2. 浙江惠嘉生物科技股份有限公司, 安吉 313300
2. Zhejiang Huijia Bio-technology Co., Ltd., Anji 313300, China
大肠杆菌病(Colibacillosis)一直是家禽养殖的难题之一,其带来肠道疾病的同时也造成了重大经济损失[1]。在家禽生产中,使用抗生素是控制大肠杆菌感染最常用的方法[2]。然而,随着抗生素在全球范围内逐渐被取缔,益生菌作为其替代物已被发现可以提高畜禽生长性能[3-4],增强免疫功能[5-7]和调节肠道微生物区系[8]。
屎肠球菌(Enterococcus faecium,简称E. faecium),是肠道中常见的产乳酸的菌种,作为益生菌已广泛应用于畜禽生产,美国饲料控制办公室已将其列为研究对象[9-10]。研究发现,屎肠球菌可提高畜禽的生长性能,改善健康,使用对象主要包括猪[11]、牛[12]、肉鸡[13-15]甚至鱼类[16-17]等。M.Levkut等[18]发现,饲喂屎肠球菌EF55对沙门氏菌感染肉鸡早期的免疫功能有调节作用。然而,很少有试验报道屎肠球菌的饲喂对大肠杆菌感染肉鸡的影响。
本研究以白羽肉鸡为试验动物,早期(7日龄)攻毒后,初步研究饲喂屎肠球菌对其生长性能、血清生化指标和盲肠菌群结构的影响。
1 材料与方法 1.1 试验动物及饲养本试验于浙江农林大学动物实验基地(中国,杭州)选取1日龄健康状况良好的白羽肉鸡360羽(平均体重(49.4±1.4) g),随机分为4组,每组肉鸡(公母各半)6个重复(每重复15只),单笼饲养。试验期间,肉鸡自由采食和饮水。鸡舍内温度第一周维持在32 ℃,随后逐渐减低至25 ℃。基础日粮参考NRC(1994)相关营养指标配制(表 1)。
|
|
表 1 基础日粮组成及营养水平(风干基础) Table 1 Composition and nutrient levels of the basal experimental diet (air-dry basis) |
试验共持续4周,第一周结束后,阳性对照组、屎肠球菌组和抗生素组肉鸡用一次性注射器口腔注射1 mL大肠杆菌菌液K88(108cfu·mL-1)。阴、阳性对照组肉鸡采食基础日粮,屎肠球菌组肉鸡采食添加109cfu·kg-1屎肠球菌的基础日粮,抗生素组肉鸡采食添加10 mg·kg-1硫酸粘杆菌素的基础日粮。
1.2 菌种制备屎肠球菌(HJEF005,鸡肠道分离所得)及大肠杆菌K88制备参照文献[19]的方法。
1.3 生长性能测定在7、10、14、21和28日龄,肉鸡每只进行称重,用于计算其体重和平均日增重。
1.4 样品收集在试验10、14、21和28天,每个重复随机挑选体重相近的2只健康状况良好的肉鸡,采用静脉割断方法进行宰杀,促凝管收集血液后离心取血清,用以检测血清生化指标。解剖并用无菌绳结扎盲肠后放入冰盒,其内容物在超净工作台内用EP管收集放入-80 ℃冰箱保存用以PCR-DGGE检测。
1.5 血清生化指标测定血清中免疫球蛋白IgA、IgE、IgM浓度利用ELISA试剂盒(武汉优尔生科技股份有限公司,武汉)检测;补体C3、C4和溶菌酶浓度检测使用南京建成试剂盒(南京建成生物工程研究所,南京);操作步骤均参照说明书。
1.6 PCR-DGGE检测肉鸡盲肠微生物菌群(16S rRNA的V3区)结构和多样性用PCR-DGGE检测。为减小样本的多样性,试验将每组中12个盲肠样品每4只合并为一个生物样本进行后续试验。微生物DNA提取采用Mobio试剂盒(Mo Bio, Laboratories, Inc., Carlsbad, CA, USA),具体提取步骤参阅美国Mobio网站。PCR引物采用GC-338F (5′-ACTCCTACGGGAGGCAGCAG-3′)和534R (5′-ATTACCGCGGCTGCTGG-3′)。试验采用50 μL反应体系:混合物包括10×PCR Buffer 5 μL,dNTP Mixture(各2.5 mmol·L-1) 4 μL,引物338F(20 μmol·L-1) 1 μL,引物534R(20 μmol·L-1) 1 μL,模板DNA 2.5 ng,TaKaRa rTaq(5 U·μL-1) 0.25 μL,ddH2O补至50 μL。PCR扩增程序:初始温度94℃10 min,30个循环(94 ℃ 60 s, 55 ℃ 60 s,72 ℃ 90 s),最后72 ℃延伸10 min。PCR产物用2%凝胶琼脂进行鉴定。
