2. 江苏省农业科学院畜牧研究所, 南京 210014;
3. 新疆农业大学动物科学学院, 乌鲁木齐 830052
2. Institute of Animal Science, Jiangsu Academy of Agricultural Sciences, Nanjing 210014, China;
3. College of Animal Science, Xinjiang Agricultural University, Urumqi 830052, China
促卵泡素受体(Follicle stimulating hormone receptor,FSHR)是雌性哺乳动物卵巢颗粒细胞膜上一种重要的受体蛋白,其编码基因是影响哺乳动物多胎性状的主效基因,调控卵巢颗粒细胞状态(增殖和凋亡)、卵泡发育和排卵[1-2]。促卵泡素(Follicle stimulating hormone,FSH)信号转导必须通过与卵巢颗粒细胞膜上的受体FSHR结合才能作用于卵巢,从而促进卵泡发育、调控配子形成[3],因此FSHR是母畜生殖必需的。研究发现猪卵泡成熟与排卵依赖FSHR基因的表达[4],其基因编码区上3个碱基突变(c.74C>G、c.532G>A和c.1166T>C)显著影响猪排卵数,FSHR被认为是影响猪繁殖性状的主效基因[1]。在绵羊中,FSHR基因在卵巢组织不同大小(直径4~7 mm)和不同状态(优势卵泡、劣势卵泡和黄体化卵泡)的卵泡中均差异表达[5],说明FSHR基因表达水平与绵羊卵泡发育、优势化和排卵有关。FSHR基因5'-调控区和编码区SNP位点(g.-414G>A、g.-200G>A、g.-197G>A、g.-98T>C、g.-47C>T和c.1235T>C)的多态性均与绵羊多胎性状(产羔数)显著关联[6-9]。因此,FSHR是影响绵羊多胎性状的重要候选基因。
湖羊是中国著名的多胎绵羊品种,其多胎机制一直是湖羊特色性状研究和应用的热点[6, 10-12]。FSHR基因SNP位点多态性与湖羊多胎性状之间关系密切[13],是影响湖羊多胎性状的候选基因。在高繁殖力绵羊个体卵巢卵泡中,FSHR基因转录水平显著高于低繁殖力个体[5, 14],可见卵泡中FSHR基因表达水平与绵羊繁殖力有关。但目前关于绵羊FSHR基因转录调控的研究较少,特别是转录因子的调控还未见报道。本研究拟以湖羊FSHR基因为研究对象,克隆湖羊FSHR基因5'-UTR序列,了解其序列特征(如转录调控元件和转录因子结合位点),分析湖羊转录因子PAX4基因的组织表达特征和在卵巢颗粒细胞凋亡中的作用,以及对湖羊FSHR基因转录活性的影响,以期揭示湖羊FSHR基因的转录调控机制和湖羊多胎分子机制。
1 材料与方法 1.1 试验动物成年湖羊母羊(n=3)心、肝、脾、肺、肾、胃、肌肉、大肠、小肠、子宫和卵巢11种组织样采自苏州种羊场,置于液氮中保存,用于提取DNA和RNA。
1.2 核酸提取采用酚/氯仿法提取湖羊卵巢组织DNA。采用Trizol(Invitrogen公司)法提取湖羊各组织和卵巢颗粒细胞总RNA,并利用快速反转录试剂盒(TaKaRa公司)反转录成cDNA。DNA和cDNA均在-20 ℃冰箱中保存备用。
1.3 克隆测序以绵羊FSHR基因序列(GenBank序列号:NC_019460.1)为参考序列,设计引物P1用于扩增湖羊FSHR基因5'-UTR序列,引物信息和扩增条件见表 1。PCR产物用1.5%琼脂糖凝胶电泳进行分离,并采用胶回收试剂盒(Axygen公司)进行回收。回收产物与载体pMD19-T(TaKaRa公司)连接后,转化到感受态细胞DH5α(天根公司)中;挑取阳性克隆,采用质粒提取试剂盒(Axygen公司)提取质粒,由上海生物工程公司进行双向测序。
|
|
表 1 引物信息 Table 1 The primers in this study |
采用DNAMAN v5.2.2软件进行序列整理和比对分析;采用在线程序Genomatix(https://www.genomatix.de)进行转录调控元件和转录因子结合位点预测;采用在线软件Methprimer(http://www.urogene.org)预测CpG岛。
