2. 内蒙古农业大学职业技术学院, 包头 014109
2. Vocational and Technical College of Inner Mongolia Agricultural University, Baotou 014109, China
Western blot是目前定量分析生物组织蛋白质表达最常用的方法之一,但试验步骤繁杂,致使人们投入巨大精力却常常遇到条带不整齐、结果不稳定且重复性差等问题[1-2]。通常,蛋白样本的提取质量及转膜电压、功率是该技术的关键[3-5]。
有试验表明,转膜液中十二烷基磺酸钠(SDS)含量会影响转膜电压及功率[6];同时,影响转膜液工作电压及功率的另一重要因素为溶液pH,当转膜液pH高于或低于蛋白等电点2个pH时才会发生蛋白转移,过高或过低都不能使得蛋白有效转移[7-8]。但均未给出相应量级变化和影响转膜电压及功率大小的趋势。对于低丰度表达的蛋白[7],如黑皮质素-4型受体(Melanocortin-4-receptor, MC4R)等,因其发挥抑制摄食和体重增加及促进机体能量消耗的功能,成为研究的新热点,但鉴定较为困难,除受转膜电压及功率的影响外[8],还受到蛋白样本提取本身质量及效率的影响[9],对于组织蛋白提取,目前有研究表明,生物化学试剂对于组织细胞本身的裂解、破膜、及蛋白释放的作用,强于机械性破碎,高效裂解液对于鉴定低丰度蛋白表达很重要[10]。同时,对于MC4R蛋白的表达,也仅有N. X. Cawry等和L.D. Faulkner等[11-12]有所报道,但并未得到有效重复。
基于转膜液中SDS的含量及溶液pH对转膜电压及功率的影响,本试验分别以0、0.3和1 g·L-1 SDS含量的转膜液于350、380及400 mA恒流湿转90 min,检测转膜液SDS含量对转膜电压及功率的影响,同时设定pH7.6、8.0、8.3和8.6的转膜液,以研究不同pH转膜液对蛋白转膜电压及功率的影响,并在获得相关数据的基础上比较提高裂解强度对MC4R表达的作用效果,以期有助于相关操作人员对具体细节的认识,并有助于鉴定低丰度表达蛋白。
1 材料与方法 1.1 材料6~8周龄C57/BL6小鼠购于北京维通利华实验动物有限公司。颈椎脱臼法处死小鼠,并获取背肩胛棕色脂肪组织(Brown adipose tissue, BAT)材料,生理盐水洗去血迹后,混合少量裂解液冻存于-80 ℃冰箱备用。
1.2 方法常规方法制作5%浓缩胶和13%分离胶,相关药品均购置于Sigma,凝胶置于电泳液4 ℃平衡过夜后备用。
基础裂解液为RIPA(康为世纪CW2333S),组织置于冰上,添加少量裂解液用电动研磨杵研磨,然后补齐至500 μL裂解液,冰上孵育30 min,每5 min旋涡30 s,然后以超声强度95 V,单次处理15 s,共做3次(QSONICA Q700)处理,再继续孵育10 min,12 000 r·min-1离心转移上清至新管,二次以超声强度95 V,单次处理15 s,做1次处理;12 000 r·min-1离心转移上清至新管,BCA工作液测定蛋白浓度,调整浓度至2.0 μg·μL-1,上样体积20 μL。
电泳装置(Bio-Rad电泳系统)于冰中分级恒压(80 V,28 min; 140 V,90 min)电泳后,首先,分别以0、0.3和1 g·L-1 SDS的转膜液(通用pH8.3),然后根据转膜液SDS结果,以pH7.6、8.0和8.6的转膜液于350、380及400 mA恒流湿转90 min,分别记录转膜电压,电转装置于冰中及磁力搅拌器均匀搅拌转膜。
所需液体配方参见Cell Signaling Technology Western Blotting Protocol (Fluorescent)。
选取效果较好的SDS含量和pH的转膜液进行后续抗体孵育试验比较不同裂解液的效果。转印后膜用5%脱脂奶粉(Nonfat dry milk)室温封闭1 h,TBST清洗3×10 min孵育一抗及相应二抗。先后孵育一抗顺序:Anti-MC4 Receptor antibody(Abcam, Rabbit, 1:500, ab24233),pERK(CST, Mouse, 1:1 000, 4370),GAPDH (Abcam, Rabbit, 1:10 000, ab181602) 4 ℃过夜孵育,TBST清洗3×10 min后孵育二抗,IR800(1:20 000,IRDye® 800 CW Goat anti-Rabbit, Odyssey)和IR680(1:20 000, IRDye® 680LT Goat anti-Mouse, Odyssey)室温孵育1 h,TBST清洗3×10 min,于LI-COR Odyssey CLX进行扫描。
1.3 统计分析试验数据利用GraphPad Prism 5.0统计程序进行单因子方差(One-way ANOVA)分析和Tukey′s Multiple Comparison test统计分析。数据以“平均值±标准误(Mean ± SEM)”表示。*为差异显著(P<0.05),**和***为差异极显著(P<0.01,P<0.001)。