DGGE分析中聚丙烯酰胺凝胶的浓度为8%,变性剂梯度为30%~60%(100%变性剂包括7 mol·L-1尿素和40%(v/v)离子甲酰胺),电泳条件为140 V 450 min。染色采用银染方法,用UMAX Power Look 1000透射扫描仪(Techville, Inc., Dallas, USA)扫描获得的DGGE图谱图片。最终,利用BioEdit7.0 (Bio-Rad Canada, Mississauga, ON, Canada)、Phylip4.0及MEGA3.1软件对结果进行分析。
1.7 统计分析用SPSS 16.0软件对试验结果各处理进行单因素方差分析(One-Way ANOVA),同时采用Duncan多重比较进行差异显著性分析,以P < 0.05为判断差异显著的标准。
2 结果 2.1 屎肠球菌对感染大肠杆菌肉鸡生长性能的影响表 2数据显示,饲喂屎肠球菌改变了肉鸡的生长性能。21、28日龄阴性对照组肉鸡体重显著高于阳性对照组(P < 0.05);14、21、28日龄屎肠球菌组肉鸡体重显著高于阴性和阳性对照组肉鸡(P < 0.05);另外,大肠杆菌攻毒显著降低了阳性对照组10~14、15~21和22~28日龄肉鸡的平均日增重(P < 0.05)。另外,饲喂屎肠球菌显著提高了攻毒肉鸡10~28日龄的平均日增重(P < 0.05)。
|
|
表 2 屎肠球菌对肉鸡体重和平均日增重的影响 Table 2 Effects of Enterococcus faecium on body weight and average daily gain in broilers |
表 3显示了肉鸡血清中免疫球蛋白的变化,10、21和28日龄阳性对照肉鸡血清中IgA的浓度显著高于阴性对照组肉鸡(P < 0.05)。在14和21日龄,与阴性和阳性对照肉鸡相比,采食添加屎肠球菌和抗生素日粮显著提高了肉鸡血清中IgA的浓度(P < 0.05)。整个试验中,与阴性对照肉鸡相比,屎肠球菌组和抗生素组显著增加了肉鸡血清中IgG的浓度(P < 0.05)。与14和21日龄阳性对照肉鸡相比,饲喂屎肠球菌显著增加了血清中IgG的浓度(P < 0.05);与10和28日龄阴性对照肉鸡相比,阳性对照组和屎肠球菌组肉鸡血清中IgM浓度显著增加(P < 0.05),而后两者间无显著性差异。以上结果说明,饲喂屎肠球菌可以显著提高肉鸡血清中免疫球蛋白的浓度。
|
|
表 3 屎肠球菌对肉鸡血清中免疫球蛋白的影响 Table 3 Effects of Enterococcus faecium on the levels of serum immunoglobulins in broilers |
表 4显示,在21日龄时,E. f组肉鸡血清中补体C3的浓度显著高于阳性对照组(0.263 mg·mL-1 vs. 0.209 mg·mL-1, P=0.033);另外,28日龄E. f组肉鸡血清中补体C4的浓度显著高于阴性对照和抗生素组(0.162 mg·mL-1 vs. 0.122、0.118 mg·mL-1,P=0.01)。而且,饲喂屎肠球菌的肉鸡较21和28日龄抗生素组,血清中C4浓度有了显著提高(0.093 mg·mL-1 vs. 0.069 mg·mL-1, P=0.019;0.162 mg·mL-1 vs. 0.118 mg·mL-1, P=0.010)。以上结果表明,肉鸡采食添加屎肠球菌日粮可以显著提高大肠杆菌攻毒肉鸡血清中补体的浓度。
|
|
表 4 屎肠球菌对攻毒肉鸡血清中补体和溶菌酶的影响 Table 4 Effects of Enterococcus faecium on serum complement components and lysozyme in broilers |
10到21日龄,阳性对照、抗生素组及屎肠球菌组肉鸡与阴性对照组相比,血清中溶菌酶的浓度均有显著增加(1.429、1.684、1.673 ng·mL-1 vs. 1.216 ng·mL-1; 1.573、2.164、2.029 ng·mL-1 vs. 1.269 ng·mL-1; 2.059、2.012、1.997 ng·mL-1 vs. 1.548 ng·mL-1; P=0.001)。而且,10~14日龄抗生素和屎肠球菌组肉鸡较阳性对照组肉鸡,血清中溶菌酶浓度显著增加(1.684、1.673 ng·mL-1 vs. 1.429 ng·mL-1; 2.164、2.029 ng·mL-1 vs. 1.573 ng·mL-1; P=0.001)。另外,试验期间抗生素和屎肠球菌组肉鸡血清中溶菌酶的浓度均没有显著不同。试验结果表明,饲喂添加屎肠球菌的日粮可以显著增加肉鸡血清中溶菌酶的浓度。