1.5 组织表达谱以湖羊11个组织cDNA为模板,以P2(表 1)为引物进行RT-PCR扩增。扩增产物用1.5%琼脂糖凝胶电泳进行分离,用Tanon-3500凝胶成像系统拍照。以GAPDH为内参基因,采用Quantity One v4.52软件对目的基因和内参基因进行灰度分析,用目的基因/内参基因计算不同组织目的基因的相对表达量。
1.6 载体构建湖羊PAX4基因过表达载体pcDNA3.1-PAX4由南京伯津公司合成,采用双酶切鉴定法和直接测序进行确认。以P4(表 1)为引物扩增湖羊FSHR基因5'-UTR,用限制性内切酶Kpn Ⅰ和Hind Ⅲ酶切后,克隆到pGL3双荧光素酶报告基因载体(Promega公司)中,并转染感受态细胞DH5α(天根公司),采用双酶切鉴定法和直接测序进行确认,构建湖羊FSHR基因5'-UTR荧光素酶报告基因重组质粒。
1.7 细胞培养、转染和荧光素酶活性分析商品猪卵巢采自南京天环屠宰场,用于抽取卵巢颗粒细胞。猪卵巢颗粒细胞培养、转染和荧光素酶活性分析的具体方法见文献[2]。
1.8 qRT-PCRpcDNA3.1-PAX4转染体外培养的卵巢颗粒细胞,48 h后收集细胞,提取细胞总RNA,并反转录成cDNA。卵巢颗粒细胞中PAX4基因表达水平采用qRT-PCR技术进行检测,具体步骤见文献[11],退火温度见表 1。
1.9 数据分析数据用“平均数±标准误”表示,采用SPSS v18.0软件对数据进行统计分析。P < 0.05表示差异显著;P < 0.01表示差异极显著。
2 结果 2.1 湖羊FSHR基因5'调控区克隆以湖羊卵巢组织基因组DNA为模板,利用引物P1进行PCR扩增,PCR产物电泳结果见图 1。从图 1可以看出,电泳条带单一明亮。测序发现,扩增片段长度为730 bp,与引物源序列(GenBank序列号:NC_019460.1)的一致性为98.99%。
|
M.DL2000 marker;1~2.PCR扩增产物 M.DL2000 marker; 1-2.PCR product 图 1 湖羊FSHR基因5'调控区扩增产物电泳图 Figure 1 Agarose gel photograph of FSHR 5' regulatory region in Hu sheep |
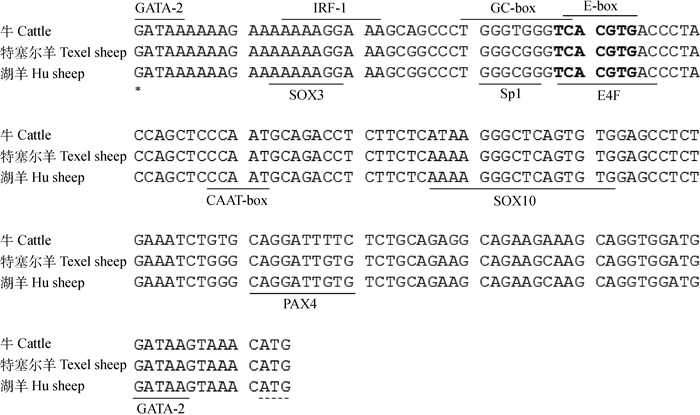
湖羊FSHR基因5'-UTR序列全长161 bp(图 2)。序列分析发现,湖羊FSHR基因5'-UTR序列中A、T、C和G等4种碱基的含量分别是32.30%、16.77%、21.74%和29.19%,其中A+T含量(49.07%)接近C+G含量(50.93%)。同源性比对发现,湖羊FSHR基因5'-UTR序列与特塞尔(Texel)绵羊序列(GenBank序列号:NC_019460.1)一致,与牛(GenBank序列号:AC_000168.1)、小鼠(GenBank序列号:NC_000083.6)和斑马鱼(GenBank序列号:NC_007123.7)的一致性分别为95.03%、61.11%和28.90%,可见FSHR基因5'-UTR核苷酸序列在哺乳动物中较为保守。通过序列比对发现,在湖羊FSHR基因5'-UTR区含有多个转录调控元件,如E-box、CAAT-box和GC-box等(图 2)。采用在线软件Genomatic对湖羊FSHR基因5'-UTR序列进行转录因子结合位点预测,结果显示,在湖羊FSHR基因5'-UTR序列含有GATA -2、SOX3、IRF-1、Sp1、E4FUSF1和SOX10转录因子结合位点(图 2)。采用在线软件Methprimer在湖羊FSHR基因5'-UTR中未检测到CpG岛,但发现多个CpG位点。
|