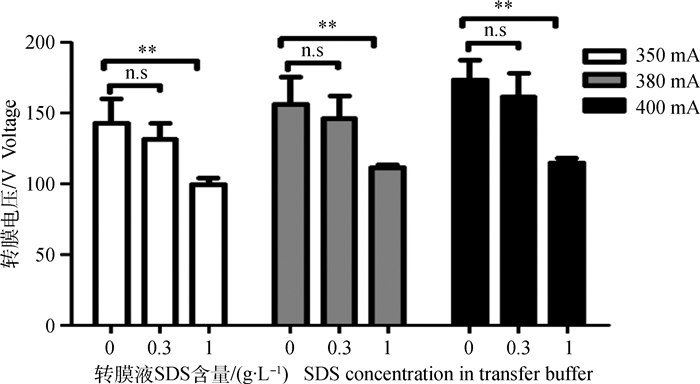
2 结果 2.1 不同SDS浓度转膜液对初始转膜电压的影响由图 1可知,350、380和400 mA恒流转膜过程中,0 g·L-1 SDS转膜液的初始转膜电压及功率极显著高于1 g·L-1 SDS转膜液(P<0.05),但0.3与0 g·L-1 SDS组间无显著性差异(n.s)。考虑到SDS影响蛋白与膜结合的关系,为保证转膜电压和蛋白转印效率(蛋白和膜的结合能力),选取0.3 g·L-1 SDS进行后续试验。
|
n.s.差异不显著。下同 n.s. No significant difference. The same as below 图 1 不同SDS浓度转膜液对初始转膜电压的影响 Figure 1 Effect of different concentration SDS of transfer buffer on initial voltage of electrotransfer |
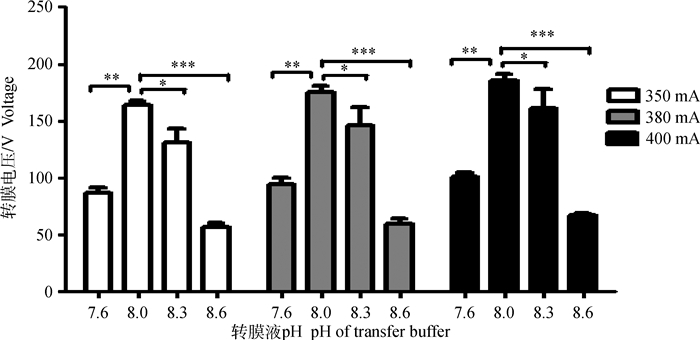
由图 2可知,pH为8.0时的初始转膜电压及功率显著高于pH 8.3组(P<0.05),极显著高于pH7.6和pH8.6的转膜液转膜电压(P<0.01,P<0.001),pH 8.6的转膜电压功率为pH8.0转膜电压功率的一半左右。恒流转膜时,随着时间延长,电压逐渐降低,转膜液的pH对初始转膜电压及功率有着积极的影响(图 2)。因此,选取pH8.0转膜液进行后续试验的抗体孵育效果比较。
|
图 2 不同pH转膜液对初始转膜电压的影响 Figure 2 Effect of different pH in transfer buffer on initial voltage of electrotransfer |
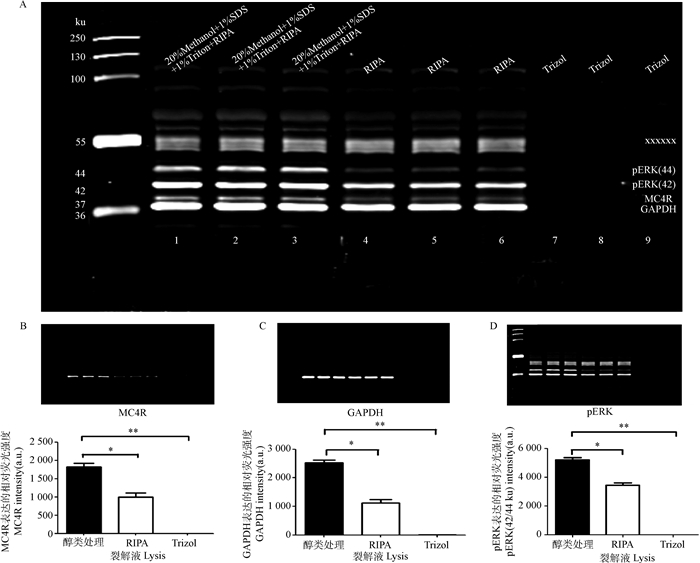
由于鉴定膜蛋白表达困难,在未知转膜液中SDS含量及pH的作用效果前,通常转膜效果时好时坏,稳定性差。在获得上述基础数据后,试验以0.3 g·L-1 SDS的转膜液(pH 8.0)、400 mA转印90 min为基础,进行蛋白提取裂解液的比较。由图 3可知,不同裂解液及处理方法对MC4R蛋白(37 ku)表达有很大影响,裂解液混合醇类的组合即(20%Methanol+1%Triton+1%SDS+RIPA)处理组显著优于单独RIPA的处理组(P<0.05),极显著优于Trizol处理组(P<0.01),且该处理组几近没有阳性条带。内参GAPDH和细胞信号通路蛋白pERK均呈现类似趋势。提示含有醇类物混合裂解液有利于脂肪蛋白的提取。图中55 ku附近为脂肪组织在鼠源荧光信号下特定的非特异性杂带。
|
A. MC4R、GAPDH、pERK结果:×××××××为非特异性杂带;B. MC4R;C. GAPDH; D.pERK A. Expression of MC4R, GAPDH, pERK: ××××××× is the non-specific bands; B. MC4R; C.GAPDH; D. pERK 图 3 不同裂解液处理组MC4R、pERK、GAPDH的表达差异 Figure 3 Expression difference of MC4R, pERK, GAPDH treated with different lysis |
作为Western blot技术的关键影响因素,转膜电压导致的转膜功率很大程度影响着最终试验结果的重复性和稳定性[13-14]。转膜液中SDS含量可影响蛋白电转的效率,同时影响蛋白和膜的结合能力,不恰当的SDS含量既不能达到提高转膜效率的目的,而且还会影响最终结合到膜上的有效蛋白,进而影响最后的结果[13]。
本试验结果表明,恒流转膜时,转膜液中SDS含量与蛋白质的转膜电压及功率呈反比,即转膜液中SDS含量越高,转膜电压及功率越低。SDS在转膜液中的含量为0.3 g·L-1时,即最终的工作浓度为0.03%,既能有效的增高转膜的电压及功率,又不影响最终蛋白和膜的结合,有效保证膜上有效蛋白的含量。
研究表明,蛋白有效转移的发生是基于蛋白本身等电点和转移缓冲液之间的pH差,只有当缓冲液pH高于或低于蛋白本身等电点时才能有效转移[3, 8, 15]。转膜液pH对转膜电压及功率的影响与SDS含量对电压的影响极为相似,需维持在一个稳定合适的范围[3, 8],本试验中,无论是pH为偏酸性的7.6,亦或是偏碱性8.6的转膜液,转膜电压及功率都明显过低,同样影响了最终转膜的效率及膜上蛋白的多少,而pH8.0的溶液,与上述两类pH的溶液相比较,转膜效率明显增高。提示,转膜液的pH应该稳定在以8.0为基准的区间内,从而保证转膜的效率。对于较大分子质量的蛋白质,也可通过适当延长转膜时间增加电流强度来提高转膜效率。