2.4 屎肠球菌对大肠杆菌感染肉鸡盲肠微生物菌群的影响表 5和图 1显示,28日龄攻毒肉鸡盲肠微生物的多样性指数。与阳性对照组相比,饲喂屎肠球菌显著增加28日龄肉鸡盲肠微生物菌群的香农指数。聚类分析显示,屎肠球菌组肉鸡盲肠微生物菌群稳定分布(图 2)。与阴性对照组相比,屎肠球菌组肉鸡盲肠菌群有显著变化。
|
|
表 5 肉鸡盲肠微生物菌群PCR-DGGE多样性指数 Table 5 Diversity index of cecal microflora community based on the PCR-DGGE DNA fingerprinting |
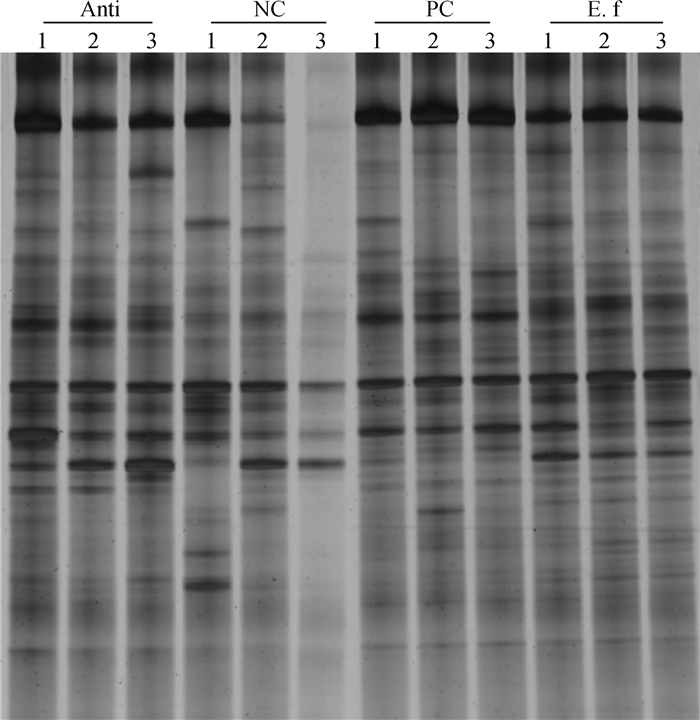
|
NC、PC、Anti和E.f分别代表阴性对照组、阳性对照组、抗生素组和屎肠球菌组;NC 1、2、3分别代表阴性对照组3个肉鸡盲肠生物样本,PC、Anti和E.f同NC。下同 NC represents negative control birds, PC represents positive control birds, E. f represents E. faecium-fed birds, Anti represents antibiotic-fed birds; NC 1, 2, 3 represent the cecal samples of birds in negative control group, which is same with PC, Anti and E.f. The same as below 图 1 肉鸡盲肠微生物16S rRNA V3区的PCR-DGGE图 Figure 1 PCR-DGGE DNA fingerprinting of the V3 region of 16S rRNA of cecal microbiota of broilers |
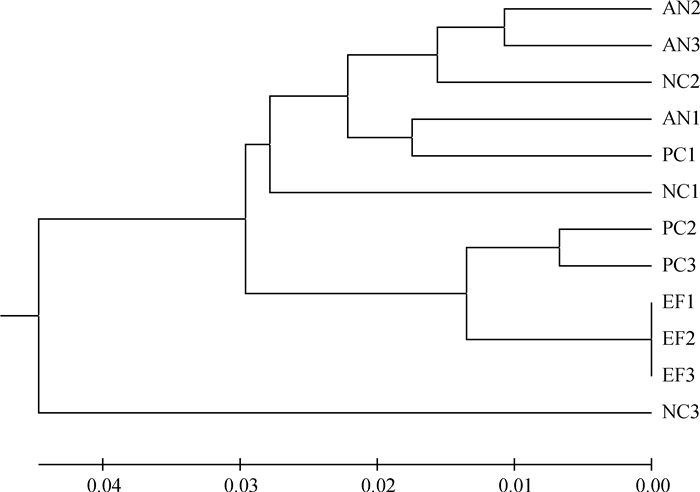
|
AN、NC、PC、EF分别代表抗生素组、阴性对照组、阳性对照组和屎肠球菌组 AN, NC, PC, EF represent the antibiotic-fed birds, negative control, positive control and E.faecium-fed birds groups, respectively 图 2 肉鸡盲肠微生物样本PCR-DGGE聚类图 Figure 2 Cluster analysis based on DGGE profiles from broiler cecal samples |
近年来,抗生素在家畜养殖方面的应用逐渐被取缔,而其替代品则引起了研究者的兴趣。我们都知道,大肠杆菌是禽类生长的慢性应激源[20],且第一周往往是大肠杆菌感染的最脆弱时期[21]。本研究结果表明,与阴性对照相比,阳性对照组肉鸡的体重和平均日增重显著减少。M.E.Cook[22]发现,动物攻毒会减少营养物质进而影响生长性能,主要表现在防御过程中营养物质的吸收减少。K.Takahashi等[23]报道称,注射免疫炎性因子(如LPS)不仅降低动物的免疫反应,同时也会导致生长的减慢。屎肠球菌已作为益生菌用以治疗动物和人类肠炎疾病很长时间[24]。当前试验显示,饲喂屎肠球菌可以显著提高大肠杆菌攻毒后肉鸡的体重和平均日增重。A.B.M.R.Bostami等[25]研究显示,肉鸡采食添加含有屎肠球菌的益生菌,平均日增重和日采食量有显著提高,且料重比显著减低。K.C.Mountzouris等[4]认为,屎肠球菌显著提高肉鸡生长性能的作用机理是其对肠道形态的有益作用。此前的研究结果显示,屎肠球菌对大肠杆菌感染的肉鸡肠道形态的发育很有益,尤其是对绒毛高度和隐窝深度[19]。但P.A.Barrow[26]推测,肠道微生物和营养物质的互作也有可能是其促进机体生长发育的主要原因。A.Zheng等[27]则对采食了屎肠球菌肉鸡的肝蛋白质组学进行了分析,认为其很有可能通过改变肉鸡的营养物质分配来促进机体的最佳养分利用。