*表示转录起始位点; 黑体字母表示USF1结合位点;虚线表示起始密码子 * indicate transcription start site. Black letters indicate binding site for USF1;Dashed line indicate start codon 图 2 湖羊FSHR基因5'-UTR序列与特塞尔绵羊、牛同源性比对 Figure 2 Alignment of FSHR 5'-UTR sequence in Hu sheep with Texel sheep and cattle |
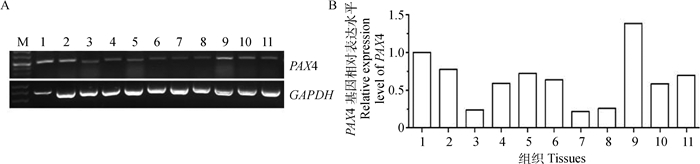
PAX4(Paired box 4)是一种重要的转录因子,主要通过与靶基因调控区结合调控基因转录[15],但关于其调控FSHR基因转录和在卵巢中作用的研究还未见报道。本研究首先以湖羊心、肝、脾、肺、肾、胃、肌肉、大肠、小肠、子宫和卵巢11种组织cDNA为模板,采用RT-PCR技术检测各组织中PAX4基因表达情况,结果见图 3。从图 3中可以看出,PAX4基因在湖羊11种组织中均有表达,其中在小肠组织表达量最高,在心和肝等组织高表达,在心、肝、肺、胃、肾、子宫和卵巢等组织中等表达,而在脾、肌肉和大肠等组织中低表达,可见PAX4是一个卵巢组织表达基因。
|
M.DL100 marker;1~11.心、肝、脾、肺、肾、胃、肌肉、大肠、小肠、子宫和卵巢 M.DL100 marker; 1-11. Heart, liver, spleen, lung, kidney, stomach, muscle, rectum, intestinal, uterus and ovary 图 3 湖羊PAX4基因组织表达谱 Figure 3 The mRNA expression profile of PAX4 gene in various tissues of Hu sheep |
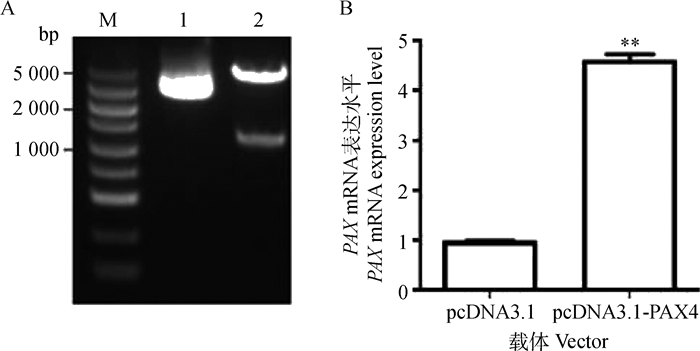
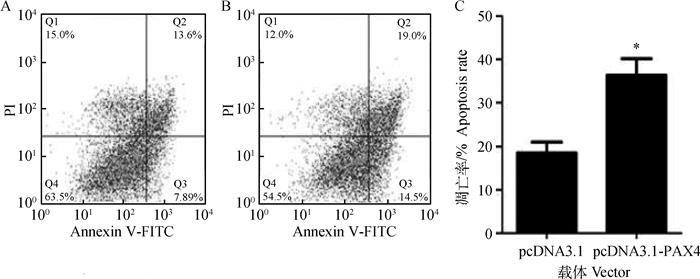
本研究合成了湖羊PAX4基因过表达载体(pcDNA3.1-PAX4)。pcDNA3.1-PAX4载体经双酶切(图 4A)和直接测序鉴定正确后,瞬时转染体外培养的卵巢颗粒细胞,qRT-PCR检测发现,PAX4过表达组(即转染pcDNA3.1-PAX4)卵巢颗粒细胞中PAX4基因mRNA表达水平极显著高于对照组(P < 0.01)(图 4B),说明构建的湖羊PAX4基因过表达质粒pcDNA3.1-PAX4可在卵巢颗粒细胞中高效表达,符合试验要求。流式细胞术检测发现,PAX4过表达组卵巢颗粒细胞凋亡率显著高于对照组(P < 0.05)(图 5),说明PAX4可促进卵巢颗粒细胞凋亡,是一个促凋亡转录因子。
|
A.pcDNA3.1-PAX4载体的酶切鉴定;B. pcDNA3.1-PAX4载体转染后PAX4基因表达。M.DL5000 marker;1.pcDNA3.1载体;2.pcDNA3.1-PAX4载体。**.P < 0.01 A.The digested results of pcDNA3.1-PAX4 vector; B.The expression of PAX4 in granulosa cells transfected with pcDNA3.1-PAX4 vector. M.DL5000 marker; 1.pcDNA3.1 vector; 2.The restructuring pcDNA3.1-PAX4 vector. **.P < 0.01 图 4 湖羊PAX4基因过表达载体在卵巢颗粒细胞中的表达 Figure 4 The expression of Hu sheep pcDNA3.1-PAX4 vector in ovarian granulosa cells |
|