MC4R具有7次跨膜结构,为G-蛋白耦联受体家族成员,是一类调节能量动态平衡的重要信号分子,广泛存在于动物机体中[16-18],通常表达丰度较低。加之现有商品化抗体效价不佳,特异性较差[19-21],其提取方式和效率的差异[22-23]最终会影响检测的灵敏性和准确性,通用蛋白裂解液仅对丰度较高、抗体特异性强的蛋白的提取效果较好,但对于像MC4R这样低丰度表达的蛋白而言,已知商品化的裂解液并不能完全达到预期效果[24-26],而现有结果表明,裂解液成分及处理方法对鉴定MC4R表达的影响有着重要作用[27-28],对于BAT组织MC4R表达而言,其中醇类物20%Methanol+1%Triton+1%SDS+RIPA处理组与RIPA及Trizol的处理组相比效果极为显著,推测,针对脂肪组织中像MC4R类似低丰度表达的蛋白提取而言,含有醇类物的混合裂解液更为适合。高丰度表达蛋白pERK和GAPDH的表达趋势也支持这种推断。
综上,转膜液SDS含量及溶液本身pH的初步确定,配合混合增强裂解液处理BAT组织的方法,为鉴定低丰度蛋白表达提供了一种选择,有效提高了该技术的灵敏性与稳定性,但必须看到,该方法并不一定适用于众多低丰度表达蛋白,鉴定特异组织、特异蛋白时仍需摸索有效步骤。
4 结论本试验探明了转膜液中SDS含量与蛋白质的转膜电压及功率呈反比的关系,并确定了工作浓度为0.03%。对于BAT组织中MC4R的表达而言,醇类物20%Methanol+1%Triton+1%SDS+RIPA处理效果最优,并提示对脂肪组织中像MC4R类似低丰度表达蛋白的抽提使用醇类物混合裂解液更为合适。上述结果有助于相关操作人员对主要细节的认识,有助于理解Western blot鉴定脂肪组织低丰度蛋白试验的灵敏及重复性。
| [1] | JIANG Y, HOU X X, GENG Z, et al. Interpretation criteria for standardized Western blot for the predominant species of Borrelia burgdorferi sensu lato in China[J]. Biomed Environ Sci, 2010, 23(5): 341–349. DOI: 10.1016/S0895-3988(10)60074-8 |
| [2] |
董燕, 张枫, 梅柱中, 等. 电转移中蛋白质的透膜现象及其对蛋白质印迹结果的影响[J]. 生物化学与生物物理进展, 2002, 29(3): 449–453.
DONG Y, ZHANG F, MEI Z Z, et al. Protein blowthrough in electrophoretic transfer and its effects on Western blotting[J]. Progress in Biochemistry and Biophysics, 2002, 29(3): 449–453. (in Chinese) |
| [3] | DEGASPERI A, BIRTWISTLE M R, VOLINSKY N, et al. Evaluating strategies to normalise biological replicates of Western blot data[J]. PLoS One, 2014, 9(1): e87293. DOI: 10.1371/journal.pone.0087293 |
| [4] | EATON S L, ROCHE S L, HURTADO M L, et al. Total protein analysis as a reliable loading control for quantitative fluorescent Western blotting[J]. PLoS One, 2013, 8(8): e72457. DOI: 10.1371/journal.pone.0072457 |
| [5] | GHOSH R, GILDA J E, GOMES A V. The necessity of and strategies for improving confidence in the accuracy of Western blots[J]. Exp Rev Proteomics, 2014, 11(5): 549–560. DOI: 10.1586/14789450.2014.939635 |
| [6] | HEDA G D, OMOTOLA O B, HEDA R P, et al. Effects of reusing gel electrophoresis and electrotransfer buffers on Western blotting[J]. J Biomol Tech, 2016, 27(3): 113–118. |
| [7] |
谢岚, 艾华. Western blot方法中不同电流强度对较大分子蛋白质转膜效率的影响[J]. 国际检验医学杂志, 2012, 33(1): 42–44.
