2. 北京市畜牧总站, 北京 102200
2. Beijing General Station of Animal Husbandry Service, Beijing 102200, China
精子发生是一种复杂的细胞分化过程,包括雄性生殖细胞的一系列分子和形态学变化,且变化过程中也有许多蛋白的参与。一些参与精子发生的关键蛋白功能缺失会引起精子活力低下,甚至导致无精子症[1-2]。其中SOX蛋白的编码基因位于雄性动物Y染色体上,在个体发育过程中,广泛参与动物早期胚胎发育[3]、性别决定和分化[4]及精子生成[5]等过程,具有重要的生物学功能。SOX蛋白由具有HMG-盒(High mobility group box,HMG-box)保守区域构成的蛋白所组成,在很多种动物的组织中均有表达。SOX家族是一种重要的转录调控因子,其保守的HMG-box能够结合特定的DNA序列。根据HMG-box盒的同源程度可将SOX分为A、B、C、D、E、F、G、H、I和J 10个不同亚族[5]。
SOX5作为SOXD亚族的重要成员[5],广泛存在于各种动物组织中,哺乳动物中包含长链(L-SOX5,6 kb)和短链(S-SOX5,2 kb)两个亚型。L-SOX5在心、肝、肌肉和脂肪等组织中存在[6-7]。S-SOX5则主要存在于睾丸等含有鞭毛细胞的组织中[8]。S-SOX5在睾丸减数分裂后的生殖细胞中表达,尤其是在精细胞阶段[8]。在家禽中,SOX5基因位于1号染色体上,含有19个外显子,序列变异会引起鸡冠形态变化[9],但SOX5是否与精子活力相关,目前尚未见报道。
本研究通过RT-qPCR和Western blot技术探讨SOX5在不同发育阶段公鸡睾丸中表达差异,并比较正常公鸡与弱精子症公鸡睾丸中SOX5的差异。同时,采用免疫组织化学技术进行蛋白表达定位,旨在从分子水平探索SOX5在公鸡睾丸精子发生和精子活力中的作用。
1 材料与方法 1.1 试验材料试验动物来自中国农业科学院北京畜牧兽医研究所昌平试验基地。选取0(出生24 h内)、5、15、40和60周龄的健康北京油鸡公鸡和40周龄弱精子症公鸡各5只,屠宰后迅速采集左侧睾丸,取中心部位样本(长1.0 cm×宽1.0 cm×高0.5 cm)放入甲醛固定液(10%福尔马林溶液)保存,用于制备组织切片,其余样本液氮速冻后,-80 ℃保存,用于后续试验。弱精子症公鸡筛选参考富丽等[10]和Y.Y.Wang等[11]方法。本研究中正常个体精子活力为75.3%,弱精子症个体精子活力为18.3%。
1.2 总RNA提取利用RNA提取试剂盒(天根,北京)提取上述公鸡睾丸组织总RNA。用核酸定量仪NanoDrop2000(Thermo scientific,美国)测定浓度,通过A260 nm/A280 nm值计算RNA纯度。
1.3 cDNA合成和RT-qPCRcDNA反转录参照Primer Script TM RT Master Mix(Perfect Real time)试剂盒(TaKaRa,美国)操作说明,反转录产物于-20 ℃保存备用。RT-qPCR以β-actin为内参基因,根据NCBI GenBank中鸡SOX5基因和β-actin基因序列设计引物,如表 1所示。采用7500型荧光定量PCR仪(ABI,美国)对SOX5和β-actin进行分析,RT-qPCR参照KAPA SYBR FAST Universal qPCR Kit(KAPA,美国)试剂盒说明书。每个样品设置2个技术重复。
|
|
