2. 农业部动物临床诊疗技术重点实验室, 呼和浩特 010018
2. Key Laboratory of Clinical Diagnosis and Treatment Technology in Animal Disease of Ministry of Agriculture, Huhhot 010018, China
绵羊肺腺瘤病毒(Jaagsiekte sheep retrovirus, JSRV)是引起绵羊肺腺瘤病(ovine pulmonary adenocarcinoma,OPA)的病原,该病是绵羊肺分泌上皮细胞发生恶性转化的一种传染性肺癌[1-2]。绵羊肺腺瘤病毒含有标准的逆转录病毒基因gag、pro、pol和env[3]。研究报道在羔羊的接种试验中,该病毒能够在10 d内迅速诱导肿瘤,类似于携带致癌基因的急性转化逆转录病毒[4]。JSRV-env基因主要编码病毒的囊膜蛋白(Env),具有表面蛋白(surface protein, SU)和跨膜蛋白(transmembrane protein, TM)两个功能区[5]。SU负责与细胞表面的特异性受体结合,并且TM负责感染时病毒与细胞膜的融合。研究表明JSRV-env可作为致癌基因,单独的JSRV Env蛋白可以转化小鼠NIH3T3细胞[6]、大鼠208F细胞[7]、鸡成纤维细胞[8]和犬上皮细胞[9],并且可以在小鼠[10-11]和绵羊[12]中诱导肺癌,因此,JSRV的囊膜蛋白(Env)具有引起细胞转化而致癌的罕见特征,但Env的致癌作用机制仍未解析清楚。
在绵羊基因组中存在大约27拷贝的与绵羊肺腺瘤病毒(JSRV)基因结构非常相似的内源性逆转录病毒(enJSRV)[13],为了与之区别,JSRV也称外源性绵羊肺腺瘤病毒(exogenous Jaagsiekte sheep retrovirus,exJSRV)。研究发现,exJSRV和enJSRV之间的序列除了三个区域的变异(VR1、VR2和VR3)以外具有高度同源性,其中VR3则映射到env基因部分,即TM蛋白的细胞质尾区[14],而TM区的YXXM基序在所有转化的JSRV中是必需的,但enJSRV的Env蛋白中不存在YXXM基序功能区,也无转化功能[15]。而且缺失和/或突变试验已经证明TM蛋白的细胞质尾区是转化必不可少的[16-17]。哺乳动物的胎盘具有物质交换、分泌激素和防御等重要功能,在胎盘中行使这些功能的主要细胞类型是滋养层细胞[18]。本实验室从正常胎盘组织中分离培养了一株永生化的滋养层细胞系用于细胞转化试验。因此本研究利用体外培养的永生化绵羊胎盘绒毛膜滋养层细胞,转染重组真核表达质粒pEGFP-C1/exJSRV-env,用平板克隆以及软琼脂集落形成试验,观察细胞的恶性转化以及细胞增殖情况,为进一步探讨exJSRV Env的致癌功能提供实验依据。
1 材料与方法 1.1 试验材料真核表达载体pEGFP-C1由本实验室保存;重组真核表达质粒pEGFP-C1/exJSRV-env以及pEGFP-C1/enJSRV-env由本实验室构建;永生化绵羊胎盘绒毛膜滋养层细胞由本实验室建立。
1.2 主要试验试剂真核细胞转染试剂LipofectamineTM LTX & PLUS购自Invitrogen公司;限制性内切酶KpnⅠ、BamHⅠ、HindⅢ和ApaⅠ购自TaKaRa公司;DMEM细胞培养液、Opti-MEM培养液,0.25%胰蛋白酶购自GIBCO公司;胎牛血清(FBS)购自澳洲;琼脂糖购自Invitrogen公司。
1.3 真核表达重组质粒的鉴定成功构建重组真核表达质粒pEGFP-C1/exJSRV-env以及pEGFP-C1/enJSRV-env。重组真核表达质粒pEGFP-C1/exJSRV-env和pEGFP-C1/enJSRV-env分别用限制性内切酶HindⅢ、ApaⅠ和KpnⅠ、BamHⅠ水浴酶切,酶切结束后利用琼脂糖凝胶电泳鉴定。