A、B.流式细胞术检测pcDNA3.1和pcDNA3.1-PAX4转染后颗粒细胞凋亡;C.凋亡率分析。*. P < 0.05 A, B.Flow cytometry was performed to detect cell apoptosis in GCs transfected with pcDNA3.1 and pcDNA3.1-PAX4;C.Apoptosis rate analysis. *. P < 0.05 图 5 湖羊PAX4在卵巢颗粒细胞凋亡中的作用 Figure 5 The role of Hu sheep PAX4 in ovarian granulosa cell apoptosis |
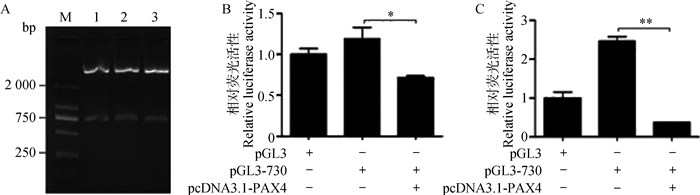
本研究构建了包含PAX4结合位点(CAGGATTG)的湖羊FSHR基因5'-UTR的双荧光素酶报告载体pGL3-730(图 6A)。将湖羊PAX4基因过表达载体pcDNA3.1-PAX4与FSHR基因5'-UTR双荧光素酶报告载体pGL3-730共转体外培养的卵巢颗粒细胞,收集细胞检测荧光素酶活性,结果见图 6B。从图 6B中可以看出,转染pcDNA3.1-PAX4后,卵巢颗粒细胞中pGL3-730载体的荧光素酶活性显著低于对照组(P < 0.05)。同样地,转染pcDNA3.1-PAX4后,COS-7细胞中pGL3-730载体的荧光素酶活性极显著低于对照组(P < 0.01)(图 6C)。结果说明转录因子PAX4可下调湖羊FSHR基因5'-UTR活性,即抑制FSHR基因的转录活性。
|
A.pGL3-730载体的酶切鉴定: M.DL2000 marker; 1~3.pGL3-730酶切产物。B.过表达PAX4对卵巢颗粒细胞中pGL3-730活性的影响; C.过表达PAX4对COS-7细胞中pGL3-730活性的影响。*.P < 0.05;**.P < 0.01 A.The digested results of pGL3-730 vector:M.DL2000 marker; 1-3.The restructuring pGL3-730 vector.B. Overexpression of PAX4 influences luciferase activity of pGL3-730 vector in granulosa cells; C.Overexpression of PAX4 influences luciferase activity of pGL3-730 vector in COS-7 cells.*.P < 0.05;**.P < 0.01 图 6 PAX4对湖羊FSHR基因转录活性的影响 Figure 6 The effect of PAX4 on the transcriptional activity of FSHR gene in Hu sheep |
FSH是促进哺乳动物卵巢卵泡生长、发育、分化和成熟的关键激素,但其生物学功能的发挥必须通过与其靶细胞膜上的受体蛋白(FSHR)结合才能传导信号进入靶细胞内[16],因此FSHR水平的高低决定了FSH对靶细胞的作用效果,以及响应的生物学特征[17]。研究发现FSHR基因转录调控的研究主要在5'调控区,其中人、模式动物(如小鼠)和主要家畜(如猪、牛)等哺乳动物FSHR基因5'-UTR和核心启动子区均已被成功鉴定,5'调控区结构和序列特征也进行了深入的研究[18-21]。在绵羊中,早在1997年就已鉴定出其FSHR基因的5'-UTR[22],其核心启动子区也于2001年被发现[23]。另外,国内著名地方绵羊品种湖羊FSHR基因核心启动子区的鉴定工作也于2014年完成[9],但目前关于绵羊特别是湖羊FSHR基因5'-UTR特征的研究相对较少。本研究通过克隆测序获得了湖羊FSHR基因5'-UTR序列,并发现其包含多个已知的转录调控元件,如E-box、CAAT-box和GC-box等。E-box是目前研究最多的FSHR基因5'-UTR的转录调控元件,在人、大鼠和绵羊等物种中已证实E-box元件可招募大量同源结合因子、上游刺激因子USF1与USF2,调控FSHR基因的转录活性[20]。I.Teino等[24]发现,AHR可通过E-box元件调控小鼠卵巢中FSHR基因的转录活性。另外,CAAT-box可能是RNA聚合酶Ⅱ(RNA polymerase Ⅱ,Pol Ⅱ)特异性结合的DNA序列元件,控制基因转录的正确起始和频率[25-26],而GC-box一般位于CAAT-box两侧,是转录因子Sp1结合区域,可激活基因转录[27-28],但目前关于这2个转录调控元件调控FSHR基因转录的研究还未见报道。湖羊FSHR基因5'-UTR序列中多个转录调控元件的发现,为进一步研究湖羊卵巢组织中FSHR基因的转录调控奠定了基础。