表 1 RT-qPCR所用基因的引物信息 Table 1 Primer information of genes used in RT-qPCR |
取100 mg左右睾丸组织,加入10倍体积的RIPA裂解液(南京建成),用高通量组织研磨器50 Hz 20 s,研磨3次。研磨后, 冰上静置15 min,不时摇晃。4 ℃,12 000 r·min-1离心15 min,取上清液即为总蛋白。所得样品总蛋白用BCA蛋白定量试剂盒(北京康为世纪)测定蛋白浓度,并用生理盐水将蛋白浓度均一化为2 μg·μL-1,分装,-80 ℃保存备用。
1.5 Western blot检测取45 μg总蛋白,95 ℃变性10 min,4%~12% SDS-PAGE电泳分离。电泳结束后,将蛋白从凝胶转至PVDF膜(0.22 μm,Millipore,美国)上,用5%脱脂奶粉(BD,美国)室温摇床封闭30 min,分别加入1.5 μg的鼠源β-actin一抗(Abcom,英国,1:5 000)和兔源SOX5一抗(Abclonal,美国,1:200),室温摇床孵育1 h。随后TBST洗涤3次,再分别加入2 μg的山羊抗鼠二抗(Abcom,英国,1:5 000)和山羊抗兔二抗(Abclonal,美国,1:200),室温摇床孵育40 min,TBST洗涤3次。ECL超敏发光液(Coolaber,美国)孵育,Image Quant LAS4000mini仪器(GE,美国)凝胶成像仪器自动曝光,采集图像。
1.6 免疫组织化学方法制作不同周龄公鸡睾丸组织石蜡切片,二甲苯脱蜡后再进行梯度酒精脱水。枸橼酸盐(柠檬酸三钠)热修复,然后加3% H2O2室温孵育,消除内源性过氧化物酶活性。5% BSA封闭1 h,PBS作为阴性对照孵育,加入兔源SOX5一抗(Abclonal,美国,1:200)室温孵化1 h,PBS洗涤后再加入山羊抗兔二抗(Abclonal,美国,1:200)室温孵化40 min。PBS洗涤后,加DAB显色2 min,PBS冲洗10 min。再使用苏木精复染,梯度酒精脱水,中性树胶封片,镜检观察。
1.7 数据分析根据荧光定量所得到的Ct值,采用2-△△Ct方法进行处理[12],利用SAS 8.0软件对数据进行单因素方差分析,Duncan’s多重比较检验组间表达量差异,P < 0.05表示差异显著。Western blot结果使用Image J图像分析软件分析,其中SOX5蛋白灰度值为SOX5蛋白IOD值和β-actin IOD值的比值。
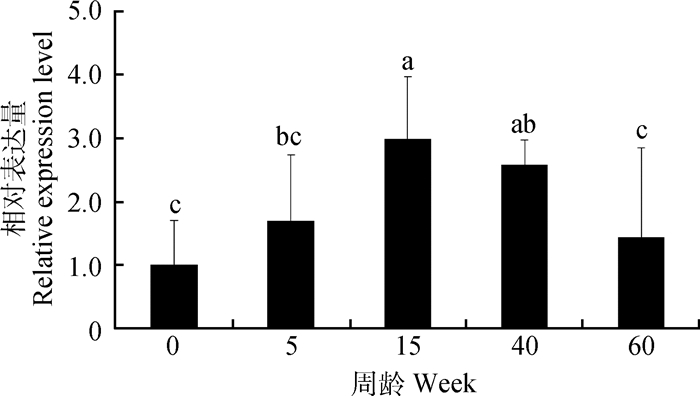
2 结果 2.1 不同周龄公鸡睾丸中SOX5 mRNA的表达如图 1所示,SOX5 mRNA在不同发育阶段睾丸中均有表达。随着周龄的增长,SOX5 mRNA表达量呈现先增长后下降的趋势,且15周龄睾丸中SOX5 mRNA表达量显著高于0、5和60周龄(P < 0.05)。
|
不同字母表示差异显著(P < 0.05);n=5。下同 Different letters mean significant difference (P < 0.05); n=5. The same as below 图 1 SOX5 mRNA在不同发育阶段公鸡睾丸中的表达 Figure 1 The expression of SOX5 mRNA in the testis of roosters of different ages |
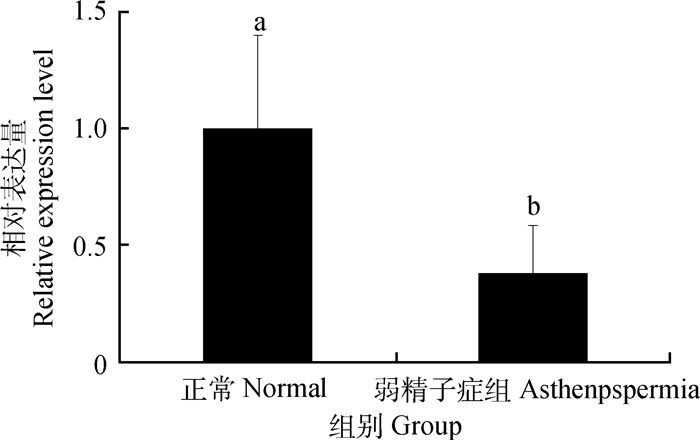