1.4 永生化绵羊胎盘绒毛膜滋养层细胞培养及转染效率的测定将永生化绵羊胎盘绒毛膜滋养层细胞复苏,放入37 ℃ 5% CO2培养箱中培养,待长到80%时传代。转染的前一天,用胰蛋白酶消化并将细胞接种在六孔板内,加入2 mL无双抗(青链霉素)的含20% FBS完全培养基。细胞密度应在转染当天汇合度应达到80%左右。按照LipofectamineTM LTX & PLUS转染试剂说明分别转染质粒pEGFP-C1/exJSRV-env、pEGFP-C1/enJSRV-env以及空载体pEGFP-C1,不作处理的正常细胞作为空白对照组。48 h后进行免疫荧光照相,以及后续软琼脂集落形成试验和平板克隆试验。
1.5 软琼脂集落形成试验转染48 h后的细胞用0.25%胰蛋白酶消化并轻轻吹打,作活细胞计数,然后根据试验要求做梯度倍数稀释。用1.2%琼脂糖溶液均匀铺制细胞6孔板底层,置CO2温箱中备用。将0.7%琼脂糖溶液与约3 000个转染后的细胞充分混匀,注入铺有琼脂糖底层的平皿中,置入37 ℃、5% CO2温箱中,培养10~14 d。显微镜下观察集落形成。
1.6 平板克隆试验对各组转染的细胞进行细胞计数,以每皿50个和100个细胞放入六孔板中,加入3 mL含20% FBS的完全培养基,每组做3组平行试验,置入37 ℃、5% CO2细胞培养箱中培养10~14 d。每3 d换一次培养液。待肉眼可见的克隆形成时,用4%多聚甲醛固定并用结晶紫染色,显微镜下计数大于50个细胞的克隆数,计算克隆形成率(克隆形成率=克隆数/接种数×100%),并用SPSS软件作统计学分析。
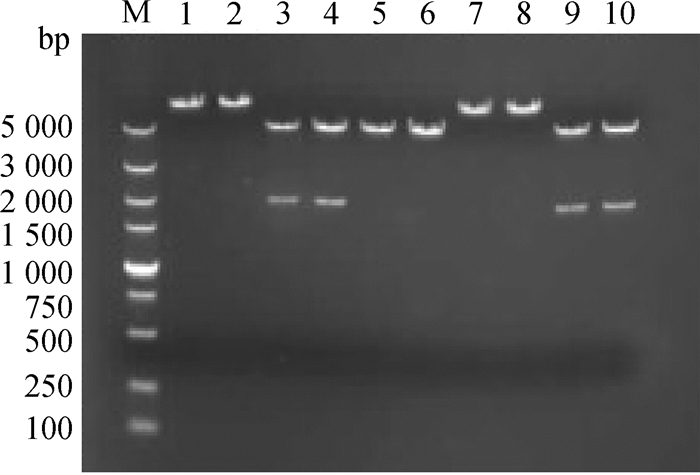
2 结果 2.1 pEGFP-C1/exJSRV-env和pEGFP-C1/enJSRV- env质粒酶切鉴定将实验室保存的重组真核表达质粒pEGFP-C1/exJSRV-env和pEGFP-C1/enJSRV-env经单、双酶切鉴定(图 1),结果显示质粒正确,可以继续使用。
|
M. DNA相对分子质量标准(DL5000); 1、2. HindⅢ单酶切pEGFP-C1/exJSRV-env重组质粒; 3、4. Hind Ⅲ和ApaⅠ双酶切pEGFP-C1/exJSRV-env重组质粒; 5. HindⅢ单酶切pEGFP-C1空质粒; 6. KpnⅠ单酶切pEGFP-C1空质粒; 7、8. KpnⅠ单酶切pEGFP-C1/enJSRV-env重组质粒; 9、10. KpnⅠ和BamHⅠ双酶切pEGFP-C1/enJSRV-env重组质粒 M. DNA marker (DL5000); 1, 2. Digested product of pEGFP-C1/exJSRV-env with HindⅢ; 3, 4. Digested product of pEGFP-C1/exJSRV-env with HindⅢ and ApaⅠ; 5. Digested product of pEGFP-C1 with HindⅢ; 6. Digested product of pEGFP-C1 with KpnⅠ; 7, 8. Digested product of pEGFP-C1/enJSRV-env with KpnⅠ; 9, 10. Digested product of pEGFP-C1/enJSRV-env with Kpn Ⅰ and BamHⅠ 图 1 重组质粒单、双酶切鉴定 Figure 1 Identification of recombinant plasmid by single and double restriction endonuclease digestion |
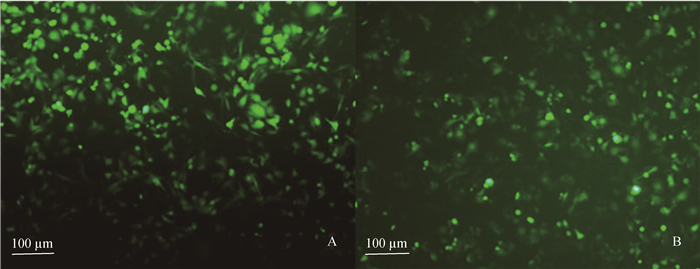
绵羊胎盘绒毛膜滋养层细胞瞬时转染重组真核表达质粒pEGFP-C1/exJSRV-env和pEGFP-C1/enJSRV-env 48 h后,荧光显微镜下观察,结果显示, 在绵羊胎盘绒毛膜滋养层细胞的细胞质内弥漫大量的绿色荧光蛋白(图 2A、B),表明重组真核表达质粒成功转染绵羊胎盘绒毛膜滋养层细胞,可用于后续研究。
|
