2. 太原市动物疫病预防控制中心, 太原 030024
2. Taiyuan Center for Disease Control and Prevention, Taiyuan 030024, China
肉鸡TD是一种家禽胫骨近端生长板成骨受阻的疾病[1]。TD可导致肉鸡骨变形[2],该病严重危害了肉鸡养殖业,导致TD的因素很多,其致病机制还不清楚,也没有有效的预防措施。福美双是一类二硫代氨基甲酸酯相关化合物,不但被广泛的用作种子处理农用杀菌剂,而且在橡胶工业中被较小范围的用作促进剂[3]。鸡慢性接触二硫代氨基甲酸酯杀虫剂,例如福美双或戒酒硫,会增加TD发病率[4-5]。福美双能有效地诱发肉鸡TD,并且与自然发生的肉鸡TD在症状上十分相似[6]。谷胱甘肽S-转移酶(glutathione S-transferase, GSTs)是Ⅱ相抗氧化家族中的一类蛋白酶,且有解毒的作用[7]。相关研究表明,GSTA3在保护正常的小鼠抵抗黄曲霉毒素毒性中起重要作用[8]。患有TD的肉鸡生长板的很多软骨细胞发生凋亡[9-10]。受到刺激的TLRs能够通过引发促凋亡信号通路而诱导凋亡[11-13]。相关研究表明,鸡红细胞不但具有免疫功能而且可以表达TLR2、TLR3、TLR4、TLR5、TLR7[14]。田文霞(W.X.Tian)等利用微阵列芯片技术筛选出TD早期的肉鸡胫骨生长板软骨细胞内差异性表达的免疫相关基因[15]。先前人们主要从生长板血管的生成、生长板细胞的代谢、成熟和凋亡方面研究了TD的发生机制[16-18],但是红细胞免疫功能和chGSTA3蛋白解毒作用对TD发生的影响尚未见报道。为研究福美双诱导的TD肉鸡中红细胞免疫基因的转录变化和重组chGSTA3对TD肉鸡红细胞免疫基因mRNA水平的影响,我们对福美双处理组肉鸡饲喂100 mg·kg-1福美双建立TD模型,并在不同的时间利用chGSTA3对TD肉鸡进行腿部肌肉注射,使用Real-time PCR检测TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、IL-7、MyD88、TRAF6、MHCⅡ和NLRC5转录水平的变化,以探明其在肉鸡TD中的作用以及chGSTA3在TD发生时对它们的影响,为更深入全面的了解TD的发病机制和预防TD的发生提供新的科学理论依据。
1 材料与方法 1.1 实验动物1日龄120只健康艾维茵肉雏鸡购自山西省大象农牧集团有限公司。
1.2 实验试剂福美双(Amresco);实验室自行制备的重组鸡GSTA3纯化蛋白[19];RNAiso plus(Trizol)(AA909-1);prime script TM RT Reagent Kit(AK3020);SYBR® Premix Ex Taq Ⅱ(TaKaRa大连宝生物工程有限公司);DEPC购于Amresco公司;6× DNA Loading Buffer,600 bp DNA ladder(中科瑞泰生物科技有限公司);50× TAE Buffer(北京博奥拓达科技有限公司);2 mL、1.5 mL离心管(Axygen)。
1.3 试验方法 1.3.1 动物处理将饲喂一周后的120只肉雏鸡随机分为基础日粮组(A、B、C组)和饲喂福美双组(D、E、F组)。参考田文霞等[20-21]试验设计方案构建肉鸡TD模型;通过肌肉注射,在肉鸡第8、10、12、14日龄时,分别给B、E组肉鸡腿部注射浓度为20 μg·kg-1的重组chGSTA3蛋白;给C、F组肉鸡腿部注射50 μg·kg-1的重组chGSTA3蛋白;给A、D组肉鸡注射等体积的PBS,对试验鸡进行常规的饲养管理。在饲喂福美双后第4和15天,对各组鸡每只采集静脉抗凝血2 mL,用于后续试验。
1.3.2 鸡红细胞分离和红细胞RNA提取及质量检测参照相关文献从上述血液样品中分离红细胞[14],使用本实验室改进的Trizol法提取红细胞总RNA,用核酸测定仪测定提取的RNA的浓度、A260/280、A260/230的值,并用1.5%琼脂糖凝胶电泳检测所提取的红细胞RNA的质量。对质量合格的RNA反转录,用于后续试验。
1.3.3 引物的设计与合成根据NCBI收录的免疫相关的基因IL-7、TLR2、TLR3、TLR5、TLR7、TLR15、NLRC5的mRNA序列,使用Primer Premier 5.0软件设计其引物,参考相关文献[22-24]得到TLR4、MyD88、MHCⅡ、TRAF6基因的引物序列,利用18S rRNA作为内参基因。由上海捷瑞生物工程有限公司合成本试验所用引物(表 1)。
|
|