40周龄弱精子症公鸡睾丸中SOX5 mRNA的表达量是正常公鸡的0.38倍,且差异显著(P < 0.05)(图 2)。
|
图 2 SOX5 mRNA在40周龄正常公鸡和弱精子症公鸡睾丸中表达 Figure 2 The expression of SOX5 mRNA in the testis of normal and asthenospermia roosters of 40 weeks of age |
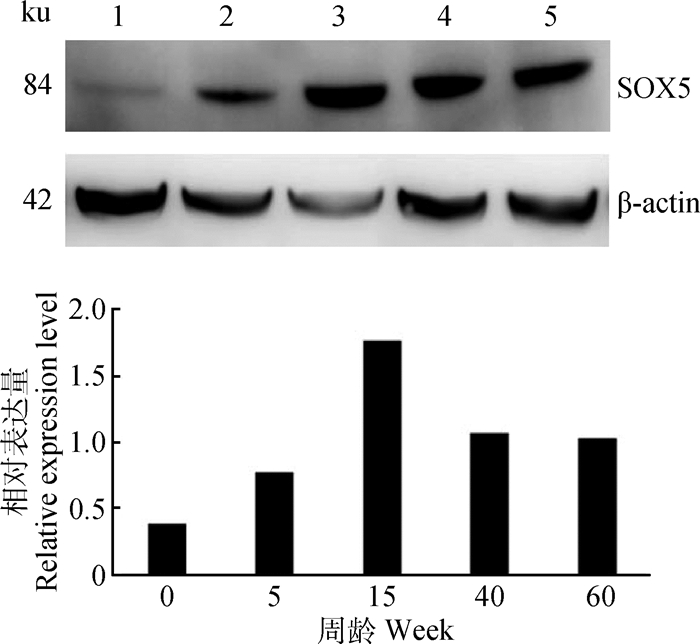
SOX5蛋白在不同发育阶段公鸡睾丸中均有表达,0周龄公鸡睾丸中,SOX5蛋白表达量最低,15周龄表达量最高,且呈现先增长后下降的趋势(图 3),与mRNA表达结果一致。
|
1~5.0、5、15、40、60周龄 1-5.0, 5, 15, 40, 60 week 图 3 SOX5蛋白在不同发育阶段公鸡睾丸中表达 Figure 3 The expression of SOX5 protein in the testis of roosters of different ages |
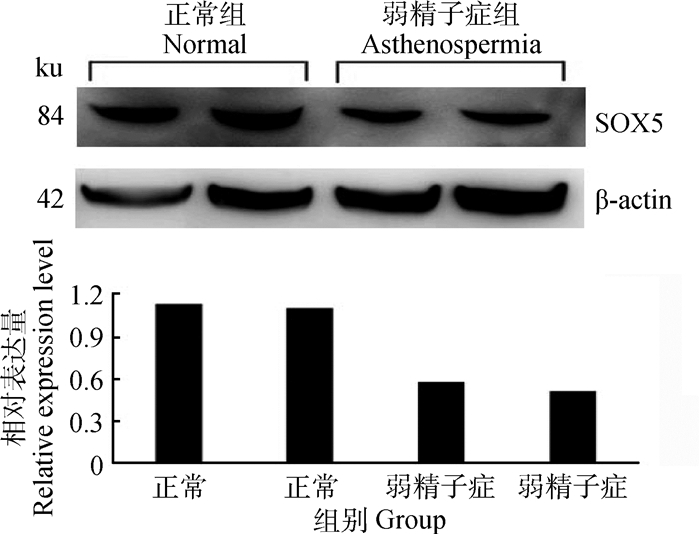
40周龄正常公鸡睾丸组织中,SOX5的蛋白表达量高于弱精子症公鸡(图 4),与mRNA结果一致。
|
图 4 SOX5蛋白在40周龄正常公鸡和弱精子症公鸡睾丸中表达 Figure 4 The expression of SOX5 protein in the testis of normal and asthenospermia roosters of 40 weeks of age |
在0和5周龄公鸡睾丸中,SOX5蛋白只在少量精原细胞和支持细胞中表达(图 5A,B);15和40周龄公鸡睾丸中,SOX5蛋白在支持细胞、初级精母细胞、次级精母细胞、圆形精子细胞和成熟的精子均表达(图 5C,D)。与40周龄正常公鸡睾丸相比,弱精子症公鸡睾丸中SOX5蛋白表达较少(图 5D,E)。
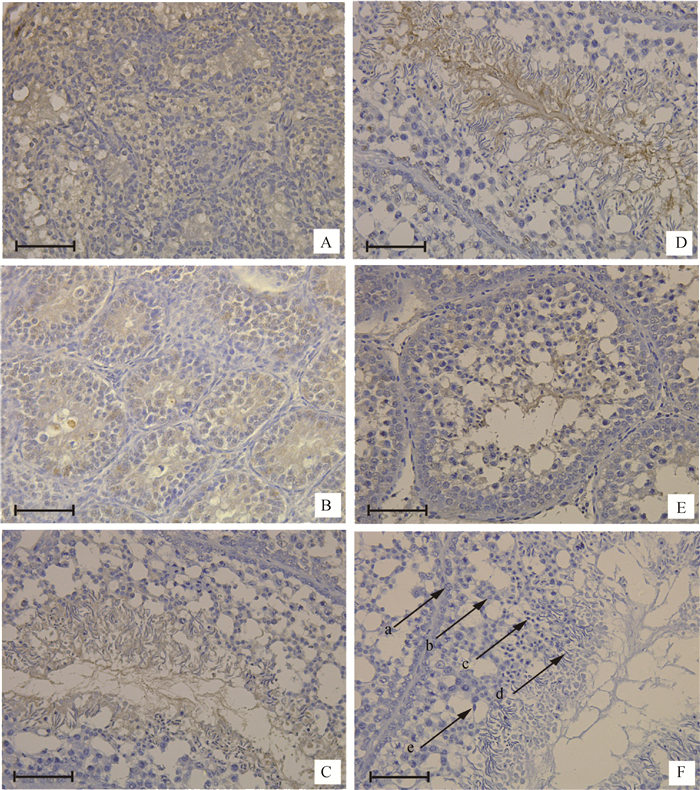
|
A~D.0、5、15和40周龄正常公鸡;E.40周龄弱精子症公鸡;F.阴性对照。a.精原细胞;b.精母细胞;c.圆形精子细胞;d.成熟精子;e.支持细胞 A-D.Normal testis at 0, 5, 15, and 40 weeks of age; E. Asthenospermia testis at 40 weeks of age; F.Negative control. a. Spermatogonia; b. Spermatocyte; c. Spermatid; d. Mature sperm; e. Sertoli cell 图 5 SOX5在不同发育阶段公鸡和弱精子症公鸡睾丸组织的免疫组织化学结果(标尺:400 μm) Figure 5 Immunohistochemistry with SOX5 using the immunoperoxidase method and hematoxylin counterstain in the testis of roosters of different ages and asthenospermia roosters of 40 week(Scale bar: 400 μm) |