转录因子是一类具有特定功能的蛋白,主要通过与靶基因5'调控区上相应的结合位点组合在一起形成顺式调控模块,共同调控靶基因转录。目前已鉴定的FSHR基因的转录调控因子主要包括转录因子如GATA-1[29]、E2F[29]、GATA-4[30]、GATA-6[30]、AR[31]、DAX-1[31]、OCT-1[32]、FOXL2[33]和YY1[9]等,和表观遗传因子如组蛋白去乙酰化酶MAT2[34]、miR-143[6]和miR-126*[35]等,其中通过作用于5'调控区对FSHR基因进行转录调控的主要还是转录因子。本研究在湖羊FSHR基因5'-UTR序列中预测到多个转录因子结合位点,如GATA-2、SOX3、IRF-1、Sp1、E4F、USF1和SOX10等,其中USF1[20]和GATA家族[32]已被证实可调控哺乳动物FSHR基因转录。PAX4是已知的促进哺乳动物胚胎期内分泌腺体发育的关键转录因子[15],但目前关于其在卵巢中的作用及对FSHR基因进行转录调控的研究还未见报道。本研究结果发现,PAX4可促进卵巢颗粒细胞凋亡,是一个促凋亡因子,这与FSHR在卵巢颗粒细胞中的作用正好相反[2]。进一步研究发现,PAX4可抑制湖羊FSHR基因转录活性,这与PAX4、FSHR在卵巢颗粒细胞中的作用[2]是一致的,说明PAX4是湖羊FSHR基因的功能性转录因子。
4 结论本研究克隆了湖羊FSHR基因5'-UTR序列,了解了5'-UTR序列特征(如转录调控元件和转录因子结合位点)。转录因子PAX4在湖羊卵巢组织中表达,可促进卵巢颗粒细胞凋亡。荧光素酶活性分析发现,PAX4可抑制湖羊FSHR基因转录活性,是湖羊FSHR基因的抑制性转录因子。
| [1] | SATO S, HAYASHI T, KOBAYASHI E. Fine mapping the number of corpora lutea quantitative trait loci on SSC3: Analysis of the porcine follicle-stimulating hormone receptor gene[J]. Anim Sci J, 2011, 82(5): 633–641. DOI: 10.1111/asj.2011.82.issue-5 |
| [2] | DU X, ZHANG L F, LI X Y, et al. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis[J]. Cell Death Dis, 2016, 7(11): e2476. DOI: 10.1038/cddis.2016.379 |
| [3] | LÓPEZ-DOVAL S, SALGADO R, LAFUENTE A. The expression of several reproductive hormone receptors can be modified by perfluorooctane sulfonate (PFOS) in adult male rats[J]. Chemosphere, 2016, 155: 488–497. DOI: 10.1016/j.chemosphere.2016.04.081 |