表 1 Real-time PCR扩增所使用的引物序列及GenBank登录号 Table 1 Primer sequences and GenBank accession numbers used in the Real-time PCR analysis |
根据SYBR® Premix Ex TaqTM Ⅱ试剂盒说明书要求进行Real-time PCR反应,采用10 μL的反应体系,体系组成:0.25 μL的上游和下游引物(10 μmol·L-1),5.0 μL SYBR Premix Ex Taq Ⅱ(2×),0.1 μL ROX Refe-rence DyeⅡ(50×),1.0 μL RT反应液,3.4 μL单蒸水(dH2O);Real-time PCR条件:预变性反应1个循环,95 ℃ 3 min,PCR反应42个循环,95 ℃ 30 s,55 ℃ 30 s;熔解曲线分析1个循环,55 ℃ 30 s,95 ℃ 30 s。
1.3.5 数据分析用2-△△Ct法计算免疫相关基因在鸡红细胞中的相对转录量,使用SPSS Statistics 17.0统计分析软件分析各组数据的差异显著性,P<0.05即为差异表达显著,P>0.05表达不显著;各组间显著差异标注不同英文小写字母。
2 结果 2.1 IL-7、TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MyD88、MHCⅡ、NLRC5和TRAF6在肉鸡红细胞中的转录试验结果表明正常肉鸡红细胞能够在mRNA水平上表达IL-7、TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MyD88、MHCⅡ、NLRC5和TRAF6,其中TLR7表达量最高,其次是IL-7、TLR5、TLR4、TLR2、TLR3、TLR15、MyD88,而MHCⅡ、NLRC5、TRAF6表达量较低,见图 1。
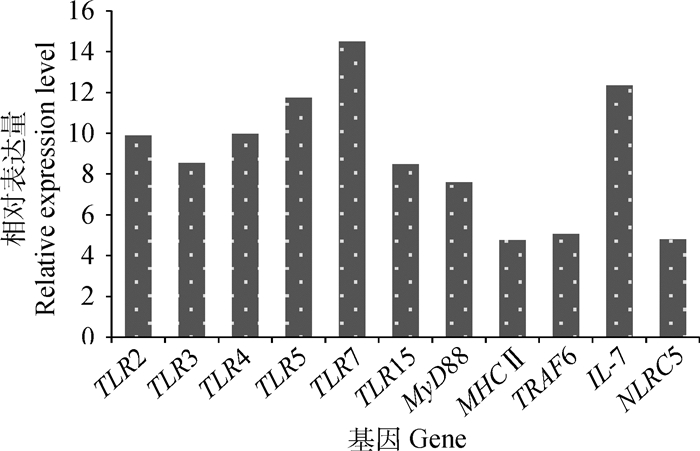
|
图 1 在正常肉鸡红细胞中TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MyD88、MHCⅡ、TRAF6、IL-7、NLRC5相对于内参基因18S的mRNA表达量 Figure 1 The mRNA expression level of TLR2, TLR3, TLR4, TLR5, TLR7, TLR15, MyD88, MHCⅡ, TRAF6, IL-7, NLRC5 in erythrocytes of healthy broiler chickens relative to reference gene 18S |
通过Real-time PCR对TD肉鸡红细胞免疫相关基因的转录水平进行检测,发现在TD损伤期的第4天,D组与A组相比,鸡红细胞中的TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MyD88、MHCⅡ、NLRC5、TRAF6基因转录水平显著上调,IL-7显著降低(P < 0.05);在饲喂福美双后第15天,D组TD肉鸡红细胞中TLR2、TLR4、MyD88和NLRC5显著下调(P < 0.05),IL-7、TLR3、TLR5、TLR7、TLR15、MHCⅡ和TRAF6变化不显著(P>0.05)。chGSTA3处理的E组和F组与D组相比,在试验第4天,除了E组中的TLR4、TLR5、MyD88,其余基因都发生了显著变化(P < 0.05),在第15天E组中的TLR4、TLR5、MyD88、NLRC5及F组中的TLR3、TLR5、MyD88、TRAF6、IL-7变化不显著,其余基因都有显著差异(P < 0.05),见图 2。
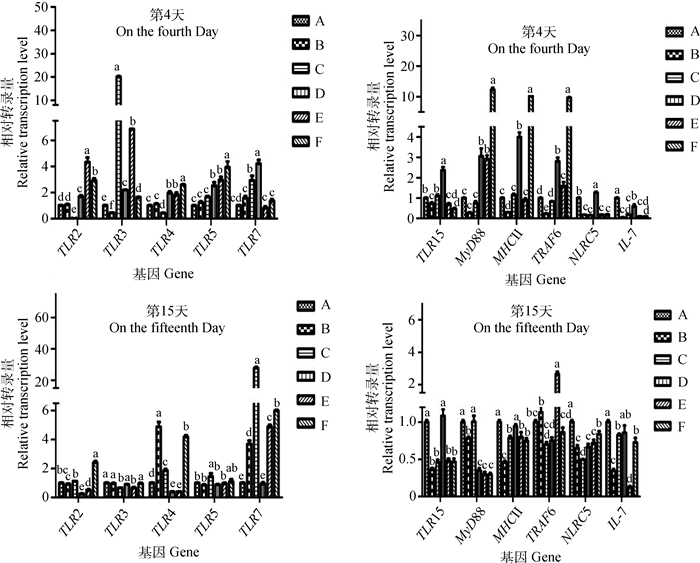
|
A. PBS组;B. 20 μg·kg-1 chGSTA3组;C. 50 μg·kg-1 chGSTA3组;D. 100 mg·kg-1福美双+PBS组;E. 100 mg·kg-1福美双和20 μg·kg-1 chGSTA3组;F. 100 mg·kg-1福美双+50 μg·kg-1 chGSTA3组;不同的小写字母(例如a、b、c、d、e、f)表示各组间差异显著(P < 0.05) A. PBS group; B. chGSTA3 20 μg·kg-1 group; C. chGSTA3 50 μg·kg-1 group; D. Thiram 100 mg·kg-1+PBS group; E. Thiram 100 mg·kg-1 + chGSTA3 20 μg·kg-1 group; F. Thiram 100 mg·kg-1 + chGSTA3 50 μg·kg-1 