A.转染重组质粒pEGFP-C1/exJSRV-env 48 h后的绵羊胎盘绒毛膜滋养层细胞;B.转染重组质粒pEGFP-C1/enJSRV-env 48 h后的绵羊胎盘绒毛膜滋养层细胞 A. The ovine trophoblast cells transfected with recombinant plasmid pEGFP-C1/exJSRV-env after 48 h; B. The ovine trophoblast cells transfected with recombinant plasmid pEGFP-C1/enJSRV-env after 48 h 图 2 转染重组质粒后的绵羊胎盘绒毛膜滋养层细胞 Figure 2 The ovine trophoblast cells transfected with recombinant plasmid |
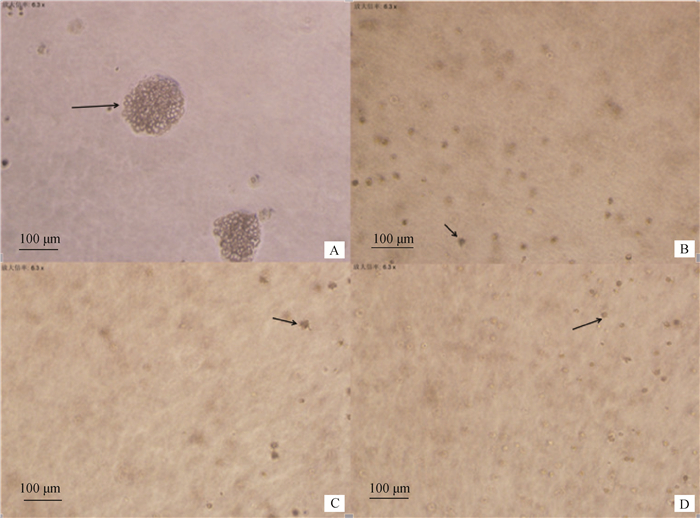
转染重组真核表达质粒pEGFP-C1/exJSRV-env的绵羊胎盘绒毛膜滋养层细胞接触抑制性消失,且能够在软琼脂上形成集落(图 3A),说明绵羊胎盘绒毛膜滋养层细胞发生了恶性转化;而转染内源性病毒的重组真核表达质粒pEGFP-C1/enJSRV-env、空载体pEGFP-C1以及空白对照组细胞均不能在琼脂上形成集落(图 3B、C、D),说明绵羊胎盘绒毛膜滋养层细胞并未发生恶性转化。
|
A.转染重组质粒pEGFP-C1/exJSRV-env 的细胞;B.转染重组质粒pEGFP-C1/enJSRV-env 的细胞;C.转染空载体的细胞;D.空白对照 A. Cells tranfected with recombinant plasmid pEGFP-C1/exJSRV-env; B.Cells transfected with recombinant plasmid pEGFP-C1/enJSRV-env; C. Cells transfected with plasmid pEGFP-C1; D. Blank control 图 3 集落形成试验(×100) Figure 3 Colony growth in soft agar(×100) |
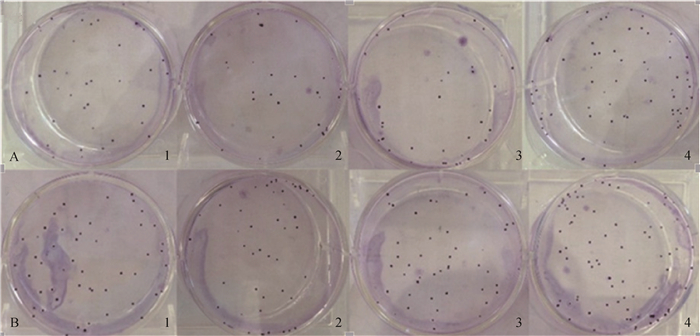
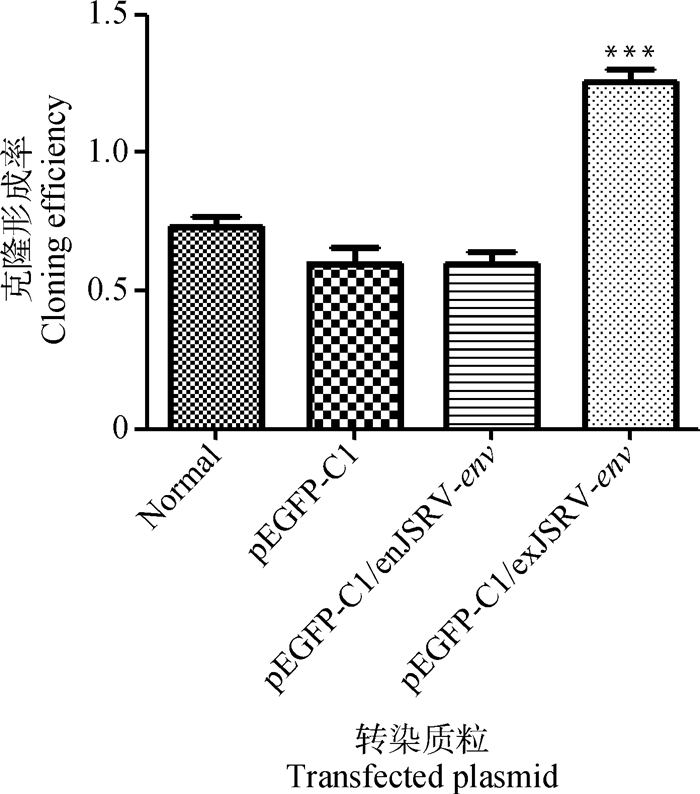
统计转染pEGFP-C1/exJSRV-env的细胞、转染pEGFP-C1/enJSRV-env、pEGFP-C1以及未转染的细胞形成的克隆数(图 4),克隆形成率结果经SPSS软件分析,转染pEGFP-C1/exJSRV-env的绵羊胎盘绒毛膜滋养层细胞的克隆形成率极显著高于其他三组,差异有统计学意义(P < 0.01)(图 5),转染pEGFP-C1/enJSRV-env以及pEGFP-C1的细胞与未转染的细胞之间无显著差异(P>0.05)(图 5)。
|
A.每孔50个细胞;B.每孔100个细胞;1.未转染的绵羊胎盘绒毛膜滋养层细胞; 2.转染空质粒pEGFP-C1的细胞; 3.转染重组质粒pEGFP-C1/enJSRV-env的细胞; 4.转染重组质粒pEGFP-C1/exJSRV-env的细胞 A. 50 cells per well; B. 100 cells per well; 1. The sheep trophoblast cells without treatment; 2. Cells transfected with pEGFP-C1; 3. Cells transfected with pEGFP-C1/enJSRV-env; 4. Cells transfected with pEGFP-C1/exJSRV-env 图 4 转染后细胞平板克隆试验 Figure 4 Cells plate cloning experiments after transfection |
|
各组间比较,***.P < 0.01 Asterisks indicate significand differences (***.P < 0.01) when compared among the different groups 图 5 克隆形成率柱状图 Figure 5 Cloning efficiency histograms |