| [4] | CAI L P, SUN A D, LI H, et al. Molecular mechanisms of enhancing porcine granulosa cell proliferation and function by treatment in vitro with anti-inhibin alpha subunit antibody[J]. Reprod Biol Endocrinol, 2015, 13: 26. DOI: 10.1186/s12958-015-0022-3 |
| [5] | REGAN S L, MCFARLANE J R, O'SHEA T, et al. Flow cytometric analysis of FSHR, BMRR1B, LHR and apoptosis in granulosa cells and ovulation rate in Merino sheep[J]. Reproduction, 2015, 150(2): 151–163. DOI: 10.1530/REP-14-0581 |
| [6] | PAN X Y, LIU S J, LI F D, et al. Molecular characterization, expression profiles of the ovine FSHR gene and its association with litter size[J]. Mol Biol Rep, 2014, 41(12): 7749–7754. DOI: 10.1007/s11033-014-3666-8 |
| [7] | CHU M X, GUO X H, FENG C J, et al. Polymorphism of 5' regulatory region of ovine FSHR gene and its association with litter size in Small Tail Han sheep[J]. Mol Biol Rep, 2012, 39(4): 3721–3725. DOI: 10.1007/s11033-011-1147-x |
| [8] |
王金鑫. 不同繁殖力绵羊FSHR基因结构和表达的差异研究[D]. 北京: 中国农业科学院, 2013.
WANG J X. Study on differences of FSHR gene structure and expression in sheep with different fecundity[D]. Beijing: Chinese Academy of Agricultural Sciences, 2013. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-82101-1013357296.htm |
| [9] |
郭晶. 湖羊FSHR和TGF-β1基因核心启动子区鉴定与表达调控[D]. 南京: 南京农业大学, 2014.
GUO J. Identification and regulation of the FSHR and TGF-β1 gene core promoter region in Hu sheep[D]. Nanjing: Nanjing Agricultural University, 2014. (in Chinese) http://cdmd.cnki.com.cn/Article/CDMD-10307-1016037530.htm |
| [10] | CHU M X, ZHUANG H B, ZHANG Y J, et al. Polymorphism of inhibin βB gene and its relationship with litter size in sheep[J]. Anim Sci J, 2011, 82(1): 57–61. DOI: 10.1111/asj.2011.82.issue-1 |
| [11] |
郭晶, 李新宇, 李隐侠, 等. 湖羊TGF-β1基因特征、表达及其与排卵数的相关性分析[J]. 中国农业科学, 2013, 46(21): 4586–4593.