XIE L, AI H. Effects of different current densities on efficiency of electrotransfer for the large-molecular-weight protein in Western blot assay[J]. International Journal of Laboratory Medicine, 2012, 33(1): 42–44. (in Chinese) |
| [8] | PENNA A, CANHALAN M. Western blotting using the Invitrogen NuPage Novex Bis Tris MiniGels[J]. J Vis Exp, 2007(7): 264. |
| [9] | ABEYRATHNE P D, LAM J S. Conditions that allow for effective transfer of membrane proteins onto nitrocellulose membrane in Western blots[J]. Can J Microbiol, 2007, 53(4): 526–532. DOI: 10.1139/W07-007 |
| [10] | COLLINS M A, AN J Y, PELLER D, et al. Total protein is an effective loading control for cerebrospinal fluid Western blots[J]. J Neurosci Methods, 2015, 251: 72–82. DOI: 10.1016/j.jneumeth.2015.05.011 |
| [11] | CAWRY N X, YANIK T, WORONOWICA A, et al. Obese carboxypeptidase E knockout mice exhibit multiple defects in peptide hormone processing contributing to low bone mineral density[J]. Am J Physiol Endocrinol Metab, 2010, 299(2): E189–E197. DOI: 10.1152/ajpendo.00516.2009 |
| [12] | FAULKNER L D, DOWLING A R, STUART R C, et al. Reduced melanocortin production causes sexual dysfunction in male mice with POMC neuronal insulin and leptin insensitivity[J]. Endocrinology, 2015, 156(4): 1372–1385. DOI: 10.1210/en.2014-1788 |
| [13] | KURIEN B T, SCOFIELD R H. Western blotting of high and low molecular weight proteins using heat[M]//KURIENR B T, SCOFIELD R H. Western Blotting: Methods and Protocols. New York, NY: Humana Press, 2015: 247-255. |
| [14] | JIN S, FURTAW M D, CHEN H X, et al. Multiplexed Western blotting using microchip electrophoresis[J]. Anal Chem, 2016, 88(13): 6703–6710. DOI: 10.1021/acs.analchem.6b00705 |
| [15] | GARCÍA-BEA A, WALKER M A, HYDE T M, et al. Metabotropic glutamate receptor 3 (mGlu3; mGluR3; GRM3) in schizophrenia: Antibody characterisation and a semi-quantitative Western blot study[J]. Schizophr Res, 2016, 177(1-3): 18–27. DOI: 10.1016/j.schres.2016.04.015 |
| [16] | MORGAN D A, MCDANIEL L N, YIN T, et al. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus[J]. Diabetes, 2015, 64(6): 1976–1987. DOI: 10.2337/db14-1257 |
| [17] |
黄萌, 贤明, 史明艳, 等. MC4R基因沉默对牛成纤维细胞内基因表达的影响[J]. 畜牧兽医学报, 2017, 48(3): 403–415.