精子鞭毛结构决定精子的运动能力,鞭毛也参与精子的形成过程,因此在精子发生周期中,鞭毛的正确构建较为关键[13]。在哺乳动物中,很多精子鞭毛基因已经被验证[14]。与哺乳动物不同,家禽是内部受精的卵生动物,有储存精子和多卵受精能力。所以了解其精子形成和活力的调控机理,有助于提高其繁殖能力。前期通过iTARQ蛋白质组学技术筛选出SOX5和SOX9等蛋白在正常公鸡和弱精子症公鸡睾丸中表达存在差异[15-16]。
SOX5在哺乳动物睾丸的生殖细胞中表达,并能调节细胞增殖、分化和精子发生等过程[8]。本试验发现SOX5在刚出生的公鸡睾丸中就表达,且随着周龄的增加,SOX5在睾丸中的表达呈现先上升后降低的趋势。同时SOX5在睾丸中的表达具有细胞特异性,在支持细胞、精原细胞、各级精母细胞、圆形精子细胞及成熟精子中都存在,且圆形精子细胞中表达量最高。刚出生公鸡睾丸中含有大量的支持细胞,而此阶段SOX5主要在支持细胞中表达。C.F.Liu等[17]研究发现,SOX5可激活SOX9,而SOX9主要存在睾丸的支持细胞中[18],参与睾丸细胞增殖分化及维持睾丸功能等过程。SOX5和SOX9在鸡胚早期发育时期均表达[19],对性别分化起决定性作用[20]。SOX5在15周龄公鸡睾丸中表达量最高,主要是由于公鸡在15周龄时处于性成熟期,睾丸含有大量的圆形精子细胞,并有少量的精子生成;30~50周龄是种鸡繁殖高峰期,SOX5表达量维持在较高水平;60周龄时,睾丸萎缩,精子生成减少[21],这可能是SOX5表达相对较低的原因,本研究中SOX5基因和蛋白随着公鸡周龄增加的表达趋势与M.Daigle等[5]在小鼠上的研究结果一致。因此,早期发育过程中, SOX5表达量与生精细胞增殖分化有关,性成熟后,其表达量与精子生成呈正相关。
SOX5在40周龄正常公鸡睾丸的表达量显著高于弱精子症公鸡。在生精小管中,精原干细胞增殖分化、精母细胞减数分裂后形成成熟精子。其中CATSPER1、SPAG6和SPAG16基因敲除的细胞肌动蛋白骨架断裂[22-23],从而影响纤毛迁移,小鼠精子丧失直线运动能力,精子活力差[24]。SPAG6在精母细胞、圆形精子细胞和成熟精子中均表达,而SOX5通过与CATSPER1和SPAG6等精子活力候选基因的启动子区域结合,进而调节精子活力[25-27]。因此,SOX5是精子鞭毛功能调节基因,鞭毛结构变化时精子生成、形态和运动都受到影响。由于弱精子症公鸡睾丸初级精母细胞、次级精母细胞、圆形精子细胞和成熟的精子比正常公鸡少,所以SOX5在40周龄弱精子症公鸡睾丸的表达显著低于正常公鸡,因此SOX5可以作为弱精子症潜在的生物标记。
4 结论本研究表明,SOX5在公鸡各周龄的睾丸组织中均表达,SOX5表达量与生精细胞增殖分化有关;此外,SOX5的表达量可能会影响精子活力。这为进一步研究SOX5对精子发生和睾丸功能调节机制提供参考,也为治疗精子活力低下和睾丸损伤等疾病提供理论依据。
| [1] | EDDY E M. Role of heat shock protein HSP70-2 in spermatogenesis[J]. Rev Reprod, 1999, 4(1): 23–30. DOI: 10.1530/ror.0.0040023 |
| [2] | WANG F B, ZHANG Q G, CAO J L, et al. The microtubule plus end-binding protein EB1 is involved in Sertoli cell plasticity in testicular seminiferous tubules[J]. Exp Cell Res, 2008, 314(1): 213–226. DOI: 10.1016/j.yexcr.2007.09.022 |