GUO J, LI X Y, LI Y X, et al. Characterization, expression of TGF-β1 gene and its association with ovulation rate in Hu sheep[J]. Scientia Agricultura Sinica, 2013, 46(21): 4586–4593. DOI: 10.3864/j.issn.0578-1752.2013.21.022 (in Chinese) |
| [12] | HU X J, POKHAREL K, PEIPPO J, et al. Identification and characterization of miRNAs in the ovaries of a highly prolific sheep breed[J]. Anim Genet, 2016, 47(2): 234–239. DOI: 10.1111/age.2016.47.issue-2 |
| [13] | WANG W M, LIU S J, LI F D, et al. Polymorphisms of the ovine BMPR-IB, BMP-15 and FSHR and their associations with litter size in two Chinese indigenous sheep breeds[J]. Int J Mol Sci, 2015, 16(5): 11385–11397. |
| [14] | GOYAL S, AGGARWAL J, DUBEY P K, et al. Expression analysis of genes associated with prolificacy in FecB carrier and noncarrier Indian sheep[J]. Anim Biotechnol, 2017, 28(3): 220–227. DOI: 10.1080/10495398.2016.1262869 |
| [15] | VOLODIN A, KOSTI I, GOLDBERG A L, et al. Myofibril breakdown during atrophy is a delayed response requiring the transcription factor PAX4 and desmin depolymerization[J]. Proc Natl Acad Sci U S A, 2017, 114(8): E1375–E1384. DOI: 10.1073/pnas.1612988114 |
| [16] | ILGAZ N S, AYDOS O S, KARADAG A, et al. Impact of follicle-stimulating hormone receptor variants in female infertility[J]. J Assist Reprod Genet, 2015, 32(11): 1659–1668. DOI: 10.1007/s10815-015-0572-5 |
| [17] | BRAMBLE M S, GOLDSTEIN E H, LIPSON A, et al. A novel follicle-stimulating hormone receptor mutation causing primary ovarian failure: A fertility application of whole exome sequencing[J]. Hum Reprod, 2016, 31(4): 905–914. DOI: 10.1093/humrep/dew025 |
| [18] | GROMOLL J, DANKBAR B, GUDERMANN T. Characterization of the 5' flanking region of the human follicle-stimulating hormone receptor gene[J]. Mol Cell Endocrinol, 1994, 102(1-2): 93–102. DOI: 10.1016/0303-7207(94)90102-3 |
| [19] | LEVALLET J, KOSKIMIES P, RAHMAN N, et al. The promoter of murine follicle-stimulating hormone receptor: Functional characterization and regulation by transcription factor steroidogenic factor 1[J]. Mol Endocrinol, 2001, 15(1): 80–92. DOI: 10.1210/mend.15.1.0583 |
| [20] | GEORGE J W, DILLE E A, HECKERT L L. Current concepts of follicle-stimulating hormone receptor gene regulation[J]. Biol Reprod, 2011, 84(1): 7–17. DOI: 10.1095/biolreprod.110.085043 |
| [21] | WU W J, HAN J, CAO R, et al. Sequence and regulation of the porcine FSHR gene promoter[J]. Anim Reprod Sci, 2015, 154: 95–104. DOI: 10.1016/j.anireprosci.2014.11.023 |
| [22] | SAIRAM M R, SUBBARAYAN V S. Characterization of the 5' flanking region and potential control elements of the ovine follitropin receptor gene[J]. Mol Reprod Dev, 1997, 48(4): 480–487. DOI: 10.1002/(ISSN)1098-2795 |
| [23] | XING W, SAIRAM M R. Characterization of regulatory elements of ovine follicle-stimulating hormone (FSH) receptor gene: The role of E-box in the regulation of ovine FSHreceptor expression[J]. Biol Reprod, 2001, 64(2): 579–589. DOI: 10.1095/biolreprod64.2.579 |