目前,绵羊肺腺瘤逆转录病毒引起绵羊肺腺瘤病的致瘤转化机制尚不清楚。M. Borobia等[19]将携带双链DNA的JSRV质粒转染到NIH 3T3细胞中导致转化,证明JSRV携带致癌基因。将pCMV2JS21真核表达质粒转染NIH 3T3细胞,形成转化灶,而转染对照组pCDNA3.1(-)质粒未有转化灶形成[19]。本实验室张宇飞等[20]证实将重组真核表达质粒pEGFP-C1/exJSRV-env转染到293T细胞中也引起恶性转化。以上研究结果均证明JSRV囊膜蛋白(Env)是肿瘤形成的主要因素。
3.1 Env引起绵羊绒毛膜滋养层细胞恶性转化为了更好地阐述JSRV Env蛋白的转化机制,利用重组质粒pEGFP-C1/exJSRV-env转染绵羊胎盘绒毛膜滋养层细胞,通过软琼脂集落形成试验对该细胞发生的形态变化进行分析。正常的绒毛膜滋养层细胞不能在半固体软琼脂上悬浮生长形成集落,而恶性转化的细胞获得了锚定非依赖性生长能力可以生长形成集落。而本研究显示转染重组真核表达质粒pEGFP-C1/exJSRV-env的细胞形成集落,而转染重组质粒pEGFP-C1/enJSRV-env、空质粒pEGFP-C1以及空白对照组细胞却不能形成集落,这提示exJSRV囊膜蛋白(Env)促使绵羊绒毛膜滋养层细胞发生恶性转化。M. Borobia等[19]研究显示将缺失gag、pro、pol编码序列的表达质粒pCMVJS21(pCMVJS21ΔGP,唯一完整的基因是env)转染NIH3T3细胞产生转化灶,并且将exJSRV的细胞质尾区与enJSRV交换的嵌合体却不能转化NIH3T3细胞,说明引起细胞发生转化的必需是完整的囊膜蛋白,而本试验所采用的真核表达质粒pEGFP-C1/exJSRV-env就是完整表达了SU和TM的囊膜蛋白所发挥的作用。
3.2 Env对绵羊绒毛膜滋养层细胞增殖的影响细胞增殖是检测分裂中的细胞数量或者细胞群体发生的变化,克隆形成率反应细胞的增值能力。据报道地方性鼻病毒(ENTV)[21]、禽血管瘤病毒(AHV)[22]的囊膜蛋白(Env)也具有类似的致癌功能,AHV对NIH3T3细胞的增殖作用也通过AHV env基因介导, 通过将AHV env基因克隆到基于MuLV的逆转录病毒载体中证明了这一点,并且用该重组质粒转染NIH3T3细胞诱导细胞增殖和表型改变[22]。S. L. Liu等[9]将JSRV Env转染犬肾细胞(MDCK),发现转染后的细胞增殖要显著高于对照组。enJSRV-env对绒毛膜滋养层细胞的细胞融合也有一定的促进作用[23]。本实验室杜方原等[24]将重组质粒pcDNA4/myc-His/exJSRV-env转染到NIH3T3细胞也发生细胞增殖。而本研究中转染重组质粒pEGFP-C1/exJSRV-env的细胞平板克隆试验的克隆形成率也显著高于对照组,说明了细胞在exJSRV囊膜蛋白(Env)的作用下,细胞发生增殖,与上述研究结果一致。目前研究表明在exJSRV囊膜蛋白致癌过程中可能有P53蛋白、表面蛋白A(surfactant protein A, SP-A)、增殖细胞核抗原(proliferating cell nuclear antigen, PCNA)、JSRV基质蛋白(JSRV matrix protein, MA)[25]、锌指蛋白111(Zinc Finger Protein, Zfp111)[26]的参与,以及囊膜蛋白致癌过程也与细胞自噬[27]和信号通路[28]相关,本试验结果为后续的研究提供了理论依据。
4 结论exJSRV囊膜蛋白能够引起绵羊胎盘绒毛膜滋养层细胞发生恶性转化以及细胞增殖。
| [1] | ZHANG Y F, SHI J, LIU S Y. Recent advances in the study of active endogenous retrovirus envelope glycoproteins in the mammalian placenta[J]. Virol Sin, 2015, 30(4): 239–248. DOI: 10.1007/s12250-015-3617-0 |