| [3] | DU Z W, CONG H C, YAO Z. Identification of putative downstream genes of Oct-4 by suppression-subtractive hybridization[J]. Biochem Biophys Res Commun, 2001, 282(3): 701–706. DOI: 10.1006/bbrc.2001.4636 |
| [4] | FOSTER J W, GRAVES J A. An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene[J]. Proc Natl Acad Sci U S A, 1994, 91(5): 1927–1931. DOI: 10.1073/pnas.91.5.1927 |
| [5] | DAIGLE M, ROUMAUD P, MARTIN L J. Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development[J]. Mol Cell Biochem, 2015, 407(1-2): 209–221. DOI: 10.1007/s11010-015-2470-7 |
| [6] | HIRAOKA Y, OGAWA M, SAKAI Y, et al. The mouse Sox5 gene encodes a protein containing the leucine zipper and the Q box[J]. Biochim Biophys Acta, 1998, 1399(1): 40–46. DOI: 10.1016/S0167-4781(98)00086-4 |
| [7] | LEFEBVRE V, LI P, DE CROMBRUGGHE B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type Ⅱ collagen gene[J]. EMBO J, 1998, 17(19): 5718–5733. DOI: 10.1093/emboj/17.19.5718 |
| [8] | DENNY P, SWIFT S, CONNOR F, et al. An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein[J]. EMBO J, 1992, 11(10): 3705–3712. |
| [9] | WRIGHT D, BOIJE H, MEADOWS J R S, et al. Copy number variation in intron 1 of SOX5 causes the Pea-comb phenotype in chickens[J]. PLoS Genet, 2009, 5(6): e1000512. DOI: 10.1371/journal.pgen.1000512 |
| [10] |
富丽, 孙研研, 薛夫光, 等. 公鸡弱精症相关候选基因的表达分析[J]. 畜牧兽医学报, 2015, 46(6): 889–895.