group; Different lower-case letters (such as a, b, c, d, e, f) indicate the significant difference between groups (P < 0.05) 图 2 TD肉鸡红细胞中免疫相关基因的转录变化及chGSTA3对其的影响 Figure 2 The transcription level of immune related genes in erythrocytes of TD affected-broiler chickens and the effection of recombinant chGSTA3 on the transcription |
田文霞等研究发现,肉鸡TD的发生可能与福美双影响GSTs的解毒功能有关[25-26],同时利用cDNA芯片技术筛选出1 630个差异表达基因,经生物信息学分析,这些基因与免疫应答、抗凋亡和氧化应激等都有关[15]。本试验对肉鸡TD红细胞中免疫基因的转录水平进行检测,发现注射重组chGSTA3蛋白后,IL-7、TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MHCⅡ、MyD88、NLRC5、TRAF6有差异,表明chGSTA3在福美双诱导的肉鸡TD中发挥一定的作用,但其作用的分子机制有待进一步研究。研究表明,福美双能改变神经元样PC12细胞的钙稳态,从而使其凋亡[27];N. C. Rath等[16]和张宁等[17]通过TUNEL法检测技术发现TD肉鸡胫骨生长板内许多软骨细胞和血管内皮细胞发生凋亡,最终导致TD发生。
有研究发现,机体细胞中的TLR3基因被激活后,通过信号转导激活核因子κB(NF-κB),使有生物活性的细胞因子和化学介质被大量生成和释放,导致干扰素等蛋白的活化,进而使感染病毒的细胞发生凋亡[28],而卡介苗能通过活化TLR7而引起浅表膀胱癌细胞的凋亡[29];鸡红细胞也可能通过上调IFNs的表达,促进病毒感染的细胞凋亡[14]。本试验结果显示,在试验第4天,饲喂福美双D组TLR3和TLR7显著上调,可能使软骨细胞凋亡,而诱导TD发生[17]。
研究表明,鞭毛蛋白诱导活化的TLR5也能导致足细胞凋亡[30],银纳米颗粒通过TLR2信号转导通路也会引起软骨细胞的凋亡[31],活化的TLR4-MyD88信号通路也参与了血管紧张素Ⅱ诱导的肾小球系膜细胞的凋亡过程[32]。本试验结果显示,在试验第4天D组TLR2、TLR4和TLR5与A组相比显著上调,可能通过一系列信号转导,激活caspases信号通路,最终导致软骨细胞凋亡。
白细胞介素7能通过使促凋亡蛋白Bad失活而促进T细胞的存活[33]。本研究显示,在饲喂福美双后第4天,IL-7基因显著下调,可能在TD发生中也有促进软骨细胞凋亡的作用。
在试验第15天,D组与对照组A相比,IL-7、MHCⅡ、TRAF6、TLR3、TLR5、TLR7、TLR15不显著,MyD88、NLRC5、TLR2、TLR4下调,这可能与减轻软骨损伤有关。
4 结论鸡红细胞能转录IL-7、TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MHCⅡ、MyD88、NLRC5、TRAF6;鸡红细胞TLR2、TLR3、TLR4、TLR5、TLR7、TLR15、MHCⅡ、MyD88、NLRC5、TRAF6基因mRNA水平升高,IL-7降低后,可能通过一系列信号转导,促进软骨细胞凋亡,而在后期可减轻TD症状;chGSTA3可改变红细胞免疫相关基因的转录,这将为预防TD和深入研究其发病机制提供理论支持。
| [1] | RASAPUTRA K S, LIYANAGE R, LAY J O Jr, et al. Tibial dyschondroplasia-associated proteomic changes in chicken growth plate cartilage[J]. Avian Dis, 2010, 54(4): 1166–1171. DOI: 10.1637/9384-050110-Reg.1 |
| [2] | LYNCH M, THORP B H, WHITEHEAD C C. Avian tibial dyschondroplasia as a cause of bone deformity[J]. Avian Pathol, 1992, 21(2): 275–285. DOI: 10.1080/03079459208418842 |