3.2 屎肠球菌对大肠杆菌感染肉鸡血清生化指标的影响研究发现,益生菌也可增加肠黏膜抗炎和促炎因子的浓度[13]。I.Ahmad[28]证实,益生菌可能通过增加抗体IgM和IgG的产生来提高机体的免疫功能。此前的试验发现,饲喂屎肠球菌可以显著提高早期肉鸡回肠黏膜IL-4、TNF-α和S-IgA的浓度[19]。本试验结果表明,与阳性和阴性对照组肉鸡相比,屎肠球菌组肉鸡显著增加了血清中IgA浓度(14~28日龄);另一方面,21日龄屎肠球菌组肉鸡血清中C3浓度显著高于阳性对照,28日龄C4浓度显著高于阴性对照。以上结果表明,大肠杆菌K88或屎肠球菌促进了血清中IgA和IgG的分泌,与R.R.Kulkarni等[29]的研究结果相似。大量试验结果也表明,益生菌可以促进肉鸡机体的免疫反应[7, 14, 30]。但K.C.Mountzouris等[6]发现,多种益生菌(包括肠球菌)的饲喂对肉鸡血浆中免疫球蛋白的浓度没有显著作用。M.Levkut等[18]试验则发现,屎肠球菌EF 55对沙门氏菌攻毒肉鸡的外周血中淋巴细胞亚群(CD4、CD8、CD4和IgM)数量没有明显的影响。还需后续试验进一步探究屎肠球菌如何增加感染大肠杆菌肉鸡血清中免疫蛋白的浓度。
3.3 屎肠球菌对大肠杆菌感染肉鸡盲肠菌群结构的影响本试验发现,攻毒肉鸡采食添加屎肠球菌日粮后,盲肠微生物的种类有显著增加。J.W.Park等[31]发现,蛋鸡饲喂屎肠球菌后,粪便中大肠杆菌的数量有显著的减少;粪便中菌群的改变伴随着体内营养物质吸收能力的提高和粪便中氨气含量的降低。但M.M.Gheisar等[32]试验结果却显示,采食添加屎肠球菌日粮的肉鸡粪便中大肠杆菌和乳酸菌的数量没有发生显著变化。此前的试验结果发现,屎肠球菌增加了肉鸡盲肠中有益微生物乳酸菌和双气杆菌的数量,减少了大肠杆菌的数量,与其他试验结果类似[10, 19, 33]。综上所述,屎肠球菌是通过增加肉鸡肠道中有益菌的种类,减少有害菌的数量来调节微生物菌群的种类和多样性。
4 结论日粮中添加屎肠球菌提高了大肠杆菌感染肉鸡的生长性能,提高了免疫相关指数,改善了盲肠内菌群结构的多样性;肉鸡饲喂屎肠球菌相较于抗生素组,整个试验周期平均日增重显著增加,试验末血清中IgA浓度有显著提高,盲肠中菌群的多样性和丰度均有显著增加。
| [1] | ALONSO M Z, PADOLA N L, PARMA A E, et al. Enteropathogenic Escherichia coli contamination at different stages of the chicken slaughtering process[J]. Poult Sci, 2011, 90(11): 2638–2641. DOI: 10.3382/ps.2011-01621 |
| [2] | BARNES H J, VAILLANCOURT J P, GROSS W B, et al. Colibacillosis[M]//SAIF Y M. Diseases of poultry. 11th Ed. Ames, IA: Iowa State Press, 2003: 631-656 |
| [3] | SAMLI H E, SENKOYLU N, KOC F, et al. Effects of Enterococcus faecium and dried whey on broiler performance, gut histomorphology and intestinal microbiota[J]. Arch Anim Nutr, 2007, 61(1): 42–49. DOI: 10.1080/17450390601106655 |
| [4] | MOUNTZOURIS K C, TSISTSIKOS P, KALAMARA E, et al. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities[J]. Poult Sci, 2007, 86(2): 309–317. DOI: 10.1093/ps/86.2.309 |
| [5] | HUANG M K, CHOI Y J, HOUDE R, et al. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens[J]. Poult Sci, 2004, 83(5): 788–795. DOI: 10.1093/ps/83.5.788 |
| [6] | MOUNTZOURIS K C, TSITRSIKOS P, PALAMIDI I, et al. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition[J]. Poult Sci, 2010, 89(1): 58–67. DOI: 10.3382/ps.2009-00308 |