| [24] | TEINO I, KUUSE S, INGERPUU S, et al. The aryl hydrocarbon receptor regulates mouse Fshr promoter activity through an e-box binding site[J]. Biol Reprod, 2012, 86(3): 77. |
| [25] | BARBASH Z S, WEISSMAN J D, CAMPBELL J A Jr, et al. Major histocompatibility complex class Ⅰ core promoter elements are not essential for transcription in vivo[J]. Mol Cell Biol, 2013, 33(22): 4395–4407. DOI: 10.1128/MCB.00553-13 |
| [26] | YANG Z S, YOSHIOKA H, MCCARREY J R. Sequence-specific promoter elements regulate temporal-specific changes in chromatin required for testis-specific activation of the Pgk2 gene[J]. Reproduction, 2013, 146(5): 501–516. DOI: 10.1530/REP-13-0311 |
| [27] | TA A, THAKUR B K, DUTTA P, et al. Double-stranded RNA induces cathelicidin expression in the intestinal epithelial cells through phosphatidylinositol 3-kinase-protein kinase Cζ-Sp1 pathway and ameliorates shigellosis in mice[J]. Cell Signal, 2017, 35: 140–153. DOI: 10.1016/j.cellsig.2017.03.016 |
| [28] | THAKUR B K, DASGUPTA N, TA A, et al. Physiological TLR5 expression in the intestine is regulated by differential DNA binding of Sp1/Sp3 through simultaneous Sp1 dephosphorylation and Sp3 phosphorylation by two different PKC isoforms[J]. Nucleic Acids Res, 2016, 44(12): 5658–5672. DOI: 10.1093/nar/gkw189 |
| [29] | KIM J S, GRISWOLD M D. E2F and GATA-1 are required for the Sertoli cell-specific promoter activity of the follicle-stimulating hormone receptor gene[J]. J Androl, 2001, 22(4): 629–639. |
| [30] | BENNETT J, WU Y G, GOSSEN J, et al. Loss of GATA-6 and GATA-4 in granulosa cells blocks folliculogenesis, ovulation, and follicle stimulating hormone receptor expression leading to female infertility[J]. Endocrinology, 2012, 153(5): 2474–2485. DOI: 10.1210/en.2011-1969 |
| [31] | LU C L, YANG W, CHEN M, et al. Inhibin A inhibits follicle-stimulating hormone (FSH) action by suppressing its receptor expression in cultured rat granulosa cells[J]. Mol Cell Endocrinol, 2009, 298(1-2): 48–56. DOI: 10.1016/j.mce.2008.09.039 |
| [32] | HERMANN B P, HECKERT L L. Silencing of Fshr occurs through a conserved, hypersensitive site in the first intron[J]. Mol Endocrinol, 2005, 19(8): 2112–2131. DOI: 10.1210/me.2004-0244 |
| [33] | QIN N, FAN X C, XU X X, et al. Cooperative effects of FOXL2 with the members of TGF-β superfamily on FSH receptor mRNA expression and granulosa cell proliferation from hen prehierarchical follicles[J]. PLoS One, 2015, 10(10): e0141062. DOI: 10.1371/journal.pone.0141062 |
| [34] | ZHANG S, LI W, ZHU C C, et al. Sertoli cell-specific expression of metastasis-associated protein 2 (MTA2) is required for transcriptional regulation of the follicle-stimulating hormone receptor (FSHR) gene during spermatogenesis[J]. J Biol Chem, 2012, 287(48): 40471–40483. DOI: 10.1074/jbc.M112.383802 |
| [35] | DU X, LI Q, PAN Z, et al. Androgen receptor and miRNA-126* axis controls follicle-stimulating hormone receptor expression in porcine ovarian granulosa cells[J]. Reproduction, 2016, 152(2): 161–169. DOI: 10.1530/REP-15-0517 |