| [3] | CERESER C, BOGET S, PARVAZ P, et al. Thiram-induced cytotoxicity is accompanied by a rapid and drastic oxidation of reduced glutathione with consecutive lipid peroxidation and cell death[J]. Toxicology, 2001, 163(2-3): 153–162. DOI: 10.1016/S0300-483X(01)00401-2 |
| [4] | VARGAS M I, LAMAS J M, ALVARENGA V. Tibial dyschondroplasia in growing chickens experimentally intoxicated with tetramethylthiuram disulfide[J]. Poult Sci, 1983, 62(7): 1195–1200. DOI: 10.3382/ps.0621195 |
| [5] | EDWARDS H M Jr. Efficacy of several vitamin D compounds in the prevention of tibial dyschondroplasia in broiler chickens[J]. J Nutr, 1990, 120(9): 1054–1061. DOI: 10.1093/jn/120.9.1054 |
| [6] | RATH N C, HUFF W E, BALOG J M, et al. Comparative efficacy of different dithiocarbamates to induce tibial dyschondroplasia in poultry[J]. Poult Sci, 2004, 83(2): 266–274. DOI: 10.1093/ps/83.2.266 |
| [7] | SHEEHAN D, MEADE G, FOLEY V M, et al. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily[J]. Biochem J, 2001, 360(1): 1–16. |
| [8] | ILIC Z, CRAWFORD D, EGNER P A, et al. Glutathione-S-transferase A3 knockout mice are sensitive to acute cytotoxic and genotoxic effects of aflatoxin B1[J]. Toxicol Appl Pharmacol, 2010, 242(3): 241–246. DOI: 10.1016/j.taap.2009.10.008 |
| [9] | RATH N C, BAYYARI G R, BALOG J M, et al. Physiological studies of turkey tibial dyschondroplasia[J]. Poult Sci, 1994, 73(3): 416–424. DOI: 10.3382/ps.0730416 |
| [10] | RATH N C, HUFF W E, BAYYARI G R, et al. Cell death in avian tibial dyschondroplasia[J]. Avian Dis, 1998, 42(1): 72–79. DOI: 10.2307/1592578 |
| [11] | LÓPEZ M, SLY L M, LUU Y, et al. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2[J]. J Immunol, 2003, 170(5): 2409–2416. DOI: 10.4049/jimmunol.170.5.2409 |
| [12] | ALIPRANTIS A O, YANG R B, WEISS D S, et al. The apoptotic signaling pathway activated by Toll-like receptor-2[J]. EMBO J, 2000, 19(13): 3325–3336. DOI: 10.1093/emboj/19.13.3325 |