| [2] | HULL S, LIM J, HAMIL A, et al. Analysis of Jaagsiekte sheep retrovirus (JSRV) envelope protein domains in transformation[J]. Virus Genes, 2012, 45(3): 508–517. DOI: 10.1007/s11262-012-0793-y |
| [3] | HOFACRE A, FAN H. Jaagsiekte sheep retrovirus biology and oncogenesis[J]. Viruses, 2010, 2(12): 2618–2648. DOI: 10.3390/v2122618 |
| [4] | LIU S L, MILLER A D. Oncogenic transformation by the Jaagsiekte sheep retrovirus envelope protein[J]. Oncogene, 2007, 26(6): 789–801. DOI: 10.1038/sj.onc.1209850 |
| [5] | DEMARTINI J C, BISHOPP J V, ALLEN T E, et al. Jaagsiekte sheep retrovirus proviral clone JSRVJS7, derived from the JS7 lung tumor cell line, induce ovine pulmonary carcinoma and is integrated into the surfactant protein A gene[J]. J Virol, 2001, 75(9): 4239–4246. DOI: 10.1128/JVI.75.9.4239-4246.2001 |
| [6] | YOUSSEF G, WALLACE W A H, DAGLEISH M P, et al. Ovine pulmonary adenocarcinoma:a large animal model for human lung cancer[J]. ILAR J, 2015, 56(1): 99–115. DOI: 10.1093/ilar/ilv014 |
| [7] | RAI S K, DUH F M, VIGDOROVICH V, et al. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation[J]. Proc Natl Acad Sci U S A, 2001, 98(8): 4443–4448. DOI: 10.1073/pnas.071572898 |
| [8] | ALLEN T E, SHERRILL K J, CRISPELL S M, et al. The Jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain[J]. J Gen Virol, 2002, 83(11): 2733–2742. DOI: 10.1099/0022-1317-83-11-2733 |
| [9] | LIU S L, MILLER A D. Transformation of Madin-Darby canine kidney epithelial cells by sheep retrovirus envelope proteins[J]. J Virol, 2005, 79(2): 927–933. DOI: 10.1128/JVI.79.2.927-933.2005 |
| [10] | WOOTTON S K, HALBERT C L, MILLER A D. Sheep retrovirus structural protein induces lung tumours[J]. Nature, 2005, 434(7035): 904–907. DOI: 10.1038/nature03492 |