| [7] | YANG C M, CAO G T, FERKET P R, et al. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens[J]. Poult Sci, 2012, 91(9): 2121–2129. DOI: 10.3382/ps.2011-02131 |
| [8] | KONG Q, HE G Q, JIA J L, et al. Oral administration of Clostridium butyricum for modulating gastrointestinal microflora in mice[J]. Curr Microbiol, 2011, 62(2): 512–517. DOI: 10.1007/s00284-010-9737-8 |
| [9] | EMMANUEL D G V, JAFARI A, BEAUCHEMIN K A, et al. Feeding live cultures of Enterococcus faecium and Saccharomyces cerevisiae induces an inflammatory response in feedlot steers[J]. J Anim Sci, 2007, 85(1): 233–239. DOI: 10.2527/jas.2006-216 |
| [10] | LUO J J, ZHENG A J, MENG K, et al. Proteome changes in the intestinal mucosa of broiler (Gallus gallus) activated by probiotic Enterococcus faecium[J]. J Proteomics, 2013, 91: 226–241. DOI: 10.1016/j.jprot.2013.07.017 |
| [11] | MACHA M, TARAS D, VAHJEN W, et al. Specific enumeration of the probiotic strain Enterococcus faecium NCIMB 10415 in the intestinal tract and in faeces of piglets and sows[J]. Arch Anim Nutr, 2004, 58(6): 443–452. DOI: 10.1080/00039420400020058 |
| [12] | FLEIGE S, PREIBINGER W, MEYER H H D, et al. The immunomodulatory effect of lactulose on Enterococcus faecium fed preruminant calves[J]. J Anim Sci, 2009, 87(5): 1731–1738. DOI: 10.2527/jas.2007-0494 |
| [13] | CHICHLOWSKI M, CROOM W J, EDENS F W, et al. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin[J]. Poult Sci, 2007, 86(6): 1121–1132. DOI: 10.1093/ps/86.6.1121 |
| [14] | CAPCAROVA M, WEIS J, HMRNCAR C, et al. Effect of Lactobacillus fermentum and Enterococcus faecium strains on internal milieu, antioxidant status and body weight of broiler chickens[J]. J Anim Physiol Anim Nutr, 2010, 94(5): e215–e224. DOI: 10.1111/jpn.2010.94.issue-5 |
| [15] | AWAD W, GHAREEB K, BOHM J. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides[J]. Int J Mol Sci, 2008, 9(11): 2205–2216. DOI: 10.3390/ijms9112205 |
