭鹏, 孙兴, 等. HBx基因上调组织蛋白酶S对HepG2细胞的影响[J]. 中华医学杂志, 2017, 97(30): 2357–2361.
ZHANG Z, GUO P, SUN X, et al. Effects of up-regulation of cathepsin S by HBx gene on HepG2 cell[J]. National Medical Journal of China, 2017, 97(30): 2357–2361. DOI: 10.3760/cma.j.issn.0376-2491.2017.30.009 (in Chinese) |
| [13] | GUO C H, ZHU Z B, GUO Y, et al. Heparanase upregulation contributes to porcine reproductive and respiratory syndrome virus release[J]. J Virol, 2017, 91(15): e00625–17. |
| [14] |
尚仁福, 吴立刚. RNA干扰的机制及其应用[J]. 生命科学, 2016, 28(5): 576–583.
SHANG R F, WU L G. Mechanism and application of RNA interference[J]. Chinese Bulletin of Life Sciences, 2016, 28(5): 576–583. (in Chinese) |
| [15] | MOHR S E, SMITH J A, SHAMU C E, et al. RNAi screening comes of age:improved techniques and complementary approaches[J]. Nat Rev Mol Cell Biol, 2014, 15(9): 591–600. DOI: 10.1038/nrm3860 |
| [16] |
董骎, 高洪, 严玉霖, 等. shRNA介导的基因沉默表达载体构建方法及应用[J]. 上海畜牧兽医通讯, 2015(5): 18–19, 22.
DONG Q, GAO H, YAN Y L, et al. Construction and application of gene silencing expression vector mediated by shRNA[J]. Shanghai Journal of Animal Husbandry and Veterinary Medicine, 2015(5): 18–19, 22. (in Chinese) |
| [17] |
梁艳. 慢病毒载体在RNA干扰中的应用进展[J]. 国际检验医学杂志, 2010, 31(11): 1282–1284.
LIANG Y. Advances in the application of lentiviral vector in RNA interference[J]. International Journal of Laboratory Medicine, 2010, 31(11): 1282–1284. DOI: 10.3969/j.issn.1673-4130.2010.11.035 (in Chinese) |
| [18] | DYKXHOORN D M, NOVINA C D, SHARP P A. Killing the messenger:short RNAs that silence gene expression[J]. Nat Rev Mol Cell Biol, 2003, 4(6): 457–467. DOI: 10.1038/nrm1129 |
| [19] | WU J Y, HUANG W Z, HE Z Y. Dendrimers as carriers for siRNA delivery and gene silencing:a review[J]. Sci World J, 2013, 2013: 630654. |
| [20] | JEONG J, CHOI K, KANG I, et al. Evaluation of a 20 year old porcine reproductive and respiratory syndrome (PRRS) modified live vaccine (Ingelvac® PRRS MLV) against two recent type 2 PRRS virus isolates in South Korea[J]. Vet Microbiol, 2016, 192: 102–109. DOI: 10.1016/j.vetmic.2016.07.006 |
| [21] | FABLET C, MAROIS-CRÉHAN C, GRASLAND B, et al. Factors associated with herd-level PRRSV infection and age-time to seroconversion in farrow-to-finish herds[J]. Vet Microbiol, 2016, 192: 10–20. DOI: 10.1016/j.vetmic.2016.06.006 |
| [22] | ROTOLO M L, GIMÉNEZ-LIROLA L, JI J, et al. Detection of porcine reproductive and respiratory syndrome virus (PRRSV)-specific IgM-IgA in oral fluid samples reveals PRRSV infection in the presence of maternal antibody[J]. Vet Microbiol, 2018, 214: 13–20. DOI: 10.1016/j.vetmic.2017.11.011 |
| [23] | ROBINSON S R, RAHE M C, GRAY D K, et al. Porcine reproductive and respiratory syndrome virus neutralizing antibodies provide in vivo cross-protection to PRRSV1 and PRRSV2 viral challenge[J]. Virus Res, 2018, 248: 13–23. DOI: 10.1016/j.virusres.2018.01.015 |
| [24] |
田德斌, 袁世山. 猪繁殖与呼吸综合征病毒侵入细胞过程研究进展[J]. 中国动物传染病学报, 2011, 19(3): 68–74.
TIAN D B, YUAN S S. Research progress on how porcine reproductive and respiratory syndrome virus enter into cell[J]. Chinese Journal of Animal Infectious Diseases, 2011, 19(3): 68–74. (in Chinese) |
| [25] | YANG H Q, ZHANG J, ZHANG X W, et al. CD163 knockout pigs are fully resistant to highly pathogenic porcine reproductive and respiratory syndrome virus[J]. Antiviral Res, 2018, 151: 63–70. DOI: 10.1016/j.antiviral.2018.01.004 |
| [26] |
宋晓晖, 王淑娟, 张衍海, 等. CD163受体在PRRSV入侵过程中的作用机制[J]. 动物医学进展, 2013, 34(4): 111–115.
SONG X H, WANG S J, ZHANG Y H, et al. Mechnism of receptor CD163 in PRRSV invasion process[J]. Progress in Veterinary Medicine, 2013, 34(4): 111–115. (in Chinese) |
| [27] | POHL M O, VON RECUM-KNEPPER J, RODRIGUEZ-FRANDSEN A, et al. Identification of Polo-like kinases as potential novel drug targets for influenza a virus[J]. Sci Rep, 2017, 7: 8629. DOI: 10.1038/s41598-017-08942-7 |
| [28] | REISER J, ADAIR B, REINHECKEL T. Specialized roles for cysteine cathepsins in health and disease[J]. J Clin Invest, 2010, 120(10): 3421–3431. DOI: 10.1172/JCI42918 |
| [29] | HSING L C, RUDENSKY A Y. The lysosomal cysteine proteases in MHC class Ⅱ antigen presentation[J]. Immunol Rev, 2005, 207(1): 229–241. DOI: 10.1111/imr.2005.207.issue-1 |
| [30] | TURK V, STOKA W, VASILJEVA O, et al. Cysteine cathepsins:from structure, function and regulation to new frontiers[J]. Biochim Biophys Acta, 2012, 1824(1): 68–88. DOI: 10.1016/j.bbapap.2011.10.002 |
| [31] |
彭舟扬帆, 李亚培, 曾萍玉, 等. 组织蛋白酶在高血压中作用的研究进展[J]. 中南大学学报:医学版, 2017, 42(12): 1447–1451.
PENG Z Y F, LI Y P, ZENG P Y, et al. Progress in the study on roles of cathepsin in hypertension[J]. Journal of Central South University:Medical Science, 2017, 42(12): 1447–1451. (in Chinese) |
| [32] |
郑贤, 邓勇志. 组织蛋白酶在心脏重塑中作用的研究进展[J]. 中华实验外科杂志, 2016, 33(3): 858–861.
ZHENG X, DENG Y Z. Role of cathepsins in cardiac remodeling[J]. Chinese Journal of Experimental Surgery, 2016, 33(3): 858–861. (in Chinese) |