| [11] | LINNERTH-PETRIK N M, SANTRY L A, YU D L, et al. Adeno-associated virus vector mediated expression of an oncogenic retroviral envelope protein induces lung adenocarcinomas in immunocompetent mice[J]. PLoS One, 2012, 7(12): e51400. DOI: 10.1371/journal.pone.0051400 |
| [12] | CAPORALE M, COUSENS C, CENTORAME P, et al. Expression of the Jaagsiekte sheep retrovirus envelope glycoprotein is sufficient to induce lung tumors in sheep[J]. J Virol, 2006, 80(16): 8030–8037. DOI: 10.1128/JVI.00474-06 |
| [13] | LEROUX C, GIRARDI N, COTTIN S, et al. Jaagsiekte sheep retrovirus (JSRV): from virus to lung cancer in sheep[J]. Vet Res, 2007, 38(2): 211–228. DOI: 10.1051/vetres:2006060 |
| [14] | SISTIAQA-POVEDA M, LARRUSKAIN A, MATEO-ABAD M, et al. Lack of association between polymorphic copies of endogenous Jaagsiekte sheep retrovirus (enJSRVs) and ovine pulmonary adenocarcinoma[J]. Vet Microbiol, 2016, 185: 49–55. DOI: 10.1016/j.vetmic.2016.01.019 |
| [15] |
张亚坤, 刘淑英. 绵羊肺腺瘤病毒囊膜蛋白致癌作用的研究进展[J]. 中国兽医科学, 2012, 42(12): 1315–1320.
ZHANG Y K, LIU S Y. Advance in tumorigenesis of Jaagsiekte sheep retrovirus' envelope protein[J]. Chinese Veterinary Science, 2012, 42(12): 1315–1320. (in Chinese) |
| [16] | PALMARINI M, MAEDA N, MURGIA C, et al. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells[J]. J Virol, 2001, 75(22): 11002–11009. DOI: 10.1128/JVI.75.22.11002-11009.2001 |
| [17] | HOFACRE A, FAN H. Multiple domains of the Jaagsiekte sheep retrovirus envelope protein are required for transformation of rodent fibroblasts[J]. J Virol, 2004, 78(19): 10479–10489. DOI: 10.1128/JVI.78.19.10479-10489.2004 |
| [18] |
张宇飞. 绵羊胎盘绒毛膜滋养层细胞中enJSRV囊膜蛋白的细胞生物学作用[D]. 呼和浩特: 内蒙古农业大学, 2016.