HAUNG M, XIAN M, SHI M Y, et al. Effects of MC4R gene silencing on gene expression in bovine fibroblast cells[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(3): 403–415. (in Chinese) |
| [18] |
张菊, 杜立新, 李宏滨, 等. 绵羊MC4R基因的半定量RT-PCR及生物信息学分析[J]. 畜牧兽医学报, 2010, 41(7): 804–810.
ZHANG J, DU L X, LI H B, et al. Semi-quantitative RT-PCR and bioinformatics analysis of sheep MC4R gene[J]. Acta Veterinaria et Zootechnica Sinica, 2010, 41(7): 804–810. (in Chinese) |
| [19] | TREINDL F, RUPRECHT B, BEITER Y, et al. A bead-based western for high-throughput cellular signal transduction analyses[J]. Nat Commun, 2016, 7: 12852. DOI: 10.1038/ncomms12852 |
| [20] | HEMMATZADEH F, KAZEMIMANESH M. Detection of specific antigens of Newcastle disease virus using an absorbed Western blotting method[J]. Iran J Vet Res, 2017, 18(2): 92–96. |
| [21] | GILDA J E, GHOSH R, CHEAH J X, et al. Western blotting inaccuracies with unverified antibodies: need for a Western blotting minimal reporting standard (WBMRS)[J]. PLoS One, 2015, 10(8): e0135392. DOI: 10.1371/journal.pone.0135392 |
| [22] | LEIMGRUBER R M, MALONE J P, RADABAUGH M R, et al. Development of improved cell lysis, solubilization and imaging approaches for proteomic analyses[J]. Proteomics, 2002, 2(2): 135–144. DOI: 10.1002/(ISSN)1615-9861 |
| [23] | BURGHI V, FERNÁNDEZ N C, GÁNDOLA Y B, et al. Validation of commercial mas receptor antibodies for utilization in Western blotting, immunofluorescence and immunohistochemistry studies[J]. PLoS One, 2017, 12(8): e0183278. DOI: 10.1371/journal.pone.0183278 |
| [24] | SINKALA E, SOLLIER-CHRISTEN E, RENIER C, et al. Profiling protein expression in circulating tumour cells using microfluidic Western blotting[J]. Nat Commun, 2017, 8: 14622. DOI: 10.1038/ncomms14622 |
| [25] | OKITA N, HIGAMI Y, FUKAI F, et al. Modified Western blotting for insulin and other diabetes-associated peptide hormones[J]. Sci Rep, 2017, 7: 6949. DOI: 10.1038/s41598-017-04456-4 |
| [26] | MERPHY R M, LAMB G D. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes[J]. J Physiol, 2013, 591(23): 5823–5831. DOI: 10.1113/jphysiol.2013.263251 |
| [27] | HEDA G D, OMOTOLA O B, HEDA R P, et al. Effects of reusing gel electrophoresis and electrotransfer buffers on Western blotting[J]. J Biomol Tech, 2016, 27(3): 113–118. |
| [28] | MARANGONI A, FOSCHI C, CAPRETTI M G, et al. Contribution of a comparative Western blot method to early postnatal diagnosis of congenital syphilis[J]. Clin Vaccine Immunol, 2016, 23(5): 410–416. DOI: 10.1128/CVI.00032-16 |