| [16] | KIM Y, KIM E Y, CHOI S Y, et al. Effect of a Probiotic Strain, Enterococcus faecium, on the immune responses of Olive Flounder (Paralichthys olivaceus)[J]. J Microbiol Biotechnol, 2012, 22(4): 526–529. DOI: 10.4014/jmb |
| [17] | SUN Y Z, YANG H L, MA R L, et al. Culture-independent characterization of the autochthonous gut microbiota of grouper Epinephelus coioides following the administration of probiotic Enterococcus faecium[J]. Aquacult Int, 2012, 20(4): 791–801. DOI: 10.1007/s10499-012-9503-y |
| [18] | LEVKUT M, REVAJOVÁ V, LAUKOVÁ A, et al. Leukocytic responses and intestinal mucin dynamics of broilers protected with Enterococcus faecium EF55 and challenged with Salmonella Enteritidis[J]. Res Vet Sci, 2012, 93(1): 195–201. DOI: 10.1016/j.rvsc.2011.06.021 |
| [19] | CAO G T, ZENG X F, CHEN A G, et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88[J]. Poult Sci, 2013, 92(11): 2949–2955. DOI: 10.3382/ps.2013-03366 |
| [20] | BUTLER E J, CURTIS M J, HARRY E G. Escherichia coli endotoxin as a stressor in the domestic fowl[J]. Res Vet Sci, 1977, 23(1): 20–23. |
| [21] | GOREN E. Observations on experimental infection of chicks with Escherichia coli[J]. Avian Pathol, 1978, 7(2): 213–224. DOI: 10.1080/03079457808418274 |
| [22] | COOK M E. Triennial growth symposium:A review of science leading to host-targeted antibody strategies for preventing growth depression due to microbial colonization[J]. J Anim Sci, 2011, 89(7): 1981–1990. DOI: 10.2527/jas.2010-3375 |
| [23] | TAKAHASHI K, YODOGAWA S, AKIBA Y, et al. Effect of dietary protein concentration on responses to Escherichia coli endotoxin in broiler chickens[J]. Br J Nutr, 1995, 74(2): 173–182. DOI: 10.1079/BJN19950121 |
| [24] | BELLOMO G, MANGIAGLE A, NICASTRO L, et al. A controlled double-blind study of SF68 strain as a new biological preparation for the treatment of diarrhoea in pediatrics[J]. Curr Ther Res, 1980, 28(6): 927–936. |