ZHANG Y F. Cell biological function of enJSRV envelope proteins in sheep trophoblast cells[D]. Hohhot: Inner Mongolia Agricultural University, 2016. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10129-1016250450.htm |
| [19] | BOROBIA M, ORTIN A, FERRER L M, et al. Cells infected with Jaagsiekte sheep retrovirus are detected in the bone marrow of asymptomatic sheep[J]. Can J Vet Res, 2014, 78(3): 237–240. |
| [20] |
张宇飞, 刘月, 王专家, 等. 绵羊肺腺瘤病毒pEGFP-C1/exJSRV-env构建及引起NIH3T3细胞恶性转化的研究[J]. 病毒学报, 2014, 30(3): 268–277.
ZHANG Y F, LIU Y, WANG Z J, et al. Research on construction of sheep lung adenomas virus pEGFP-C1/exJSRV-env and induction of malignant transformation in NIH3T3[J]. Chinese Journal of Virology, 2014, 30(3): 268–277. (in Chinese) |
| [21] | MAEDA N, FAN H. Signal transduction pathways utilized by enzootic nasal tumor virus (ENTV-1) envelope protein in transformation of rat epithelial cells resemble those used by Jaagsiekte sheep retrovirus[J]. Virus Genes, 2008, 36(1): 147–155. DOI: 10.1007/s11262-007-0193-x |
| [22] | ZHANG Y F, SHI J, LIU S Y. Establishment and characterization of a Telomerase-Immortalized sheep trophoblast cell line[J]. Biomed Res Int, 2016, 2016: 5808575. |
| [23] |
张宇飞, 石晶, 刘淑英. enJSRV囊膜蛋白及其受体的瞬时表达与绵羊绒毛膜滋养层细胞融合的诱导研究[J]. 畜牧兽医学报, 2015, 46(11): 1924–1933.
ZHANG Y F, SHI J, LIU S Y. Study on the Transient expression of enJSRV envelope protein and its receptor and the induction of trophoblast cell fusion in sheep[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(11): 1924–1933. (in Chinese) |
| [24] |
杜方原, 陈大勇, 张宇飞, 等. 绵羊肺腺瘤病毒囊膜蛋白的表达可促进NIH3T3细胞增殖[J]. 细胞与分子免疫学杂志, 2016, 32(9): 1188–1192.
DU F Y, CHEN D Y, ZHANG Y F, et al. Envelope protein of Jaagsiekte sheep retrovious expressed in NIH3T3 cells promotes cell proliferation[J]. Chinese Journal of Cellular and Molecular Immunology, 2016, 32(9): 1188–1192. (in Chinese) |
| [25] | ILHAN F, VURAL S A, YILDIRIM S, et al. Expression of p53 protein, Jaagsiekte sheep retrovirus matrix protein, and surfactant protein in the lungs of sheep with pulmonary adenomatosis[J]. J Vet Diagn Invest, 2016, 28(3): 249–256. DOI: 10.1177/1040638716636939 |
| [26] | HSU T, PHUNG A, CHOE K, et al. Role for a zinc finger protein (Zfp111) in transformation of 208F rat fibroblasts by Jaagsiekte sheep retrovirus envelope protein[J]. J Virol, 2015, 89(20): 10453–10466. DOI: 10.1128/JVI.01631-15 |
| [27] | SUN X L, DU F Y, LIU S Y. Modulation of autophagy in exJSRV-env-transfected cells through the Akt/mTOR and MAPK signaling pathway[J]. Biochem Biophys Res Commun, 2017, 485(3): 672–678. DOI: 10.1016/j.bbrc.2017.02.099 |
| [28] |
孙晓林, 刘淑英. 外源性绵羊肺腺瘤病毒囊膜蛋白激活Akt/mTOR信号通路及调控Beclin1的研究[J]. 中国预防兽医学报, 2017, 39(4): 251–256.
SUN X L, LIU S Y. Modulation of Beclin 1 in exogenous Jaagsiekte sheep retrovirus envelope gene transfected cells through the Akt/mTOR signaling pathway[J]. Chinese Journal of Preventive Veterinary Medicine, 2017, 39(4): 251–256. (in Chinese) |

 图 1(Figure 1)
图 1(Figure 1)