| [13] | INTO T, KIURA K, YASUDA M, et al. Stimulation of human Toll-like receptor (TLR) 2 and TLR6 with membrane lipoproteins of Mycoplasma fermentans induces apoptotic cell death after NF-κB activation[J]. Cell Microbiol, 2004, 6(2): 187–199. DOI: 10.1046/j.1462-5822.2003.00356.x |
| [14] | ST PAUL M, PAOLUCCI S, BARJESTEH N, et al. Chicken erythrocytes respond to Toll-like receptor ligands by up-regulating cytokine transcripts[J]. Res Vet Sci, 2013, 95(1): 87–91. DOI: 10.1016/j.rvsc.2013.01.024 |
| [15] | TIAN W X, LI J K, QIN P, et al. Screening of differentially expressed genes in the growth plate of broiler chickens with tibial dyschondroplasia by microarray analysis[J]. BMC Genomics, 2013, 14: 276. DOI: 10.1186/1471-2164-14-276 |
| [16] | RATH N C, RICHARDS M P, HUFF W E, et al. Changes in the tibial growth plates of chickens with thiram-induced dyschondroplasia[J]. J Comp Pathol, 2005, 133(1): 41–52. DOI: 10.1016/j.jcpa.2005.01.005 |
| [17] |
张宁, 李欣, 宁官保, 等. 福美双诱导的肉鸡胫骨软骨发育不良软骨细胞凋亡[J]. 畜牧兽医学报, 2017, 48(12): 2421–2428.
ZHANG N, LI X, NING G B, et al. Apoptosis of chondrocytes in tibial dyschondroplasia induced by thiram in broilers[J]. Acta Veterinaria et Zootechnica Sinica, 2017, 48(12): 2421–2428. (in Chinese) |
| [18] | RATH N C, HUFF W E, HUFF G R. Thiram-induced changes in the expression of genes relating to vascularization and tibial dyschondroplasia[J]. Poult Sci, 2007, 86(11): 2390–2395. DOI: 10.3382/ps.2007-00219 |
| [19] |
卢晓晓. 肉鸡谷胱甘肽S-转移酶A3基因的克隆及蛋白的表达和纯化[D]. 太谷: 山西农业大学, 2016.
LU X X. Cloning, expression and purification of broilers Glutathione S-Transferase A3[D]. Taigu: Shanxi Agricultural University, 2016. (in Chinese) |
| [20] |
田文霞, 李家奎, 王瑞, 等. 四甲基秋兰姆二硫化物对肉鸡胫骨生长板超微结构的影响[J]. 畜牧兽医学报, 2013, 44(6): 965–971.
TIAN W X, LI J K, WANG R, et al. The ultrastructure changes of tibia growth plate in broiler chickens with a thiram-feeding procedure[J]. Acta Veterinaria et Zootechnica Sinica, 2013, 44(6): 965–971. DOI: 10.11843/j.issn.0366-6964.2013.06.020 (in Chinese) |
| [21] |
田文霞, 李家奎, 毕丁仁, 等. 福美双对肉鸡生长性能和胫骨软骨发育不良组织病理学变化的影响[J]. 畜牧兽医学报, 2008, 39(6): 733–738.