FU L, SUN Y Y, XUE F G, et al. The expression of the six candidate genes for asthenospermia of the rooster[J]. Acta Veterinaria et Zootechnica Sinica, 2015, 46(6): 889–895. (in Chinese) |
| [11] | WANG Y Y, XUE S Y, LIU X R, et al. Analyses of Long Non-Coding RNA and mRNA profiling using RNA sequencing during the pre-implantation phases in pig endometrium[J]. Sci Rep, 2016, 6: 20238. DOI: 10.1038/srep20238 |
| [12] | LIVAK K J, SCHMITTGEN T D. Analysis of relative gene expression data using real-time quantitative PCR and the method[J]. Methods, 2001, 25(4): 402–408. DOI: 10.1006/meth.2001.1262 |
| [13] | HOROWITZ E, ZHANG Z B, JONES B H, et al. Patterns of expression of sperm flagellar genes: early expression of genes encoding axonemal proteins during the spermatogenic cycle and shared features of promoters of genes encoding central apparatus proteins[J]. Mol Hum Reprod, 2005, 11(4): 307–317. DOI: 10.1093/molehr/gah163 |
| [14] | BORG C L, WOLSKI K M, GIBBS G M, et al. Phenotyping male infertility in the mouse: how to get the most out of a 'non-performer'[J]. Hum Repord Update, 2010, 16(2): 205–224. DOI: 10.1093/humupd/dmp032 |
| [15] |
薛夫光. 利用iTRAQ蛋白质组学技术筛选与种公鸡繁殖力相关的候选蛋白[D]. 北京: 中国农业科学院, 2015.
XUE F G. iTRAQ-based proteomic analyses for identification of candidate proteins underlying the fecundity of breeder cocks[D]. Beijing: Chinese Academy of Agricultural Sciences, 2015. (in Chinese) |
| [16] | ESCALIER D. Knockout mouse models of sperm flagellum anomalies[J]. Hum Reprod Update, 2006, 12(4): 449–461. DOI: 10.1093/humupd/dml013 |
| [17] | LIU C F, LEFEBVRE V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis[J]. Nucleic Acids Res, 2015, 43(17): 8183–8203. DOI: 10.1093/nar/gkv688 |
| [18] | KENT J, WHEATLEY S C, ANDREWS J E, et al. A male-specific role for SOX9 in vertebrate sex determination[J]. Development, 1996, 122(9): 2813–2822. |
| [19] | BOIJE H, HARUN-OR-RASHID M, LEE Y J, et al. Sonic Hedgehog-signalling patterns the developing chicken comb as revealed by exploration of the pea-comb mutation[J]. PLoS One, 2012, 7(12): e50890. DOI: 10.1371/journal.pone.0050890 |
| [20] | AKIYAMA H, CHABOISSIER M C, MARTIN J F, et al. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6[J]. Genes Dev, 2002, 16(21): 2813–2828. DOI: 10.1101/gad.1017802 |
| [21] | SARABIA FRAGOSO J, PIZARRO DíAZ M, ABAD MORENO J C, et al. Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels)[J]. Reprod Domest Anim, 2013, 48(2): 345–352. DOI: 10.1111/j.1439-0531.2012.02161.x |
| [22] | PARK T J, HAIGO S L, WALLINGFORD J B. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling[J]. Nat Genet, 2006, 38(3): 303–311. DOI: 10.1038/ng1753 |
| [23] | PAN J H, YOU Y J, HUANG T, et al. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1[J]. J Cell Sci, 2007, 120(11): 1868–1876. DOI: 10.1242/jcs.005306 |
| [24] | JAROW J P. A sperm ion channel required for sperm motility and male fertility[J]. J Urol, 2002, 168(5): 2309. DOI: 10.1016/S0022-5347(05)64376-5 |
| [25] | MATA-ROCHA M, HERNÁNDEZ-SÁNCHEZ J, GUARNEROS G, et al. The transcription factors Sox5 and Sox9 regulate Catsper1 gene expression[J]. FEBS Lett, 2014, 588(18): 3352–3360. DOI: 10.1016/j.febslet.2014.07.024 |
| [26] | ZHANG L, LIU Y H, LI W, et al. Transcriptional regulation of human sperm-associated antigen 16 gene by S-SOX5[J]. BMC Mol Biol, 2017, 18: 2. DOI: 10.1186/s12867-017-0082-3 |
| [27] | KISELAK E A, SHEN X N, SONG J M, et al. Transcriptional regulation of an axonemal central apparatus gene, sperm-associated antigen 6, by a SRY-related high mobility group transcription factor, S-SOX5[J]. J Biol Chem, 2010, 285(40): 30496–30505. DOI: 10.1074/jbc.M110.121590 |