| [25] | BOSTAMI A B M R, ISLAM M M, AHMED S T, et al. Effect of beneficial microorganisms on growth performance, mortality and intestinal microflora in broilers[J]. Global J Microbiol Res, 2015, 3(2): 126–133. |
| [26] | BARROW P A. Probiotics for chickens[M]//Fuller R. Probiotics: The scientific basis. London: Chapman and Hall, 1992: 255-257. |
| [27] | ZHENG A, LUO J, MENG K, et al. Probiotic (Enterococcus faecium) induced responses of the hepatic proteome improves metabolic efficiency of broiler chickens (Gallus gallus)[J]. BMC genomics, 2016, 17: 89. DOI: 10.1186/s12864-016-2371-5 |
| [28] | AHMAD I. Effect of probiotics on broilers performance[J]. Int J Poult Sci, 2006, 5(6): 593–597. DOI: 10.3923/ijps.2006.593.597 |
| [29] | KULKARNI R R, PARREIRA V R, SHARIF S, et al. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis[J]. Clin Vaccine Immunol, 2007, 14(9): 1070–1077. DOI: 10.1128/CVI.00162-07 |
| [30] |
靳二辉, 陈耀星, 王群, 等. 芽孢杆菌类益生素对肉鸡血细胞及免疫器官组织结构的影响[J]. 畜牧兽医学报, 2013, 44(5): 778–787.
JIN E H, CHEN Y X, WANG Q, et al. Effect of the bacillus probiotics on blood cells and structure of immune organs in broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(5): 778–787. (in Chinese) |
| [31] | PARK J W, JEONG J S, LEE S I, et al. Effect of dietary supplementation with a probiotic (Enterococcus faecium) on production performance, excreta microflora, ammonia emission, and nutrient utilization in ISA brown laying hens[J]. Poult Sci, 2016, 95(12): 2829–2835. DOI: 10.3382/ps/pew241 |
| [32] | GHEISAR M M, HOSSEINDOUST A, KIM I H, et al. Effects of dietary Enterococcus faecium on growth performance, carcass characteristics, faecal microbiota, and blood profile in broilers[J]. Veterin Med, 2016, 61(1): 28–34. DOI: 10.17221/VETMED |
| [33] | CHEN K, GAO J, LI J X, et al. Effects of probiotics and antibiotics on diversity and structure of intestinal microflora in broiler chickens[J]. Afr J Microbol Res, 2012, 6(37): 6612–6617. |

 图 1(Figure 1)
图 1(Figure 1)