TIAN W X, LI J K, BI D R, et al. Effect of Thiram on Growth Performance and Histopathologic Changes of tibial dyschondroplasia in Broiler[J]. Acta Veterinaria et Zootechnica Sinica, 2008, 39(6): 733–738. (in Chinese) |
| [22] | XU M, ZHANG H M, LEE L, et al. Gene expression profiling in rMd5-and rMd5Δmeq-infected chickens[J]. Avian Dis, 2011, 55(3): 358–367. DOI: 10.1637/9608-120610-Reg.1 |
| [23] | KOGUT M H, IQBAL M, HE H Q, et al. Expression and function of Toll-like receptors in chicken heterophils[J]. Dev Comp Immunol, 2005, 29(9): 791–807. DOI: 10.1016/j.dci.2005.02.002 |
| [24] | JIE H, LIAN L, QU L J, et al. Differential expression of Toll-like receptor genes in lymphoid tissues between Marek's disease virus-infected and noninfected chickens[J]. Poult Sci, 2013, 92(3): 645–654. DOI: 10.3382/ps.2012-02747 |
| [25] |
宁官保, 田文霞, 王瑞, 等. 福美双诱发肉鸡胫骨软骨发育不良早期生长板消减cDNA文库的构建[J]. 中国农业科学, 2011, 44(4): 829–834.
NING G B, TIAN W X, WANG R, et al. Construction of subtracted cDNA library of the early growth plate in broiler chickens with thiram-induced tibial dyschondroplasia[J]. Scientia Agricultura Sinica, 2011, 44(4): 829–834. (in Chinese) |
| [26] |
田文霞, 刘红霞, 喻进, 等. cDNA芯片筛选肉鸡胫骨软骨发育不良相关基因[J]. 中国畜牧杂志, 2014, 50(17): 17–20.
TIAN W X, LIU H X, YU J, et al. Screening of associated genes in the growth plate of broiler chickens with tibial dyschondroplasia by cDNA microarrays[J]. Chinese Journal of Animal Science, 2014, 50(17): 17–20. DOI: 10.3969/j.issn.0258-7033.2014.17.005 (in Chinese) |
| [27] | SOOK HAN M, SHIN K J, KIM Y H, et al. Thiram and ziram stimulate non-selective cation channel and induce apoptosis in PC12 cells[J]. Neurotoxicology, 2003, 24(3): 425–434. DOI: 10.1016/S0161-813X(03)00013-5 |
| [28] | KALAI M, VAN LOO G, VANDEN BERGHE T, et al. Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA[J]. Cell Death Differ, 2002, 9(9): 981–994. DOI: 10.1038/sj.cdd.4401051 |
| [29] | YU D S, WU C L, PING S Y, et al. Bacille Calmette-Guerin can induce cellular apoptosis of urothelial cancer directly through toll-like receptor 7 activation[J]. Kaohsiung J Med Sci, 2015, 31(8): 391–397. DOI: 10.1016/j.kjms.2015.05.005 |
| [30] | LIN X, HUANG H T, YOU Y W, et al. Activation of TLR5 induces podocyte apoptosis[J]. Cell Biochem Funct, 2016, 34(2): 63–68. DOI: 10.1002/cbf.3165 |
| [31] | KIM A S, CHAE C H, KIM J, et al. Silver nanoparticles induce apoptosis through the Toll-like receptor 2 pathway[J]. Oral Surg Oral Med Oral Pathol Oral Radiol, 2012, 113(6): 789–798. DOI: 10.1016/j.oooo.2012.01.019 |
| [32] | LV J L, JIA R H, YANG D P, et al. Candesartan attenuates Angiotensin Ⅱ-induced mesangial cell apoptosis via TLR4/MyD88 pathway[J]. Biochem Biophys Res Commun, 2009, 380(1): 81–86. DOI: 10.1016/j.bbrc.2009.01.035 |
| [33] | LI W Q, JIANG Q, KHALED A R, et al. Interleukin-7 inactivates the pro-apoptotic protein bad promoting T cell survival[J]. J Biol Chem, 2004, 279(28): 29160–29166. DOI: 10.1074/jbc.M401656200 |



